Abstract
Magnetite iron oxide (Fe3O4) nanoparticles (NPs) are key materials applied in many different fields of modern technology. The potential environmental impact of these NPs is of great concern. In this study, initially the effect of Fe3O4 NPs size (20 and 40 nm) as well as bulk (>100 nm) at 200 mg L−1 on Picochlorum sp. (Trebouxiophyceae, Chlorophyta) is investigated during the different growth phases. The most inhibitory NPs were then chosen to assess their effects at different concentrations. The 20 nm NPs at 200 mg L−1 were found to significantly reduce the viable cell concentration and chlorophyll a content during the exponential growth phase compared to the other particle sizes. However, the 20 nm NPs at different concentrations were found to promote algal growth during the late growth stages (stationary and decline phases) compared to the control. Additionally, algae were found to accelerate the aggregation and sedimentation of nanoparticles into the medium and therefore can be considered as potential organisms for bioremediation of nano-pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the use of metal oxide nanoparticles (NPs) has increased exponentially. These NPs are the base for manufacturing new materials that project and materialize a variety of applications (Garcia et al. 2011). Engineered Fe3O4 NPs are key multifunctional materials applied in many different fields of modern technology, including environmental remediation (Mueller and Nowack 2010), food industry (Fidler et al. 2004), wastewater purification (Shen et al. 2009), cleanup polluted waters, soils and sediments (D’Autréaux and Toledano 2007), and medical applications (Berry and Curtis 2003; Cheng et al. 2005; Petri-Fink et al. 2005; Figuerola et al. 2010). Due to the widespread production and usage of Fe3O4 NPs, they eventually reach aquatic ecosystems, which are already enriched with colloidal iron NPs (Whang and Dei 2003). Iron-based NPs can produce a range of reactive oxygen species (ROS) via Fenton type reactions (LeBel et al. 1992) that cause oxidative injury to cells (Li et al. 2009). In opposite, iron is also an essential micronutrient for phytoplankton as it is required in essential cellular functions like photosynthesis and respiration, and therefore its availability controls phytoplankton productivity, community structure, and ecosystem functioning in large regions of the global ocean (Gledhill and Buck 2012). In addition to that, research has shown that nanoscale iron particles are very effective for the transformation and detoxification of a wide variety of common environmental contaminants, such as chlorinated organic solvents, organochlorine pesticides (Zhang 2003). Accordingly, it became essential to have lab-scale experimental studies focusing on the metabolic role of trace elements as limiting nutrients and toxicants to phytoplankton (Kadar et al. 2012).
The majority of investigations on aquatic NPs has been carried out on freshwater species; only few studies have been conducted on marine organisms. It should be noted that the properties of NPs will change according to exposure media, as well as the biological, behavioral, and respiration characteristics of marine organisms (Baker et al. 2013).
There is a concern of the potential release of large amounts of NPs to soil, water, or as byproducts of their use in remediation strategies of removal of toxins (Limbach et al. 2008). Therefore, the benefits of using some NPs for environmental remediation have to be balanced with their potential risks (Garcia et al. 2011).
During the past years, several contradictory observations have been reported, which highlight the great need for a more thorough understanding of cell and Fe3O4 NPs interactions (Soenen et al. 2012). For example, Chen et al. (2012) showed that Fe3O4 (35 nm) induce oxidative stress and an alteration of photosynthetic activity based on the absorbed CO2 fixation of the green alga Chlorella vulgaris treated 72 h to a nominal concentration range from 0 to 1600 μg mL−1. Additionally, Barhoumi and Dewez (2013) reported that superparamagnetic NPs (SPION) suspensions deteriorate the photochemical activities of photosynthesis, induce oxidative stress and inhibit cell division. To the contrary, Kadar et al. (2012) found that there was a normal algal growth of three microalgae species in the presence of three types of zero-valent nano-iron compounds, and there was an improvement in lipid content in treated cultures compared to algae grown in f/2 medium with EDTA-Fe (control). Prokaryotic and eukaryotic algae serve as a base for primary productivity and food web chain equilibrium, and thus the nanotoxicity assessment becomes predictable (Herrero and Flores 2008). Additionally, it was found that algae are more sensitive to NPs compared to other organisms (Aruoja 2011). It is also worth mentioning that algae not only provide the basic nourishment for aquatic food web, but also contribute to the self-purification of polluted water (Ji et al. 2011).
