Abstract
Discharge of dye-containing wastewater by the textile industry can adversely affect aquatic ecosystems and human health. Bioremoval is an alternative to industrial processes for detoxifying water contaminated with dyes. In this work, active and inactive biomass of the microalga Chlorella vulgaris was assayed for the ability to remove Congo Red (CR) dye from aqueous solutions. Through biosorption and biodegradation processes, Chlorella vulgaris was able to remove 83 and 58 % of dye at concentrations of 5 and 25 mg L−1, respectively. The maximum adsorption capacity at equilibrium was 200 mg g−1. The Langmuir model best described the experimental equilibrium data. The acute toxicity test (48 h) with two species of cladocerans indicated that the toxicity of the dye in the effluent was significantly decreased compared to the initial concentrations in the influent. Daphnia magna was the species less sensitive to dye (EC50 = 17.0 mg L−1), followed by Ceriodaphnia dubia (EC50 = 3.32 mg L−1). These results show that Chlorella vulgaris significantly reduced the dye concentration and toxicity. Therefore, this method may be a viable option for the treatment of this type of effluent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
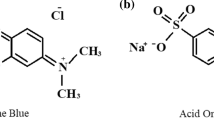
Azo dyes represent the largest group of dyes produced by industry (60–70 %) (Ong et al. 2010). These xenobiotic chemicals are characterized by the presence of one or more azo groups (–N=N–). They are frequently used in the textile and paper industries due to their low cost, color persistence, and variety of colors in comparison with natural dyes. Effluents containing azo dyes can significantly affect the photosynthetic activity of aquatic life, as they modify and reduce the penetration of light, affecting photosynthesis (Hernández-Zamora et al. 2014) and therefore the concentration of dissolved O2. There is also concern because many of the colors are made from carcinogenic compounds, such as benzidine (Golka et al. 2004). One of these dyes is Congo Red or Direct Red 28, used for dyeing cotton, jute, leather, paper, silk, and wool. The European Union banned its use in 1999 because the metabolic conversion of benzidine generates carcinogenic amines (Sponza and Isik 2005). However, this dye is still used in the textile industry because of its low cost.
Azo dyes are particularly difficult to degrade. Methods such as adsorption, neutralization, coagulation, chemical degradation, photocatalytic degradation, ozonation, membrane filtration, and ion exchange have been used for their removal from wastewater (Saratale et al. 2011; Sudha et al. 2014). Although these physical and chemical processes produce quality effluent, they have a high cost and limited application in most cases (Kumar and Ahmad 2011). Biological treatment, in contrast, is more economical. For this reason, there is increasing interest in applying biological processes as viable, effective, and inexpensive alternatives (Hazrat 2010). It has been reported that fungi, bacteria, yeast, and algae can discolor and even mineralize some azo dyes under certain environmental conditions (Pandey et al. 2007).
Two mechanisms are used by microorganisms for azo dye bioremoval: adsorption and/or biodegradation. The adsorption of dyes is carried out by using the biomass of living and dead microbial cells. In this process, the original structure of the dye remains intact. However, biodegradation is performed when microorganisms alter the original structure of the dye and sometimes mineralize it to water and carbon dioxide (Hazrat 2010).
It has been shown that microalgae respond quickly to environmental changes due to their short cell-doubling time compared to higher plants, and they are important because of their role as major primary producers in aquatic ecosystems. Algae such as Chlorella vulgaris, Chlorella pyrenoidosa, Spirogyra sp., Oscillatoria tenuis, Oscillatoria rubescens, and Elakatothrix viridis can remove azo dyes depending on their molecular structure and the species of alga used (Jinqi and Houtian 1992; Hanan 2008; El-Sheekh et al. 2009). For example, Jinqi and Houtian (1992) reported that Eriochrome Blue was completely biodegraded by Chlorella vulgaris and Oscillatoria tenuis. Acuner and Dilek (2004) showed that Chlorella vulgaris was able to remove 69, 66, and 63 % of the mono-azo dye Tectilon Yellow G at concentrations of 50, 200, and 400 mg L−1, respectively. This alga then converts this dye into aniline, although this product has been reported to be toxic. For this reason, it is important to conduct research on the toxicity of compounds that could be generated in the bioremoval processes. Although the elimination of xenobiotics is important, it is also imperative to ensure that the degradation products are safe. In addition to chemical assessment of the bioremoval of pollutant compounds, it is important and even necessary to determine whether their toxic characteristics have been reduced or eliminated. This evaluation is done by controlled exposure of selected test organisms, which should be representative of the communities in the aquatic environment. Because of their ecological importance, cladocerans are often used as test organisms because they are a critical component of the zooplankton community (Martínez-Jerónimo et al. 2000). The cladocerans Daphnia magna and Ceriodaphnia dubia have been recognized as reference species for ecotoxicological studies (Blaise and Férard 2005).
