Abstract
Currently, char substrates gain a lot of interest since soils amended with such substrates are being discussed to increase in fertility and productivity, water retention, and mitigation of greenhouse gases. Char substrates can be produced by carbonization of organic matter. Among different process conditions, temperature is the main factor controlling the occurrence of organic and inorganic contaminants such as phenols and furfurals, which may affect target and non-target organisms. The hydrochar produced at 200 °C contained both furfural and phenol with concentrations of 282 and 324 mg kg−1 in contrast to the 300 °C hydrochar, which contained only phenol with a concentration of 666 mg kg−1. By washing with acetone and water, these concentrations were significantly reduced. In this study, the potential toxic effects of hydrochars on the free-living nematode Caenorhabditis elegans were investigated via gene transcription studies using the following four matrices: (i) raw rice husk, (ii) unwashed rice char, (iii) acetone/water washed rice char, and (iv) the wash water of the two rice chars produced at 200 and 300 °C via hydrothermal carbonization (HTC). Furthermore, genetically modified strains, where the green fluorescent protein (GFP) gene sequence is linked to a reporter gene central in specific anti-stress regulations, were also exposed to these matrices. Transgenic worms exposed to hydrochars showed very weak, if any, fluorescence, and expression of the associated RNAs related to stress response and biotransformation genes was surprisingly downregulated. Similar patterns were also found for the raw rice husk. It is hypothesized that an unidentified chemical trigger exists in the rice husk, which is not destroyed during the HTC process. Therefore, the use of GFP transgenic nematode strains cannot be recommended as a general rapid monitoring tool for farmers treating their fields with artificial char. However, it is hypothesized that the observed reduced transcriptional response with the subsequent lack of energy-consuming stress response is an energy-saving mechanism in the exposed nematodes. If this holds true in future studies, this finding opens the window to an innovative new field of stress ecology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
New management practices for organic residues such as rice husk include the generation of char substrates, which hold promise as soil amendments for carbon sequestration, reduction in the emission of greenhouse gases, and improving soil fertility (Atkinson et al. 2010; Mao et al. 2012). The enhancement of soil fertility by the amendment of char substrates has been recognized by studies of terra preta de índio soils and their functioning (Glaser et al. 2001). In addition to biochars, which are derived from pyrolysis in an oxygen-depleted atmosphere, other char substrates gain interest, as for example the so-called hydrochars (Mumme et al. 2011). These substrates are generated by hydrothermal carbonization (HTC) (Libra et al. 2011; Sevilla et al. 2011; Titirici et al. 2007). The basis of hydrochars is organic material that is converted under wet conditions at temperatures of 180–250 °C (Diakité et al. 2013).
Although the benefits of char substrate applications have often been reported, there is only little knowledge about potential adverse effects. They may derive from the chemical composition of the feedstock itself or from the carbonization process. Both processes, pyrolysis and HTC, may be associated with the generation of toxicants and increased persistence of xenobiotic chemicals (Kookana 2010) and metals and metalloids (Agrafioti et al. 2013; Pellera et al. 2012). For instance, incomplete combustion and pyrolysis may generate polycyclic aromatic hydrocarbons (PAHs) and other persistent organic pollutants (POPs) (Keiluweit et al. 2012; Wiedner et al. 2013). As a consequence, adverse effects to terrestrial organisms have to be anticipated. Toxicity toward plants and microorganisms has already been reported in some cases (Oleszczuk et al. 2014), and standard model animals are being developed as new promising tools for future ecotoxicological assessment of charcoal compounds (Linhares et al. 2012). In addition, through direct contact with soil-dwelling animals, PAHs and other POPs adsorbed on char substrates can easily attach to microorganisms (Thion et al. 2012) that serve as food for these invertebrates, and thereby can enter the food chain.
In a recent study with the earthworm Eisenia fetida Savigny, several tests (avoidance, growth and reproduction, anti-oxidative status) were carried out to assess the potential toxicity of soil amended with biochar produced from wood chips (Li et al. 2011). The authors found that earthworms avoided soils containing 100 and 200 g kg−1 of dry biochar. In this case, the behavior of the worms was attributed mainly to insufficient moisture, but not to potentially harmful contaminants of the amended soils.
Comparing natural humic substrates and two char substrates of different origins with biomolecular techniques, it was demonstrated that humic substrates increased the transcription of a homolog to the human anticancer gene p53 in the nematode Caenorhabditis elegans Maupas. In contrast, a hydrochar made of poplar wood did not show a clear effect (Chakrabarti et al. 2011). Therefore, more comprehensive studies are needed to better elucidate the effects of chars to soil organisms. Being the most abundant metazoans in soils, nematodes play a central role in the microbial loop and nutrient bioavailability (Coleman 1994); therefore, any reduction in nematode biomass and/or activity may, in turn, carry the risk to reduce the bioavailability of key nutrients to plants.
During the hydrochar production, source material loses weight and potential toxic substances migrate into the wash water (Kalderis et al. 2014). Therefore, the char products as well as the different wash waters were tested for toxicity and analyzed for potentially toxic chemical compounds. In biochars, polycyclic aromatic hydrocarbons (PAHs) form as the result of secondary thermochemical reactions at temperatures over 700 °C (Libra et al. 2011; McGrath et al. 2007; Norinaga et al. 2009). Since the production temperatures of the hydrochars did not exceed 300 °C, the products were not analyzed for this class of chemicals, but for furfurals, including hydroxymethylfurfural (HMF), and phenols. Recent comparative studies support this selection of xenobiotics analyzed. For instance, Wiedner et al. (2013) compared the xenobiotic contamination of biochars and hydrochars and found that hydrochars have only very low proportions of aromatic compounds compared to biochars but are richer in functional groups compared to biochars. Although it cannot be excluded, it is very unlikely that minor contaminants, such as highly condensed PAHs, may function as triggers of gene transcription. Therefore, the current study focused on potentially water-soluble toxic compounds such as furfurals and phenols.