In this study, the microalga Picochlorum sp. was cultured and incubated with magnetite Fe3O4 (nanosized and bulk) particles. This microalga species is chosen due to its nutritional values and use in mariculture. Indeed, it was proposed as a new candidate for aquaculture, food, and biofuel exploitation (Tran et al. 2014). Foflonker et al. (2014) demonstrated that Picochlorum sp. strain SE3 was able to tolerate a wide range of salinities, suggesting a wide distribution of this marine alga. Additionally, Picochlorum sp. belongs to the small unicellular algae (photoautotrophic picoplankton) which are vital in all aquatic environments (Callieri 2008). The phytoplankton biomass and primary production are principally contributed by the picoplankton in oligotrophic lakes and oceans and they can play an important role in more productive aquatic environments (Li 1994). Recently, it has been shown that these algae are among the main picoplankton contributors in many ecosystems (Díez et al. 2004; Not et al. 2005), highlighting their abilities to adapt to different ecosystem characteristics and their pertinent role in ecosystem structure and functioning (Fuller et al. 2006; Dimier et al. 2007). Regardless of the apparent ecological significance of picoplankton, comparatively little is known for their diversity in the marine environment, particularly in the open ocean (Fuller et al. 2006). This has been attributed primarily to difficulties in identification by light microscopy. Only recently, with the advent of molecular techniques, has picophytoplankton diversity begun to be revealed (Moreira and Lopez- Garcia 2002). Some studies have reported a summer peak of picoeukaryotes (Nagata 1986; Søndergaard 1990). Though autotrophic picoplankton shows characteristic seasonal dynamics in the temperate zone: picocyanobacteria dominate the picoplankton in summer, whereas picoeukaryotes are dominant in autumn, spring (Callieri 2008), and winter (Somogyi et al. 2009).
The overall aim of this research was to examine the effect of Fe3O4 NPs (different sizes and concentrations) on the growth and photosynthetic pigment content of the marine alga Picochlorum sp. The Muse™ Cell Analyzer was used for the first time in this work to give a precise viable cell count after inoculating cells with NPs. This instrument uses patent-pending, miniaturized fluorescent detection and micro-capillary technology to deliver quantitative cell analysis of both suspension and adherent cells 2 to 60 μm in diameter.
Materials and methods
Fe3O4 NPs and structural characterization
Two nanosized Fe3O4 NPs were purchased from US Research Nanomaterials, Inc, Houston, USA. The particle size specified by the manufacturer is 20 and 40 nm. Bulk Fe3O4 (>100 nm) was purchased from BDH Chemicals Ltd, England. Stock suspensions of nano and bulk Fe3O4 particles were prepared in Walne’s algal medium (Walne 1970) immediately before each experiment.
For the characterization of the purchased Fe3O4 nanoparticles, XRD, SEM, TEM, and EDS analyses were performed. The size was determined through XRD, whereas information about the morphology and exact composition of the NPs were obtained by SEM, TEM, and EDS, respectively. The purity of the NPs and bulk Fe3O4 were all above 99 %.
Phase analysis was carried out from X-ray diffraction measurements using high-resolution Rigaku Ultima IV diffractometer equipped with Cu-K radiation (λ = 1.5418 Å). Qualitative and quantitative phase analyses were performed using PDXL program. The refinements were carried out using magnetite Fe3O4 phase with cubic (spinel type) crystal structure (space group Fd3m, No. 227). During refinements, phase composition, lattice parameters, and microstructural parameters (crystallite size and microstrain) were refined.
Biotoxicity assay
Picochlorum sp. culture
A pure culture of Picochlorum sp. (Trebouxiophyceae, Chlorophyta) was obtained from the National Mariculture Centre, Ministry of Municipalities and Urban Planning, Kingdom of Bahrain.
The microalga was grown in 2-L Erlenmeyer flasks containing 1 L of sterilized medium which were capped with loose cotton and these flasks were then placed in an illuminated incubator. Walne’s medium was used with an initial pH value of 8. The culture was kept at 18 °C under continuous illumination of approximately 100 μmol m−2 s−1. The cell density of the culture was monitored microscopically every 24 h and the different growth phases were determined by counting with a hemocytometer and Olympus CX21 microscope. Morphological observations were conducted with a Zeiss HBO 100 microscope.
Algal growth assays
The Organization for Economic Co-operation and Development (OECD) 201 algal growth inhibition test guidelines were followed with some modifications. The cultures were incubated with Fe3O4 NPs at the lag phase and the viable cell counts and measurements of chlorophyll a concentrations were performed at 24, 48, 72, 240, 408, and 576 h (4 weeks from inoculation time).
The following sizes of Fe3O4 (20, 40 and >100 nm (bulk)) were used at 200 mg L−1, with three replicates for each size in addition to the control. The cultures (control and treated algae) were incubated in an illuminated incubator under the above mentioned conditions with continuous shaking (180 r/min). High shaking speed was selected to reduce aggregation and settlement of the NPs over the incubation period. Changes in viable cell concentration were recorded using Muse™ Cell analyzer (Millipore, USA). The Muse™ Cell Analyzer uses patent-pending, miniaturized fluorescent detection and micro-capillary technology to deliver quantitative cell analysis of both suspension and adherent cells 2 to 60 μm in diameter.
Additionally, chlorophyll a concentrations were determined by collecting 30 mL of each treated algal culture. Chlorophyll a was extracted using 90 % acetone, followed by measurement by spectrophotometer (Perkin Elmer UV spectrophotometer) following UNESCO protocol (Vohra 1966).
The nanoparticles showing toxic effects were then selected for a second experiment.
The second experiment was designed to investigate the dose-response relationship between the selected particles (20 nm NPs) and the algae with particle concentrations of 0, 50, 100, 200, 400, 800, and 1600 mg L−1. Changes in the viable cells concentration and chlorophyll a contents during 4 weeks were measured as mentioned above.
TEM observation of algal cells
The ultrastructural changes of Picochlorum sp. induced by Fe3O4 NPs were observed with TEM (Jeol JEM 2100). Several algae samples from different experiments were fixed using 4 % buffered formalin, dehydrated, embedded in Epon epoxy, and sectioned. The grids were analyzed using a Bruker Quantax EDS under STEM mode.
Statistical analysis
Data are presented as mean ± SDEV (standard deviation) and were tested for statistical significance using analysis of variance (one-way analysis of variance, ANOVA) followed by Tukey’s pairwise comparison using Minitab version 16. All statistical analysis used the default 5 % rejection level.
Results and discussion
Preliminary characterization
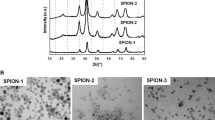
The preliminary characterizations of Fe3O4 particles were carried out by SEM, EDS, and XRD analyses before introducing them to the test organism. The SEM images of Fe3O4 NPs and EDS spectra are shown in Fig. 1. Irregular-shaped NPs in agglomerated conditions can be observed.
X-ray diffraction patterns were refined using the Rietveld method and the results are reported in Table 1 and Fig. 2. It can be noticed that the estimated crystallite size (calculated from peak broadening) is lower than the values given by the supplier (measured experimentally); the fact is a particle is usually formed by many crystallites.
X-ray diffraction qualitative and quantitative phase analysis revealed the presence of both magnetite Fe3O4 (82 %) and Goethite FeOOH (28 %). However, bulk Fe3O4 was confirmed to be of >100 nm in size with high purity of Fe3O4 phase (data not shown).
Effect of Fe3O4 on algae
Effect of Fe3O4 particle size on algae
The effects of nanoscale (20–40 nm) and bulk (>100 nm) Fe3O4 particles (200 mg L−1) on the algal growth varied with the particle size. Treated and untreated cultures displayed similar concentrations of viable cells during the lag phase (72 h) and stationary phase (406 h). During the lag phase, there is a negligible cell growth. The 40 nm NPs and the bulk particles exhibited higher concentration of viable cells compared to the control during the exponential growth phase (at 240 h). To the contrary, the lowest number of viable cells was recorded with the 20 nm NPs at 240 h (exponential growth phase). There are many studies that support the fact that metal oxide NPs are more toxic for many organisms, including Chlorella compared to their bulk counterpart (Manzo et al. 2013). Moreover, bulk Fe3O4 is known to be used as an algal fertilizer which serves as an important source of iron for the algae (WHOI 2007). The addition of iron promotes the algal growth, as it is necessary for a number of cellular functions including the synthesis of chlorophyll (Allsopp et al. 2007). Therefore, the high growth rate of the culture treated with bulk Fe3O4 is not unexpected. Sadiq et al. (2011) showed that alumina (Al2O3) NPs were toxic to algal species, while their bulk counterparts were less toxic; they assigned this behavior to the difference in particle size in suspension.
Cell walls in algae mainly consist of cellulose and semipermeable membrane, permitting the passage of small molecules while limiting the passage of larger ones (Navarro et al. 2008). It should be noted here that the diameter of pores across the cell wall, which has a thickness ranging from 5 to 20 nm eventually determines the sieving properties (Madigan et al. 2003). Accordingly, only NPs and aggregates with a size smaller than that of the largest pore are expected to pass through the cell wall and reach the plasma membrane. This is a possible explanation that the 20 nm NPs might be able to enter the algal cells and cause a negative effect. Therefore, there was a significant decrease in the number of viable cells during the exponential growth phase. Additionally, the permeability of the cell wall might change during reproduction, with the newly synthesized cell wall during the exponential phase will be more permeable to NPs (Ovecka et al. 2005). Furthermore, the interactions of cells with NPs might induce the formation of new pores, bigger than usual and hence increase the internalization of NPs through the cell wall (Navarro et al. 2008).
The control culture reached the decline phase during the fourth week (576 h) due to the depletion of the nutrients in the medium (0.002 × 106 cells mL−1). Nevertheless, the NPs-treated cultures showed growth promotion and displayed high concentration of viable cells (1.53 × 106 and 1.50 × 106 cells mL−1 in the 20 and 40 nm treated cultures, respectively). The bulk particles showed a decline in the cell number during the decline phase (0.005 × 106 cells mL−1) (Fig. 3). Similar finding was reported by Kadar et al. (2012). They found that all physiological parameters of algae grown with Fe3O4 NPs were comparable or superior to controls, which may indicate similar or enhanced bioavailability of the metal in the form of NPs compared to the soluble form added as EDTA-Fe in the f2 media. The dissolution of NPs may have provided Fe+2/Fe+3 for phytoplankton growth with rates similar as controls that were supplied with EDTA-Fe (Kadar et al. 2012). It is also possible that electrochemical interaction of NPs with exopolymeric substances from some microalgae (e.g., Isochrysis galbana) may be involved in the entrapment and internalization of NPs via endocytosis, which may be a more widespread cellular uptake mechanism than previously thought (Kadar et al. 2010). On the other hand, some organisms including algae may excrete compounds as a feedback response to alter the NPs toxicity (Navarro et al. 2008). Furthermore, algae can produce substances that can induce NPs flocculation or metal ion chelation and thus reduce the bioavailability of both NPs and metal ions they released (Soldo et al. 2005). Exopolymeric substances production may increase in algae upon exposure to NPs and may thus contribute to detoxification mechanisms (Miao et al. 2007; Quigg et al. 2013).