As first step, this study tested the toxicity of selected hydrochars with 11 different transgenic nematode strains prepared to produce luminescence in the presence of stressors. These strains were transfected with different reporter genes coupled to the green fluorescence protein gene. The selected reporter genes are central in various stress defense pathways, for instance, general stress, hsp genes and daf-16; biotransformation of lipophilic chemicals, cyp genes; biotransformation of functionalized lipophilic chemicals, gst genes; oxidative stress, sod genes, etc. Increased transcription of the corresponding genes should result in green fluorescing worms and provide hints of the agonist chemicals. To confirm the results obtained with the genetically modified worms, in a second step, quantitative PCR (qPCR) was performed to identify potential alterations in the corresponding mRNAs. Based on this set of tools, we hypothesized that (1) hydrochars independent of their feedstock will adversely affect C. elegans, and, if present, these effects can be monitored via transcription of key genes, and (2) therefore, transgenic nematodes could be applied in monitoring programs of char-amended agricultural sites.
Material, methods, and test design
Hydrochar production and post-treatment
Char material was produced at the Technological Educational Institute of Crete, Greece, via HTC of rice husk (Oryza sativa L.) at 200 and 300 °C with residence times between 2 and 16 h. It could be shown that between 34 and 42 % of the hydrochar produced at 200 °C and between 33 and 64 % of the hydrochar produced at 300 °C were removed by acetone in the post-treatment (Kalderis et al. (2014). For further examination of the hydrochars, the residence time of 6 h was selected in this study, since this condition resulted in the greatest difference in loss of weight after the wash process regarding the temperature setting (200 or 300 °C).
Post-treatments were conducted at the Leibniz Institute for Agricultural Engineering Potsdam-Bornim (ATB) in Potsdam. Part of the obtained hydrochars and rice husks was washed with acetone and water to remove dissolvable compounds, which may be toxic. Ten grams of hydrochars was mixed with 200 ml of acetone (99.8 p.a.) in a flask, covered with aluminum foil to prevent evaporation, and shaken for 5.5 h at 120 rpm at room temperature. Thereafter, the mixture was filtered (folded filter paper MN 280 ¼, Machery-Nagel, Düren, Germany). To remove traces of acetone from the char, the wet mixture was washed two times with 300 ml of distilled water, given in several small quantities. These two water fractions were chemically characterized. The second wash water was also used for exposition of the nematode test organisms. Filters and char material were dried at 60 °C overnight before it was removed from the filter. The same procedure was performed for the non-carbonized rice husk.
Preparation of rice husk and hydrochars
For feeding of the worms, 1 g of each test substrate, the rice husk, and the washed and the unwashed char material were dissolved in weak sodium hydroxide solution (39.6 ml aqua bi-distilled/0.4 ml NaOH 10 M) to increase the solubility of the char material (Chakrabarti et al. 2011), shaken overnight at room temperature, and then centrifuged at 2500 g for 2 min and neutralized. To ensure sterile conditions, the solvent was filtered through a 0.2-μm membrane (Sarstedt) and stored at 4 °C. The sterile char solution was thereafter mixed with an up concentrated solution of Escherichia coli bacteria and poured onto the nematode growth medium (NGM) plates. As tested before in several works, this ensures the worm’s uptake of the substance via their food.
Chemical characterization of hydrochars and washing water
A comprehensive characterization of elemental composition, metal contents, H/C atomic ratios, and combustion profiles of hydrochars produced by hydrothermal carbonization of rice husks is available in Kalderis et al. (2014). The authors also described nitrogen adsorption and desorption isotherms of the produced hydrochars.
For the biotests, dry matter was determined by drying subsamples at 105 °C for 12 h. The ash content of the dry matter was obtained after ignition at 550 °C. Electrical conductivity and pH were measured in a 1:10 biochar water mixture, after shaking at 150 rpm for 15 min. Rice husk and carbonized products were analyzed for total C, H, and S content with an elemental analyzer (Vario EL III, Elementar, Germany). Total P and Kjeldahl-N were derived after acid digestion using a selenium catalyst. Flow injection analysis was applied to measure NH4 +-N.
Phenols (phenol, cresol, catechol) and furfurals (furfural and hydroxymethylfurfural (HMF)) were quantified using high-pressure liquid chromatography (HPLC, Dionex™ ICS-3000, Thermo Scientific, US). Ten grams of solid samples was homogenized and dispersed in distilled water (Heidoph DIAX 900, Schwabach, Germany). Then, the samples were extracted via steam distillation (Gerhardt Vapodest 20, Königswinter, Germany). From the liquid samples of the washing steps with acetone and water, only those of the wash water could be analyzed. The samples were filtrated with PTFE syringe filter (Neolab PTFE 0.2 μm green, Heidelberg, Germany) prior to measurement. For the measurement, sampler temperature was set to 10 °C and the volumetric flow rate to 1 ml min−1, and materials were separated using a C18 column (Knauer Eurospher II, 150*4 mm with precolumn, Berlin, Germany), which was set to 23 °C. Eluents were ultrapure water and acetonitril ultrapure water mix (1:1). During the run, the composition of the solvent was adjusted at several steps (multistep gradient).
Test organism
C. elegans, a free-living bacterivorous nematode with 1-mm length, was introduced as model organism by Brenner (1974). It is very suitable for biological research due to its complete sequenced genome and its easy handling. C. elegans lives in composts in moderate climate zones, within a temperature range between 4 and 30 °C (Kiontke and Sudhaus 2006). Other advantages of this model organism are the low cost for strains and the short life cycle reaching sexual maturity within about 5 days.