To our best knowledge, no previous study has compared the effect of Fe3O4 NPs size on algae. However, a previous study that determined the effect of TiO2 NPs to algae demonstrated that the toxicity decreased with increasing particle size (Clément et al. 2013), which is similar to our findings.
The unexpected increase in the cell viability in the fourth week further confirms the fact that the NPs have actually helped the algal growth rather than inhibiting it. At this stage, the algae might have acquired some adaptive mechanism to thrive as a response to the presence of NPs. And this mechanism allowed the algae to keep growing even after its decline phase. This observation also revealed that the duration of NPs exposure also affects the toxicity. A previous study that evaluated the effect of gold (Au) NPs on a higher organism also concluded that the effect of NPs depends on the exposure duration (Abdel Halim 2012).
Figure 4 depicts the effects of Fe3O4 particle size on the chlorophyll a content in Picochlorum sp. The treated and untreated cultures exhibited similar concentrations during the lag phase. However, chlorophyll a content was higher using the 20 nm and the bulk particles during the exponential phase (240 h). During the decline phase, these particles (20 nm and bulk) caused a decrease in chlorophyll a concentration compared to the 40 nm particles, although there was no significant difference between the control and the treated cultures (p > 0.05). A previous research performed to test the effect of Fe3O4 NPs on a higher plant’s (soybean) chlorophyll also showed that there was no evidence for photosynthetic toxicity, rather the chlorophyll levels increased in the presence of NPs (Ghafariyan et al. 2013). Nevertheless, the literature also suggested that there are evidences that NPs can have negative effects on chlorophyll a concentration of different organisms. For example, Al2O3 and SiO2 NPs are proven to be toxic for chlorophyll of a unicellular green algae Pseudokirchneriella subcapitata (Metzler et al. 2012).
Effect of the 20 nm NPs concentration
In the second experiment, it became obvious that all the tested concentrations of Fe3O4 NPs had a negative effect on the number of viable cells during the early phases of growth (Fig. 5). Similar results have been reported by Stevenson et al. (2013) who showed that Ag NPs were found to be more toxic to the alga Chlamydomonas reinhardtii during the early phases of its growth (i.e., lag and exponential phases). The concentration of viable cells in treated samples was very similar to the control during the stationary phase. This might be a result of certain adaptation mechanism that algae have acquired over time. Generally, there is no notable difference in the viable cell concentration between the treated cultures. However, at the end of the decline phase, an inverse relationship between the concentration of viable cells and concentration of NPs was observed. The lowest number of viable cells was recorded in the control culture during the decline phase due to the depletion of nutrients in the medium (2.8 × 106 cells mL−1) (Fig. 5). Many previous reports have proved that the increase in NPs concentration increased their toxic effects e.g., Naqvi et al. (2010). Conversely, evidence for decreasing toxicity with increasing NPs concentration is also present. This could be based on the fact that a higher concentration of NPs might enhance the particles aggregation, and therefore the toxic effect is reduced as compared to the lower concentrations of the same NPs (Rajkishore et al. 2013).
The effect of the 20 nm NPs concentration on the chlorophyll a content is displayed in Fig. 6. There was no significant differences between the treated and untreated cultures during the lag and decline phases. However, the 200 mg L−1 showed a significant decrease in the chlorophyll a concentration during the exponential phase (p < 0.05). Additionally, there was a significant decrease in chlorophyll a concentration in the control culture compared to the treated cultures during the stationary phase (p < 0.05). For instance, chlorophyll a concentration was 1.4 and 3.5 μg mL−1 in the control and 100 mg L−1 treated culture, respectively. Like the viable cell concentration, the effect of Fe3O4 NPs on chlorophyll a content in the treated samples was higher in the early growth stages. In the later stages, there is no clear difference on the effect of Fe3O4 NPs concentration. The control culture exhibited an abrupt decrease in chlorophyll a concentration after the exponential phase, as its viable cell concentration also started to decrease after this point (Fig. 5). The decrease in the chlorophyll a of the treated cultures appeared at a later stage than the control. Furthermore, the decrease in chlorophyll a concentration in treated samples is gradual unlike the control (in which there is an abrupt decrease). A possible explanation for this trend is that the samples that are not fully transparent (treated cultures), absorb the light energy that is required for algal growth and may therefore reduce the shading effect caused by the presence of aggregated NPs. Hund-Rinke and Simon (2006) as well as Arouja et al. (2009) also reported that the contribution of shading effect by NPs to the toxicity was negligible. Nevertheless, Gong et al. (2011) found that NPs aggregation behavior affect the growth of algae in the aquatic systems due to shading effects.
The 20 nm Fe3O4 (200 mg L−1) exert primary toxicity effect on viable cells with a much less harmful effect on chlorophyll a content (Figs. 5 and 6). Similar results were reported using metals such as Zn, Cd, Cu, Hg, and Pb with C. vulgaris. Rosko and Rachlin (1977) observed that there was an inverse relationship between cell division and chlorophyll a content using those metals.