Wild-type strain N2 (var. Bristol) and transgenic GFP strains, except of the Pcyp35A3::GFP strain, and the Escherichia coli strain OP50 were obtained from the Caenorhabditis elegans Center CGC (University of Minnesota) and cultivated in the Laboratory of Freshwater and Stress Ecology of the Biological Department at Humboldt-Universität zu Berlin. The Pcyp35A3::GFP was constructed and provided by Dr. Ralph Menzel, Humboldt-Universität zu Berlin. The transgenic strains contain a DNA sequence of the GFP protein ligated downstream of the target gene promoter. All strains were pre-cultivated on nematode growth medium (NGM) agar plates and fed with Escherichia coli strain OP50 ad libitum.
Positive controls of GFP strains
For the monitoring of the response to different contaminants, several genetically modified strains of C. elegans were exposed to selective chemical and physical stressors. The putative function of the respective reporter genes of the different strains with regard to responses to the selected stressors is reflected by the resulting fluorescence marked with a plus sign, as identified by visible inspection (Table 1). The putative functions of the proteins of the genes tested are listed below in Table 3.
Each strain has a green fluorescent protein (GFP) vector located in a region (mainly in the promoter region) of the specific genes given in Table 1, while the gene is regulated, also the GFP is synthesized. Under a fluorescent microscope, the nematode glows green. Positive controls (chemical and physical model stressors; Table 1) were used to validate induction of a specific genetic construct as reported by Menzel et al. (2001). Nematodes at all developmental stages were exposed to the test substances given via food bacteria for 24 to 48 h and approximately 100 individuals per test were inspected. The fluorescent signal was visualized via a Keyence digital microscope camera with a GFP filter (Kosel et al. 2011). Non-exposed strains of the nematode were analyzed for auto-fluorescence and served as the negative control. By visual inspection, the response of exposed worms was monitored in a “Yes/No” manner (Fig. 1). For the GFP experiments, 80–100 harvested eggs were pre-exposed to the test substrates and worms grew for 24 to 48 h. Only one NGM plate was used for inspection in Yes/No manner.
Synchronization and exposure scenario
Pre-cultivated and well-fed individuals of all GFP strains were synchronized through egg preparation according to Strange et al. (2007) with sodium hypochlorite (Sigma-Aldrich, Taufkirchen, Germany). This method implicates dissolving the worm’s membrane and structure in a sodium hypochlorite solution. Between 1000 and 3000 eggs were washed and placed on the exposure agar plate. Larvae grew for 52 h until young adults on seven different substrates and one negative control. Then, the worms were harvested and washed with cold M9 buffer from the NGM plates and collected in 1.5-ml Eppendorf tubes.
RNA preparation and cDNA synthesis
At the first day of adulthood, the nematodes were harvested by rinsing with M9 buffer, washed at least three times, shock frozen in liquid nitrogen, and stored at −80 °C. Samples were cultivated in triplicates. The samples were homogenized with 0.5-mm glass beads in a SpeedMill Plus (AnalytikJena, Germany), and thereafter, the RNA was extracted using the innuSPEED Tissue RNA kit (AnalytikJena, Germany). The extracted RNA was examined by gel electrophoresis and then quantified with a spectrophotometer. The complementary DNA (cDNA) synthesis was carried out with approximately 1500 ng ml−1 of RNA and the M-MLV revertase (Harris et al. 2010) (Promega, Madison, USA).
qRT-PCR
Gene transcription analysis was performed with cDNA samples according to the given instructions of the qRT-PCR green core kit (Jena Bioscience GmbH, Jena, Germany). The cDNA concentrations of all samples were normalized by using the two reference genes act-1 and cdc-42. Hereby, the mean threshold of both genes established the calculation of the dilution factors for all samples. Subsequently, a final normalization qRT-PCR was performed with the reference gene act-1, to which all following PCR data were referred to.
Statistics
Gene transcription studies were carried out in three biological replicates and data is shown as means +/− standard error of means (Fig. 2). Changes in transcript abundances were determined by the computer software Bio-Rad IQ5, and significance of the results were checked in two ways: (1) They were considered significant if they were at least by a factor 2 different from the control level of 1 (>2.0-fold in upregulations and <0.5 fold in downregulations); (2) a one way ANOVA was performed with Sigma Stat at significance level of p < 0.05.
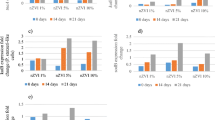
Changes in transcripts of cyp (a), gst (b), and sod (c) genes in the C. elegans sod-3::gfp strain exposed to applied hydrochars as compared to untreated controls. Data are means of four values ± SEM. Significance at p < 0.05 is marked with asterisks. Data are considered significantly different from the control if different by a factor >2.0. Additionally, asterisk indicates significantly different from the control at p < 0.05
Results
Chemical characterization of hydrochars and the feedstock
Hydrothermal carbonization by dehydration yielded chars with enhanced contents of carbon and ashes as expected, resulting in reduced hydrogen content (Table 2). The temperature of the carbonization process clearly affected the contents of nitrogen and phosphorus in the two chars. Carbonization at 200 °C decreased the P content and the NKjel content. This may be explained by acid hydrolysis of P and N compounds, since the pH was 3 units below that in the 300 °C carbonization (Table 2). The rice husk contained only 2.4 mg kg−1 furfural and no phenol, which can be considered as a derivative of lignin. The two hydrochars contained high amounts of the cyclic hydrocarbons like furfurals and phenols before they were treated by washing. Of the compounds under study, HMF, furfural, and phenol were found in the 200 °C hydrochar, in contrast to the 300 °C hydrochar, where only phenol but no furfurals could be detected (Table 2).
Alteration of hydrochars by washing
The washing of the two chars in acetone dissolved the particulate matter resulting in weight losses of 35 % (200 °C) and 57 % (300 °C) as reported by Kalderis et al. (2014). This loss can be attributed primarily to the removal of carbon and nitrogen compounds, as evidenced by the decreases in carbon content and Kjeld-N between between 9 to 29 % after washing with acetone and water (Table 2). Furthermore, the washings changed the charge of the chars as reflected by their pH values. Before washing, they were quite disparate, 3.95 (200 °C) and 7.72 (300 °C), while after washing, they were in the same range, 4.70 and 4.61, respectively. This corresponds to the strong decrease of the electrical conductivity after washing (Table 2).