Our results are contradictory to a previous research that concluded that the Fe3O4 NPs’ toxicity on Chlorella sp. is concentration-dependent (Chen et al. 2012). It was found that chlorophyll a concentration of C. vulgaris decreased as nano-Fe3O4 concentration increased. Barhoumi and Dewez (2013) observed that PS II performance index decreased significantly when C. vulgaris was exposed during 72 h to 400 μgmL−1 of SPION, compared to the control. The inconsistency in the algae responses to nano-Fe3O4 could be explained by special characters of algae (e.g., morphology, cytology, physiology, and genetics (Wei et al. 2010)). Additionally, different culture media could impact the algal growth, the characteristics, and activity of the NPs, which in turn can bring on different toxicological reactions (Ji et al. 2011). Therefore, it is very important to examine the effect of metals “including NPs” using different species of algae to gain a comprehensive view of the effect of each type of NPs (Rosko and Rachlin 1977).
In general, our study suggested that the NPs’ size has a significant effect on the concentration of viable cells and chlorophyll a concentration. Though there is no significant effect of Fe3O4 NPs concentration on viable cell concentration and chlorophyll a content. Additionally, it is observed that the effect of Fe3O4 NPs changes during the different growth stages. The negative effect of NPs is observed only at the early stages, after which the NPs showed a positive effect rather than being negative. This could be explained by the fact that during the early growth stages, Fe3O4 NPs may adhere to the cell and block critical pores and membrane functions. As an alternative, it could also enter the cell by endocytosis, by means of diffusion through pores or via ion transport systems (Baker et al. 2013), then algal cells can reverse the negative effect of NPs and acquire adaptive mechanisms during the later growth stages.
Ultrastructural changes
The ultrastructure of the Picochlorum sp. treated with 20 nm Fe3O4 NPs was investigated using TEM imaging and EDX analysis (Fig. 7). One clearly sees the cell nucleus and chloroplast (Fig. 7a). STEM analysis confirmed nanoparticles’ aggregation outside the cells. The Fe3O4 NPs were observed as black aggregates. EDX analysis of the black aggregates showed essential elements of the nanoparticles (oxygen and iron) along with other elements at lower content (Fig. 7b).
Aggregation and bioremediation of NPs by algae
In this study, it was found that the presence of algae has accelerated the aggregation of Fe3O4 NPs (Figs. 7 and 8) and consequently reduced their toxicity to free cells, which is in accordance with the high number of viable cells and chlorophyll a concentration during the stationary and decline phases. Similar findings were reported by Gong et al. (2011). NPs are expected to be more soluble in freshwater with lower pH, while most will aggregate in marine waters (Klaine et al. 2008; Batley et al. 2013; Quigg et al. 2013). Navarro et al. (2008) explained that organic matter in the seawater might affect the surface speciation and charge of NPs and therefore affect their aggregation/deposition properties. Figure 8a shows the sorption of NPs to algal cells, and that was also to be expected (Navarro et al. 2008). This will ultimately increase the cellular weight of algae and lead to their sedimentation (Huang et al. 2005). These processes of adsorption and sedimentation will help to remove pollutants from the medium.
Moreover, in saltwater, the increasing salinity and accordingly the ionic strength can reduce the electrophoretic mobility of the particles and thus favors their aggregation (Batley et al. 2013). In this study, the presence of algae and their secretions increased the organic matter of the medium which is known to affect the NPs aggregation. In addition to that, the relative high pH recorded at the end of the experiment (varied between 8.1 and 8.6) further accelerated the aggregation of NPs. Also, aggregation correlates positively with concentration most likely due to more particles being available for interactions (Miller et al. 2010). The aggregation of NPs may possibly reduce the surface area, and consequently the dissolution potential (especially at high concentration) (Baker et al. 2013). Agglomeration speed of NPs in seawater is related to the concentration of NPs and natural organic matter (NOM) (Baalousha 2009). Recent evidence demonstrated by Zhang et al. (2009) suggests that divalent cations found in natural seawaters, e.g., Ca2+ may destabilize the electrical charges on NOM-coated NPs, leading to further aggregation and sedimentation. Hence, algae may influence the sedimentation of NPs into the environment and are potential organisms for bioremediation of nano-pollution.
The capability of algae to remove NPs should be considered during the assessment of nanomaterials’ potential risks in aquatic ecosystems. For example, algae living in extreme habitats (the liquid water between snow crystals) showed the capacity to accumulate mineral particles on their cell walls (Luetz-Meindl and Luetz 2006). These mineral particles have been hypothesized to be important for the survival of these algae living in low nutrient habitats. Thus, it is expected that NPs containing essential elements might also be attached to the algae and supply nutrient for their growth. Furthermore, NPs may adsorb pollutants, which might change the transport and bioavailability of both NPs and pollutants in natural systems and alter their toxic effects (Navarro et al. 2008).
It has been demonstrated in this study that Fe3O4 NPs are not toxic and can promote algal growth especially during the late growth stages. Fe3O4 NPs have been used in many fields and are generally considered as nontoxic materials (Nam and Lead 2008; Bystrzejeska-Piotrowska et al. 2009). In addition, Yavuz et al. (2006) showed that Fe3O4 NPs have generally nontoxic character at low concentrations and proposed the use of Fe3O4 NPs for bioremediation of other pollutants.
The deposition/aggregation processes of NPs can be controlled through many parameters of the suspension, such as temperature, ionic strength, pH, as well as surface charge of NPs. This could provide guidance to remediation of nano-pollutants in aquatic systems (Gong et al. 2011).
Conclusions
In this investigation, we demonstrated that nano and bulk Fe3O4 particles did not inhibit the growth of the algae and cell content of chlorophyll a. However, the 20 nm NPs at 200 mg L−1 were found to significantly affect the chlorophyll a content of Picochlorum sp. during the exponential growth phase. To date, most studies have focused on the toxicological effects of NPs during the exponential growth phase only, whereas this study represents one of the few studies to examine the effect of Fe3O4 NPs during the different growth stages (lag, exponential, stationary, and decline phases). At the same time, we have shown that the algae can be affected negatively by the presence of Fe3O4 NPs during the early growth stages, but they acquire adaptive mechanisms to reverse these effects during the late stages of their growth. Algal cells were found to have the ability to accelerate the aggregation and consequently the sedimentation of Fe3O4 NPs. These results indicate that algae can be a promising organism for bio-remediating nano-pollution.
References
Abdel Halim MA (2012) The Influence of size and exposure duration of gold nanoparticles on gold nanoparticles levels in several rat organs in vivo. J Cell Sci Therapy 3:129. doi:10.4172/2157-7013.1000129
Allsopp M, Santillo D, Johnston P (2007) A scientific critique of oceanic iron fertilization as a climate change mitigation strategy. Greenpeace research laboratories. Technical Note 07:32pp
Aruoja V (2011) Algae Pseudokirchneriella subcapitata in environmental hazard evaluation of chemicals and synthetic nanoparticles, PhD thesis, Estonian University of Life Sciences, Tartu 114 pp
Arouja V, Dubourguier HC, Kasemets K, Kahru A (2009) Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ 407:1461–1468
Baalousha M (2009) Aggregation and disaggregation of iron oxide nanoparticles: influence of particle concentration, pH and natural organic matter. Sci Total Environ 407:2093–2101
Baker TJ, Tyler CR, Galloway TS (2013) Impacts of metal and metal oxide nanoparticles on marine organisms. Environ Pollut 186:257–271
Barhoumi L, Dewez D (2013) Toxicity of superparamagnetic iron oxide nanoparticles on green alga Chlorella vulgaris. BioMed Res Inter 2013:1–11
Batley GE, Kirby JK, McLaughlin MJ (2013) Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc Chem Res 46:854–862
Berry CC, Curtis ASG (2003) Functionalization of magnetic nanoparticles for applications in biomedicine. J Phys D 36:R198–R206
Bystrzejeska-Piotrowska G, Golimowski J, Urban JP (2009) Nanoparticles: their potential toxicity, waster and environmental management. Waste Manage 29:2587–2595
Callieri C (2008) Picophytoplankton in freshwater ecosystems: the importance of small- sized phototrophs. Freshw Rev 1:1–28
Chen X, Zhu X, Li R, Yao H, Lu Z, Yang X (2012) Photosynthetic toxicity and oxidative damage induced by nano-Fe3O4 on Chlorella vulgaris in aquatic environment. Open J Ecol 2:21–28
Cheng F, Su C, Yang Y, Yeh Y, Tsi C, Wu CL, Wu MT, Sheieh DB (2005) Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials 26:729–738
Clément L, Hurel C, Marmier N (2013) Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants—effects of size and crystalline structure. Chemosphere 90:1083–1090
D’Autréaux B, Toledano MB (2007) ROS as signaling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8:813–824
Díez B, Massana R, Estrada M, Pedrós-Alió C (2004) Distribution of eukaryotic picoplankton assemblages across hydrographic fronts in the Southern Ocean, studied by denaturing gradient gel electrophoresis. Limnol Oceanogr 49:1022–1034
Dimier C, Corato F, Saviello G, Brunet C (2007) Photophysiological properties of the marine picoeukaryote Picochlorum RCC 237 (Trebouxiophyceae, Chlorophyta). J Phycol 43:275–283
Fidler MC, Walczyk T, Davidsson L, Zeder C, Sakaguchi N, Juneja LR, Hurrell RF (2004) A micronized, dispersible ferric pyrophosphate with high relative bioavailability in man. Br J Nutr 91(1):107–112
Figuerola A, Corato RD, Manna L, Pellegrino T (2010) From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol Res 62:126–143