Cyclic hydrocarbons, which are known to be produced during HTC, have been removed to high degrees between 79 and 96 % (Table 2). Some of the removed compounds were found in the aqueous washing water. In the first water fraction of the 200 °C hydrochar, HMF and furfural were found at high concentrations, whereas the wash water of the 300 °C hydrochar contained only catechol and cresol (Table 2). The lack of phenol in the aqueous washing water of both chars must be caused by the complete removal in the first washing step with acetone, which could not be analyzed by the HPLS system applied. The second wash water fraction did not contain any of these chemicals.
Application of GFP strains
Positive controls
All tested GFP strains except the hsp-16.2::gfp strain responded to at least one chemical or physical trigger (Table 1). The putative functions of the proteins of the genes tested are listed in Table 3. For instance, PCB52 and β-naphthoflavone, well-known agonists of the aryl hydrocarbon receptor, induced fluorescence intensity of the cyp35A3::gfp strain with the latter chemical being more potent. Exposed worms showed green fluorescence particularly in the intestine (Fig. 1). The hsp-4::gfp strain responded to atropine exposure and gst-4::gfp to a variety of chemicals as well as heat. Also, daf-16::gfp responded to a variety of challenges, however, most prominently to heat stress. Overall, the selected GFP strains appeared to be applicable as monitoring tools for the positive control stressors selected in this study, with the exception of hsp-16.2::gfp. The strains gst-4::gfp and sod-3::gfp were the most responsive overall with six to seven positive reactions after chemical or physical challenges.
Exposure to hydrochars
Surprisingly, the green fluorescence signals of worms exposed to hydrochars were very weak and could most often not be distinguished from the GFP zero control individuals (Supplementary Fig. S1 and Fig. 2). Weak fluorescence signals in both the excretory cells and pharynx were most likely indicative of the constitutive activity of excretion and pumping. Excretion depends, at least partly, on GST activity. It is understood that, besides a major function in sperm maturation and migration (Kubagawa et al. 2006), the GST encoded by gst-4 is also responsive to synthetic xenobiotics (Tawe et al. 1998).
No GFP strain responded to any hydrochar and rice husk exposure, as the transcription of none of the reporter genes was increased after the worms were exposed. Consequently, transcription studies of the corresponding genes should shed light onto this unexpected phenomenon.
Gene transcription of selected genes
Gene transcription studies with the sod-3::gfp strain resulted in a significant downregulation of all selected genes with all hydrochar samples. Nearly all downregulations were considered significant, since the values fell below 0.5 times-fold of the standard transcription (Fig. 2 and Table 3, grey fields). Only two glutathione transferase genes, namely, gst-9 and gst-38, and one cytochrome gene (Fig. 2) tended to be upregulated by the unwashed 300 °C HTC.
The worms did not upregulate the superoxide dismutase gene; instead, nearly all exposure downregulated it (Fig. 2). Upon exposure to the unwashed 300 °C hydrochar, the response of the nematodes is almost like the control (Fig. 2 and Table 4).
Discussion
Since char substrates adsorb inorganic and organic contaminants (Agrafioti et al. 2013; Pellera et al. 2012), upregulation of stress-responsive genes in nematodes exposed to husks and hydrochars was expected. In particular, upregulation of biotransformation or oxidative stress genes was expected. However, the most significant result of this study was the reduced transcription of the studied stress-related genes in nematodes exposed to both the rice husks and their processed hydrochars. We exclude artifacts because other transcription studies with different exposure chemicals simultaneously carried out in the same laboratory revealed significant upregulation of core anti-stress genes (Höss et al. 2013; Liu et al. 2013). Furthermore, this surprising reduction of the transcription of genes agrees well with the lack of inducible fluorescence of the GFP strains exposed to husks and hydrochars. Only weak constitutive fluorescence signals occurred, mainly in both the excretory cells and pharynx. These signals were most likely indicative of the constitutive activity of excretion and pumping. Excretion depends, at least partly, on GST activity. It is understood that, besides a major function in sperm maturation and migration (Kubagawa et al. 2006), the GST encoded by gst-4 is also responsive to synthetic xenobiotics (Tawe et al. 1998).
The pharynx is a neuro-muscular pump in the head of the animal; its activity is energy demanding. Energy, in turn, must be provided by activation of oxygen activation. To reach redox homeostasis, reducing substrates or reducing (including dismutating) enzymes, such as super oxide dismutases, is required. Therefore, a weakly fluorescing pharynx in sod-3::gfp individuals should not be considered as an indication of oxidative stress; rather, it is indicative of an active worm. The used GFP constructs, however, responded well to classical triggers (Fig. 1 and Table 1). This is further evidence that artifacts can be excluded.
It is surprising that even the cep-1 gene analog homolog to human anticancer P53 was downregulated by all hydrochars and the feedstock (Table 4). This finding contrasts a previous study that showed that the tested hydrochars provoked an inverse biphasic response of the gene transcription in C. elegans (Chakrabarti et al. 2011). However, the feedstocks used in this and the Chakrabarti et al. (2011) studies were different: poplar wood in the previous study and rice husks in this study. The second downregulated gene, daf-21, encodes for a member of the HSP90 family of molecular chaperones (Table 4). DAF-21 activity is required for larval development, inhibition of dauer larva formation, and a number of specific chemosensory behaviors. Phenotypic life trait variables such as chemotactic behavior or lifespan were not evaluated in this study. Therefore, it could not be assessed how the down-regulation of daf-21 could have affected these life traits; however, life span could have been adversely affected, because it is understood that daf-21 is also part of a chaperone network required for the extended life span (Harris et al. 2010).