Foflonker F, Price DC, Qiu H, Palenik B, Wang S, Bhattacharya D (2014) Genome of the halotolerant green alga Picochlorum sp. reveals strategies for thriving under fluctuating environmental conditions. Environ Microbiol. doi:10.1111/1462- 2920.12541
Fuller NJ, Campbell C, Allen DJ, Pitt FD, Zwirglmaier K, Gall FL, Vaulot D, Scanlan DJ (2006) Analysis of photosynthetic picoeukaryote diversity at open ocean sites in the Arabian Sea using a PCR biased towards marine algal plastids. Aquat Microb Ecol 43:79–93
Garcia A, Espinosa R, Delgado L, Casals E, Gonzalez E, Puntes V, Barata C, Font X, Sanchez A (2011) Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests. Desalination 269:136–141
Gledhill M, Buck KN (2012) The organic complexation of iron in the marine environment: a review. Front Microbiol 3:1–17
Ghafariyan MH, Malakouti MJ, Dadpour M, Stroeve P, Mahmoudi M (2013) Effect of magnetite nanoparticles on soybean chlorophyll. Environ Sci Technol 47:10645–10652
Gong N, Shao K, Feng W, Lin Z, Liang C, Sun Y (2011) Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. Chemosphere 83:510–516
Herrero A, Flores E (2008) The cyanobacteria. In: Herrero (ed) Molecular biology, genetics and evolution. Caister, Norfolk, pp 21–34
Huang CP, Cha DK, Ismat SS (2005) Progress report: short-term chronic toxicity of photocatalytic nanoparticles to bacterial, algae and zooplankton. University of Delaware
Hund-Rinke K, Simon M (2006) Ecotoxic effect of photocatalytic active nanoparticles TiO2 on algae and daphnids. Environ Sci Pollut Res 13:225–232
Ji J, Long Z, Lin D (2011) Toxicity of oxide nanoparticles to the green algae Chlorella sp. Chem Eng J 170:525–530
Kadar E, Rooks P, Lakey C, White DA (2012) The effect of engineered nanoparticles on growth and metabolic status of marine microalgae culture. Sci Total Environ 439:8–17
Kadar E, Lowe D, Sole M, Fisher AS, Jha AN, Readman JW, Hutchinson TH (2010) The uptake and biological responses to non-Fe versus soluble FeCl3 in excised mussel gills. Anal Bioanal Chem 396:657–666
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27:1825–1851
LeBel C, Ishiropoulos H, Bondy S (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Li WKW (1994) Primary productivity of prochlorophytes, cyanobacteria, and eukaryotic ultraphytoplankton: measurements from flow cytometric sorting. Limnol Oceanogr 39:169–175
Li H, Zhou Q, Wu Y, Fu J, Wang T, Jiang G (2009) Effects of waterborne nano-iron on medaka (Oryzias latipes): antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicol Environ Saf 72:684–692
Limbach LK, Bereiter R, Muller E, Krebs R, Galli R, Stark WJ (2008) Removal of oxide nanoparticles in a model wastewater treatment plant: influence of agglomeration and surfactants on clearing efficiency. Environ Sci Technol 42:5828–5833
Luetz-Meindl U, Luetz C (2006) Analysis of element accumulation in cell wall attached and intracellular particles of snow algae by EELS and ESI. Micro 37:452–458
Madigan MT, Martinko JM, Parker J (2003) Brock biology of microorganisms. Prentice Hall/ Pearson Higher Education Group, Upper Saddle River
Manzo S, Miglietta M, Rametta G, Buono S, Francia G (2013) Toxic effects of ZnO nanoparticles towards marine algae Dunaliella tortiolecta. Sci Total Environ 445–446:371–376
Metzler DM, Erdem A, Tseng YH, Huang CP (2012) Responses of algal cells to engineered nanoparticles measured as algal cell population, chlorophyll a, and lipid peroxidation: effect of particle size and type. Journal of Nanotechnology Article ID 237284, 12 pages, doi:10.1155/2012/237284
Miao AJ, Quigg A, Schwehr K, Xu C, Santschi P (2007) Engineered silver nanoparticles (ESNs) in coastal marine environments: bioavailability and toxic effects to the phytoplankton Thalassiosira weissflogii. 2nd international conference on the environmental effects of nanoparticles and nanomaterials, 24th- 25th September, London UK
Miller RJ, Lenihan HS, Muller EB, Tseng N, Hanna SK, Keller AA (2010) Impacts of metal oxide nanoparticles on marine phytoplankton. Environ Sci Technol 44:7329–7334
Moreira D, Lopez- Garcia P (2002) The molecular ecology of microbial eukaryote, unveils a hidden world. Trends Microbiol 10:31–38
Mueller NC, Nowack B (2010) Nano zero valent iron—the solution for water and soil remediation. Report of the observatory NANO. Available at: www.observatorynano.eu
Nagata T (1986) The seasonal abundance and vertical distribution of the <3 μm phytoplankton in the North Basin of lake Biwa. Ecol Res 1:207–221
Nam J, Lead JR (2008) Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci Total Environ 400(1–3):396–414
Naqvi S, Samim M, Abdin MZ, Ahmed FJ, Maitra AN, Prashant CK, Dinda AK (2010) Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int J Nanomed 5:983–989
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao AJ, Quigg A, Santschi PH, Sigg L (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants and fungi. Ecotoxicology 17:372–386
Not F, Massana R, Latasa M, Marie D, Colson C, Eikrem W, Pedrós-Alió C, Vaulot D, Simonn N (2005) Late summer community composition and abundance of photosynthetic picoeukaryotes in Norwagian and Barents seas. Limnol Oceanogr 50:1677–1686
Ovecka M, Lang I, Baluska F, Ismail A, Illes P, Lichtscheidl IK (2005) Endocytosis and vesicle trafficking during tip growth of root hairs. Protoplasma 226:39–54
Petri-Fink A, Chastellain M, Juillerat-Jeanneret L, Ferrari A, Hofmann H (2005) Development of functionalized superparamagnetic iron oxide nanoparticles for interaction with human cancer cells. Biomaterials 26:2685–2694
Quigg A, Chin WC, Chen CS, Zhang S, Jiang Y, Miao AJ, Schwehr KA, Xu C, Santschi PH (2013) Direct and indirect toxic effects of engineered nanoparticles on algae: role of natural organic matter. ACS Sustainable Chem Eng 1:686–702
Rajkishore SK, Subramanian KS, Natarajan N, Gunasekaran K (2013) Nanotoxicity at various trophic levels—a review. Biogeosciences 8:975–982
Rosko JJ, Rachlin JW (1977) The effect of cadmium, copper, mercury, zinc and lead on cell division, growth, and chlorophyll a content of the chlorophyte Chlorella vulgaris. Bull Torrey Botan Club 104(3):226–233
Sadiq IM, Pakrashi S, Chandrasekaran N, Mukherjee A (2011) Studies on toxicity of aluminium oxide (Al2O3) nanoparticles to microalgae species; Scenedesmus sp. and Chlorella sp. J Nanopart Res 13:3287–3299
Shen YF, Tang J, Nei ZH, Wang YD, Ren Y, Zuo L (2009) Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep Purif Technol 68:312–319
Soenen SJ, Cuyper MD, Smedt SCD, Braeckmans K (2012) Investigating the toxic effects of iron oxide nanoparticles. Methods Enzym 509:195–224
Soldo D, Hari R, Sigg L, Behra R (2005) Tolerance of Oocystis nephrocytioides to copper: intracellular distribution and extracellular complexation of copper. Aquat Toxicol 71:307–317
Somogyi B, Felföldi T, Vanyovszki J, Ágyi Á, Márialigeti K, Vörös L (2009) Winter bloom of picoeukaryotes in Hungarian shallow turbid soda pans and the role of light and temperature. Aquat Ecol 43:735–744
Søndergaard M (1990) Picophytoplankton in Danish Lakes. Verth Int Ver Limnol 24:609–612
Stevenson LM, Dickson H, Klanjscek Keller A, McCauley E, Nisbet RM (2013) Environmental feedbacks and engineered nanoparticles: mitigation of silver nanoparticle toxicity to Chlamydomonas reinhardtii by algal-produced organic compounds. PLoS ONE 8:e74456
Tran D, Giordano M, Louime C, Tran N, Vo T, Nguyen D, Hoang T (2014) An isolated Picochlorum species for aquaculture, food, and biofuel. N Am J Aquacult 76(4):305–311. doi:10.1080/15222055.2014.911226
Vohra FC (1966) Determination of photosynthetic pigment in seawater. Monographs on oceanographic methodology. UNESCO, France, p 66
Walne PR (1970) Studies on food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria and Mytilus. Fish Invest Lond Ser 2 26(5):1–62
Wei CX, Zhang YB, Guo J, Han B, Yang X, Yaun JL (2010) Effects of silica nanoparticles on growth and photosynthetic pigment content of Scenedesmus obliquus. J Environ Sci 22:155–160
Whang WX, Dei RCH (2003) Bioavailability of iron complexed with organic colloids to the cyanobacteria Synechococcus and Trichodesmium. Aqaut Microb Ecol 33:247–259
WHOI (Woods Hole Oceanographic Institution) (2007) Iron fertilization in southern ocean increased growth of algae that absorb greenhouse gases, and could cool climate. Available at: http://www.whoi.edu/main/news-releases/1995-2004?tid=3622&cid=971Accessed: December, 2013
Yavuz CT, Mayo JT, Yu WW, Prakash A, Falkner JC, Yean S, Gong LL, Shipley HJ, Kan A, Tomson M, Natelson D, Colvin VL (2006) Low field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 314:964–967
Zhang WX (2003) Nanoscale iron particles for environmental remediation: an overview. J Nanopart Res 5:323–332
Zhang Y, Chen Y, Westerhoff P, Crittenden J (2009) Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res 43:4249–4257
Acknowledgments
This work was supported by grant number (11/2012) funded by Deanship of Scientific Research, University of Bahrain. The authors are grateful to Mrs. Hanan Abbas for SEM and EDS analyses, Mr Ahmed Addad for TEM/EDX analysis, Mr. Makki Daffalla, and Mr. Etienne Dewailly for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Hazeem, L.J., Waheed, F.A., Rashdan, S. et al. Effect of magnetic iron oxide (Fe3O4) nanoparticles on the growth and photosynthetic pigment content of Picochlorum sp.. Environ Sci Pollut Res 22, 11728–11739 (2015). https://doi.org/10.1007/s11356-015-4370-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4370-5