Furthermore, it is noteworthy that even the feedstock, namely, untreated rice husks, significanlty reduced the transcription of cyp-14A2, cyp-14A5, cyp-34A9, cyp-35A3, cyp-35B1, gst-9, sod-1, sod-3, sod-5, cep-1, and daf-21 (Fig. 2 and Table 3). Therefore, the following discussion explores (1) which trigger of the husks and their hydrochars might have been the cause for this downregulation, and (2) whether the downregulation might represent a “smart” energy-saving strategy unique to C. elegans. Usually, the biotransformation of xenobiotics and the reduction of oxidative stress are cost-intensive, and its products may even lead to deformities in developing organisms (Arzuaga and Elskus 2010). Therefore, the downregulation of corresponding genes and metabolic pathways save energy that may be provided for other life traits.
Identification of potential causative agents in char substrates
An interesting trend was found in the different solids and liquids under study. In the feedstock, the unwashed and washed chars, as well as in the wash water, all compounds targeted by this study were present. Furfurals occurred in the raw rice husk, the 200 °C hydrochar, and the 200 °C first wash water, whereas phenol was present in both hydrochars and catechol and cresol in the 300 °C first wash water. These results are in accordance with previous reports (Knicker et al. 2007 and references therein) who reported a change in formation of volatile compounds with enhanced temperature during pyrolysis. Generally, lower temperatures resulted mainly in furfural and HMF, and higher temperatures in phenol and catechol.
From the few available ecotoxicological studies with compounds from wood degradation, furfurals appear to be the most toxic ones (Wang et al. 2014). However, they are not likely candidates responsible for the downregulation of the tested genes in the C. elegans sod-3::gfp strain, because they do not occur in all exposures that cause the downregulation of the transcription. The lack of phenol in the aqueous washing water of both chars must be caused by the complete removal in the first washing step with acetone, which could not be analyzed by the HPLC system applied.
Although natural xenobiotic compounds such as phenols have been identified as potent allelochemicals even in unprocessed rice tissues (Kong 2008), phenols and furfurals were reduced considerably. Some residual content of these compounds could be found in the wash water of the chars, but a clear relationship between the presence of phenols and furfurals in the solid and the liquid phase is missing. The washing process was carried out in three steps: The first wash was conducted with acetone, then two times with distilled water to remove the acetone. Polar compounds like furfural can be easily washed out with water.
The washed and unwashed 300 °C hydrochars contained more phenol than the 200 °C hydrochars (Table 2). In the feedstock, however, no phenols, cresol and catechol, and only a small amount of furfural could be detected; instead, rice plants are well documented to possess a variety of allelochemicals, such as phenolic acids, cytokinins, alkyl resorcinols, momilactone B, flavonoids, and steroids (Kong 2008 with references therein). They may have acted as the causative agents also for the observed downregulation of anti-stress genes of C. elegans in this study. Phenols have been shown to have significant toxic, mutagenic, or carcinogenic potentials (Michałowicz and Duda 2007). However, phenols occurred only in several, but not all exposures that caused the downregulation and are unlikely to be the causing agent. The same applies to furfural and HMF.
The identification for the causative agents that induced the observed downregulation of the anti-stress genes gets even more complex, since in a simultaneous study with two disinfection by-products, namely, dibromoacetic acid (DBAA) and N-nitrosodimethylamine, revealed that exposure to environmentally relevant concentrations of these two compounds leads also to significantly reduced transcription of the same anti-stress genes (Baberschke et al. 2015). In addition, DBAA exposure led to eased growth and lifespan in C. elegans (Saul et al. 2014). These findings indicate that the C. elegans appears to apply this downregulation as a kind of anti-stress strategy. Future studies should include variables on vitality of the nematodes, such as lifespan, growth, or reproduction, in order to discover indications of the underlying mechanisms of this kind of stress response and of whether the saved energy is spent on other life traits.
Instead of the observed downregulation of cep-1, an upregulation by the feedstock rice husks and unwashed hydrochars could have been expected, since both hydrochars contain mutagenic phenol. Since this was not observed, a possible reason might be that extraction of the hydrochars with NaOH was incomplete with regard to capturing this compound. It is also possible that with the washing method utilized by this study, not all substances that were adsorbed to the hydrochars were removed or could be detected.
How common is the downregulation of stress genes in exposed organisms?
It is still a matter of discussion why a downregulation of the tested stress-response genes occurred. In general, organisms exposed to abiotic stressors react by an immediate general and a delayed specific response. The initial transcription comprised a set of core environmental stress response genes, which, by adjustment of the energy homeostasis, have a crucial role in various stress responses. During this initial phase, many genes primarily related to metabolic processes like catalytic and hydrolytic activities as well as peptidase activity are downregulated (Sørensen et al. 2005). Later, a much more specific response counteracts the stress and its potential damages by upregulation of chaperones and enzymes and proteins of the different biotransformation phases (Sørensen et al. 2005; Zhang et al. 2012), often combined with increased transcription of anti-apoptosis genes (Zhang et al. 2012).
It is well understood that C. elegans responds extensively on the biomolecular level to environmental stressors such as xenobiotic chemicals (Ju et al. 2013; Menzel et al. 2005a, b; Roh and Choi 2011), whereby cyp genes in general, and cyp-35 in particular, are highly responsive to natural (Menzel et al. 2005b) and synthetic xenobiotics (Menzel et al. 2005a; Roh and Choi 2011). These genes belong to the core of specific defense responding to any organic chemical challenges. This assumption is supported by a whole genomic DNA microarray study with C. elegans exposed to different contaminated German river sediments. After exposure to Rhine sediments, most of the differentially expressed genes of C. elegans were downregulated (Menzel et al. 2009). However, these genes comprised energy metabolism, but not the biotransformation; instead, the latter genes were upregulated. In the current study, the identical exposure and RNA extraction protocols as in the previous studies were applied. Hence, the observed differences are unlikely to methodological differences.
From vertebrate toxicology, it is well understood that particularly the activity of biotransformation enzymes, such as cytochrome P450 monooxygenases, can transform relatively harmless parent compounds into potentially harmful (toxic, mutagenic, carcinogenic) products (Castell et al. 2005; Pelkonen and Nebert 1982). More recent findings expanded this knowledge even to invertebrates, such as Chironomids (Schuler et al. 2004) and C. elegans (Menzel et al. 2005a). Furthermore, there is environmental evidence demonstrating that populations inhabiting contaminated sites reduce the transcription and activity of biotransformation enzymes, thereby avoiding the bioactivation of synthetic xenobiotics and the formation of harmful metabolites. This has convincingly been shown with the Atlantic killifish, Fundulus heteroclitus L., surviving and reproducing even on Superfund sites at the US east coast (Fisher and Oleksiak 2007; Meyer and Di Giulio 2002). Very recently, the same strategy has been discovered in populations of the Gulf killifish, Fundulus grandis Baird & Girard, from the Houston Ship Channel, TX, USA (Oziolor et al. 2014).
In addition to the fishes, a similar phenomenon appears to apply also to C. elegans exposed to dibromoacetic acid (DBAA) and N-nitrosodimethylamine (NDMA) as evaluated in a recent study (Baberschke et al. 2015). Both xenobiotics usually develop as inadvertent disinfection by-products during water treatment processes and are classified as toxic and carcinogenetic to vertebrates (Richardson et al. 2007). However, applying environmentally realistic concentrations of the compounds individually and in mixtures led to a downregulation of major stress-responsive genes (cyps, gsts, sods, ctl), whereas no stress-responsive gene was upregulated. Since stress defense is energy demanding, this downregulation could represent a type of energy-saving tactic, and, in case of the study by Baberschke et al. (2015), the saved energy in the exposed nematodes appeared to have been spent for body maintenance (longevity and growth) (Saul et al. 2014).
In conclusion, the chemical triggers inducing energy-saving mode in C. elegans in this study remain to be identified and may originate from different classes of xenobiotic chemicals. Nevertheless, it seems plausible that the hydrochars tested in this study switched on the same mechanism. If this assumption holds true, the ecotoxicity of hydrochars should be evaluated by another test model and should also be considered in the context of the feedstock with its own allelopathic effects; the nematode C. elegans has obviously developed a strategy to occasionally avoid intoxication and saves energy for major life traits.
References
Agrafioti E, Bouras G, Kalderis D, Diamadopoulos E (2013) Biochar production by sewage sludge pyrolysis. J Anal Appl Pyrolysis 101:72–78. doi:10.1016/j.jaap.2013.02.010
Arzuaga X, Elskus A (2010) Polluted-site killifish (Fundulus heteroclitus) embryos are resistant to organic pollutant-mediated induction of CYP1a activity reactive oxygen species, and heart deformities. Environ Toxicol Chem 29:676–682. doi:10.1002/etc.68
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18. doi:10.1007/s11104-010-0464-5
Baberschke N, Steinberg CEW, Saul N (2015) Low concentrations of dibromoacetic acid and N-nitrosodimethylamine induce several stimulatory effects in the invertebrate model Caenorhabditis elegans. Chemosphere 124:122–128. doi:10.1016/j.chemosphere.2014.12.002
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94
Castell JV, Donato MT, Gómez-Lechón MJ (2005) Metabolism and bioactivation of toxicants in the lung. The in vitro cellular approach. Exp Toxicol Pathol 57:189–204. doi:10.1016/j.etp.2005.05.008
Chakrabarti S, Kern J, Menzel R, Steinberg CEW (2011) Selected natural humic materials induce and char substrates repress a gene in Caenorhabditis elegans homolog to human anticancer P53. Ann Environ Sci 5:1–6
Coleman DC (1994) The microbial loop concept as used in terrestrial soil ecology studies. Microb Ecol 28:245–250. doi:10.1007/BF00166814
Diakité M, Paul A, Jäger C, Pielert J, Mumme J (2013) Chemical and morphological changes in hydrochars derived from microcrystalline cellulose and investigated by chromatographic, spectroscopic and adsorption techniques. Bioresour Technol 150:98–105. doi:10.1016/j.biortech.2013.09.129
Fisher MA, Oleksiak MF (2007) Convergence and divergence in gene expression among natural populations exposed to pollution. BMC Genomics 8:108. doi:10.1186/1471-2164-8-108
Glaser B, Haumaier L, Guggenberger G, Zech W (2001) The 'Terra Preta' phenomenon: a model for sustainable agriculture in the humid tropics. Naturwissenschaften 88:37–41. doi:10.1007/s001140000193
Harris TW, Antoshechkin I, Bieri T (2010) Wormbase: a comprehensive resource for nematode research. Nucleic Acids Res 38:D463–D467. doi:10.1093/nar/gkp952
Höss S, Menzel R, Gessler F, Nguyen HT, Jehle JA, Traunspurger W (2013) Effects of insecticidal crystal proteins (Cry proteins) produced by genetically modified maize (Bt maize) on the nematode Caenorhabditis elegans. Environ Pollut 178:147–151. doi:10.1016/j.envpol.2013.03.002
Ju J, Ruan Q, Li X, Liu R, Li Y, Pu Y, Yin L, Wang D (2013) Neurotoxicological evaluation of microcystin-LR exposure at environmental relevant concentrations on nematode Caenorhabditis elegans. Environ Sci Pollut Res 20:1823–1830. doi:10.1007/s11356-012-1151-2
Kalderis D, Kotti MS, Méndez A, Gascó G (2014) Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 5:477–483. doi:10.5194/se-5-477-2014
Keiluweit M, Kleber M, Sparrow MA, Simoneit BRT, Prahl FG (2012) Solvent-extractable polycyclic aromatic hydrocarbons in biochar: influence of pyrolysis temperature and feedstock. Environ Sci Technol 46:9333–9341. doi:10.1021/es302125k
Kiontke K, Sudhaus W (2006) Ecology of Caenorhabditis species. WormBook: the online review of C elegans biology. 1–14 doi:10.1895/wormbook.1.37.1
Knicker H, Müller P, Hilscher A (2007) How useful is chemical oxidation with dichromate for the determination of "Black Carbon" in fire-affected soils? Geoderma 142:178–196. doi:10.1016/j.geoderma.2007.08.010
Kong CH (2008) Rice allelopathy. Allelopath J 22:261–273
Kookana RS (2010) The role of biochar in modifying the environmental fate, bioavailability, and efficacy of pesticides in soils: a review. Aust J Soil Res 48:627–637. doi:10.1071/SR10007
Kosel M, Wild W, Bell A, Rothe M, Lindschau C, Steinberg CE, Schunck WH, Menzel R (2011) Eicosanoid formation by a cytochrome P450 isoform expressed in the pharynx of Caenorhabditis elegans. Biochem J 435:689–700. doi:10.1042/BJ20101942
Kubagawa HM, Watts JL, Corrigan C, Edmonds JW, Sztul E, Browse J, Miller MA (2006) Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat Cell Biol 8:1143–1148. doi:10.1038/ncb1476
Li D, Hockaday WC, Masiello CA, Alvarez PJJ (2011) Earthworm avoidance of biochar can be mitigated by wetting. Soil Biol Biochem 43:1732–1737. doi:10.1016/j.soilbio.2011.04.019
Libra JA, Ro KS, Kammann C, Funke A, Berge ND, Neubauer Y, Titirici MM, Fühner C, Bens O, Kern J, Emmerich KH (2011) Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2:71–106. doi:10.4155/bfs.10.81
Linhares CR, Lemke J, Auccaise R, Duó DA, Ziolli RL, Kwapinski W, Novotny EH (2012) Reproducing the organic matter model of anthropogenic dark earth of Amazonia and testing the ecotoxicity of functionalized charcoal compounds. Pesq Agrop Brasileira 47:693–698. doi:10.1590/S0100-204X2012000500009
Liu S, Saul N, Pan B, Menzel R, Steinberg CEW (2013) The non-target organism Caenorhabditis elegans withstands the impact of sulfamethoxazole. Chemosphere 93:2373–2380. doi:10.1016/j.chemosphere.2013.08.036
Mao JD, Johnson RL, Lehmann J, Olk DC, Neves EG, Thompson ML, Schmidt-Rohr K (2012) Abundant and stable char residues in soils: implications for soil fertility and carbon sequestration. Environ Sci Technol 46:9571–9576. doi:10.1021/es301107c
McGrath TE, Wooten JB, Geoffrey Chan W, Hajaligol MR (2007) Formation of polycyclic aromatic hydrocarbons from tobacco: the link between low temperature residual solid (char) and PAH formation. Food Chem Toxicol 45:1039–1050. doi:10.1016/j.fct.2006.12.010
Menzel R, Bogaert T, Achazi R (2001) A systematic gene expression screen of Caenorhabditis elegans cytochrome P450 genes reveals CYP35 as strongly xenobiotic inducible. Arch Biochem Biophys 395:158–168. doi:10.1006/abbi.2001.2568
Menzel R, Rödel M, Kulas J, Steinberg CEW (2005a) CYP35: xenobiotically induced gene expression in the nematode Caenorhabditis elegans. Arch Biochem Biophys 438:93–102. doi:10.1016/j.abb.2005.03.020
Menzel R, Stürzenbaum S, Bärenwaldt A, Kulas J, Steinberg CEW (2005b) Humic material induces behavioral and global transcriptional responses in the nematode Caenorhabditis elegans. Environ Sci Technol 39:8324–8332. doi:10.1021/es050884s
Menzel R, Swain SC, Hoess S, Claus E, Menzel S, Steinberg CEW, Reifferscheid G, Sturzenbaum S (2009) Gene expression profiling to characterize sediment toxicity – A pilot study using Caenorhabditis elegans whole genome microarrays. BMC Genomics 10:160. doi:10.1186/1471-2164-10-160
Meyer J, Di Giulio R (2002) Patterns of heritability of decreased EROD activity and resistance to PCB 126-induced teratogenesis in laboratory-reared offspring of killifish (Fundulus heteroclitus) from a creosote-contaminated site in the Elizabeth River, VA, USA. Mar Environ Res 54:621–626. doi:10.1016/S0141-1136(02)00170-8
Michałowicz J, Duda W (2007) Phenols - sources and toxicity. Pol J Environ Stud 16:347–362
Mumme J, Eckervogt L, Pielert J, Diakité M, Rupp F, Kern J (2011) Hydrothermal carbonization of anaerobically digested maize silage. Bioresour Technol 102:9255–9260. doi:10.1016/j.biortech.2011.06.099
Norinaga K, Deutschmann O, Saegusa N, Hayashi J (2009) Analysis of pyrolysis products from light hydrocarbons and kinetic modeling for growth of polycyclic aromatic hydrocarbons with detailed chemistry. J Anal Appl Pyrolysis 86:148–160
Oleszczuk P, Jośko I, Kuśmierz M, Futa B, Wielgosz E, Ligeza S, Pranagal J (2014) Microbiological, biochemical and ecotoxicological evaluation of soils in the area of biochar production in relation to polycyclic aromatic hydrocarbon content. Geoderma 213:502–511. doi:10.1016/j.geoderma.2013.08.027
Oziolor EM, Bigorgne E, Aguilar L, Usenko S, Matson CW (2014) Evolved resistance to PCB- and PAH-induced cardiac teratogenesis, and reduced CYP1A activity in Gulf killifish (Fundulus grandis) populations from the Houston Ship Channel, Texas. Aquat Toxicol 150:210–219. doi:10.1016/j.aquatox.2014.03.012
Pelkonen O, Nebert DW (1982) Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol Rev 34:189–222
Pellera FM, Giannis A, Kalderis D, Anastasiadou K, Stegmann R, Wang JY, Gidarakos E (2012) Adsorption of Cu(II) ions from aqueous solutions on biochars prepared from agricultural by-products. J Environ Manag 96:35–42. doi:10.1016/j.jenvman.2011.10.010
Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM (2007) Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res 636:178–242. doi:10.1016/j.mrrev.2007.09.001
Roh JY, Choi J (2011) Cyp35a2 gene expression is involved in toxicity of fenitrothion in the soil nematode Caenorhabditis elegans. Chemosphere 84:1356–1361. doi:10.1016/j.chemosphere.2011.05.010
Saul N, Baberschke N, Chakrabarti S, Stürzenbaum SR, Lieke T, Menzel R, Jonáš A, Steinberg CE (2014) Two organobromines trigger lifespan, growth, reproductive and transcriptional changes in Caenorhabditis elegans. Environ Sci Pollut Res 21:10419–10431. doi:10.1007/s11356-014-2932-6
Schuler LJ, Landrum PF, Lydy MJ (2004) Time-dependent toxicity of fluoranthene to freshwater invertebrates and the role of biotransformation on lethal body residues. Environ Sci Technol 38:6247–6255. doi:10.1021/es049844z
Sevilla M, Maciá-Agulló JA, Fuertes AB (2011) Hydrothermal carbonization of biomass as a route for the sequestration of CO2: chemical and structural properties of the carbonized products. Biomass Bioenergy 35:3152–3159. doi:10.1016/j.biombioe.2011.04.032
Sørensen JG, Nielsen MM, Kruhøffer M, Justesen J, Loeschcke V (2005) Full genome gene expression analysis of the heat stress response in Drosophila melanogaster. Cell Stress Chaperones 10:312–328. doi:10.1379/CSC-128R1.1
Strange K, Christensen M, Morrison R (2007) Primary culture of Caenorhabditis elegans developing embryo cells for electrophysiological, cell biological and molecular studies. Nat Protoc 2:1003–1012. doi:10.1038/nprot.2007.143
Tawe WN, Eschbach ML, Walter RD, Henkle-Dührsen K (1998) Identification of stress-responsive genes in Caenorhabditis elegans using RT-PCR differential display. Nucleic Acids Res 26:1621–1627. doi:10.1093/nar/26.7.1621
Thion C, Cébron A, Beguiristain T, Leyval C (2012) PAH biotransformation and sorption by Fusarium solani and Arthrobacter oxydans isolated from a polluted soil in axenic cultures and mixed co-cultures. Int Biodeterior Biodegrad 68:28–35. doi:10.1016/j.ibiod.2011.10.012
Titirici MM, Thomas A, Yu SH, Müller JO, Antonietti M (2007) A direct synthesis of mesoporous carbons with bicontinuous pore morphology from crude plant material by hydrothermal carbonization. Chem Mater 19:4205–4212. doi:10.1021/cm0707408
Wang W, Yang S, Hunsinger GB, Pienkos PT, Johnson DK (2014) Connecting lignin-degradation pathway with pre-treatment inhibitor sensitivity of Cupriavidus necator. Front Microbiol 5:247. doi:10.3389/fmicb.2014.00247
Wiedner K, Rumpel C, Steiner C, Pozzi A, Maas R, Glaser B (2013) Chemical evaluation of chars produced by thermochemical conversion (gasification, pyrolysis and hydrothermal carbonization) of agro-industrial biomass on a commercial scale. Biomass Bioenergy 59:264–278. doi:10.1016/j.biombioe.2013.08.026
Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, Yang P, Zhang L, Wang X, Qi H, Xiong Z, Que H, Xie Y, Holland PW, Paps J, Zhu Y, Wu F, Chen Y, Wang J, Peng C, Meng J, Yang L, Liu J, Wen B, Zhang N, Huang Z, Zhu Q, Feng Y, Mount A, Hedgecock D, Xu Z, Liu Y, Domazet-Lošo T, Du Y, Sun X, Zhang S, Liu B, Cheng P, Jiang X, Li J, Fan D, Wang W, Fu W, Wang T, Wang B, Zhang J, Peng Z, Li Y, Li N, Wang J, Chen M, He Y, Tan F, Song X, Zheng Q, Huang R, Yang H, Du X, Chen L, Yang M, Gaffney PM, Wang S, Luo L, She Z, Ming Y, Huang W, Zhang S, Huang B, Zhang Y, Qu T, Ni P, Miao G, Wang J, Wang Q, Steinberg CE, Wang H, Li N, Qian L, Zhang G, Li Y, Yang H, Liu X, Wang J, Yin Y, Wang J (2012) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490:49–54. doi:10.1038/nature11413
Acknowledgments
This study derived from cooperation between the ATB Potsdam and the Technological Educational Institute of Crete, both embedded in the EU COST Action TD1107 “Biochar as option for sustainable resource management.” The authors are very grateful to Christian Steinberg for his initiative and support to this study and to Ralph Menzel, Humboldt-Universität zu Berlin, for providing the Pcyp35A3::GFP strain. We acknowledge two anonymous reviews, helpful comments given by Markus Hecker on an earlier draft of the manuscript, and the final proofreading by Judy Libra. Furthermore, the authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Markus Hecker
Rights and permissions
About this article
Cite this article
Chakrabarti, S., Dicke, C., Kalderis, D. et al. Rice husks and their hydrochars cause unexpected stress response in the nematode Caenorhabditis elegans: reduced transcription of stress-related genes. Environ Sci Pollut Res 22, 12092–12103 (2015). https://doi.org/10.1007/s11356-015-4491-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4491-x