The objectives of this research were to evaluate the potential of Chlorella vulgaris for the bioremoval of the azo dye Congo Red (CR) from aqueous solutions and to determine the toxicity of the effluent produced after bioremoval using bioassays with the cladocerans D. magna and Ceriodaphnia dubia.
Materials and methods
Organisms
The green microalga Chlorella vulgaris LHE-Chl01 was obtained from the Laboratorio de Hidrobiología Experimental of the Escuela Nacional de Ciencias Biológicas of the Instituto Politécnico Nacional, México. The microalga was grown in Bold’s basal mineral medium (Stein 1973) using flat-sided glass bottles with a total capacity of 0.5 L and a working volume of 0.25 L. Cultures were performed using the following incubation conditions: temperature of 25 ± 3 °C, light intensity of 120 μmol m−2 s−1, 12/12 h (light/dark) photoperiod, and bubbling with air flow of 200 mL min−1.
The cladocerans Ceriodaphnia dubia and D. magna used in the bioassays were obtained from the cladoceran strain collection of the Laboratorio de Hidrobiología Experimental of the Escuela Nacional de Ciencias Biológicas of the Instituto Politécnico Nacional, México, where they have been successfully maintained through controlled cultivation for over 15 years.
Both species of cladocerans were cultured in soft water (12 mg L−1 NaHCO3, 7.5 mg L−1 CaSO4 2H2O, 7.5 mg L−1 MgSO4, and 0.5 mg L−1 KCl) with a pH of 6.8, hardness of 10–13 mg L−1 CaCO3, temperature of 25 °C, and photoperiod of 16:8 h (light/dark). Cladocerans were fed the chlorophycean microalga Pseudokirchneriella subcapitata at a concentration of 10 × 105 cells mL−1. For neonates (offspring under 24 h old) (USEPA 2002), controlled batches of known age of each species were used. Once reproduction began, adult parthenogenetic females were separated, and all hatchlings produced were carefully collected by filtering through a 125-μm sieve. Neonates were then used as test organisms in all toxicity measurements. Assays were performed in triplicate.
Dye
CR azo dye, also called Direct Red 28, was used in the experiments. The dye was obtained from Sigma-Aldrich®, USA. The molecular formula of CR azo dye is C32H22N6O6S2Na2, with a molecular weight of 696.7 g mol−1. In all the experiments, the colorant was prepared as a stock solution at 100 mg L−1. This solution was sterilized by filtration using a Millipore® membrane with pore diameter of 5.0 μm before experimental use.
Bioremoval study
Batch experiments were performed using axenic cultures of the green microalga Chlorella vulgaris in flat-sided glass bottles of 0.5 L in capacity as experimental cultivation units. The total volume of Bold’s basal mineral medium (Stein 1973) used was 0.25 L and was inoculated with 15 mL of a culture of Chlorella vulgaris in the exponential growth phase and various concentrations of CR dye (5, 10, 15, 20, and 25 mg L−1). The control used contained only growth medium and dye. This control was used to identify any reaction of the medium with the dye and to determine whether photolysis and/or adsorption occurred in the culture vessel.
In biosorption experiments, inactive biomass concentrations were used. Inactivation was performed by sterilization in an autoclave at 121 °C for 15 min. Subsequently, the biomass was dried in a lyophilizer. Each treatment system was inoculated with the various concentrations of dry cell biomass of inactive Chlorella vulgaris (11.1, 24.4, 51.1, 73.3, 108, and 146.6 mg L−1), mineral Bold’s basal medium, and the various concentrations of dye indicated previously.
The incubation conditions for experiments with live and inactive cells were as follows: 25 ± 3 °C, 120 μmol m−2 s−1 light intensity, 12/12 h photoperiod (light/dark), and air flow of 200 mL min−1. All experimental units were maintained under these conditions for 96 h. For experiments with live cells, aliquots of 15 mL of each dye concentration were taken at 0, 24, 48, 72, and 96 h to further evaluate the residual concentration of dye as described below. The activity of the azo reductase enzyme was evaluated only at the end of the time course (96 h), as per the procedure described by El-Sheekh et al. (2009).
For the biosorption experiments, samples of 5 mL from each experimental unit were collected at pre-determined time intervals to evaluate the residual dye concentration.
Acute toxicity bioassays with cladocerans
The toxic effects of the influent and effluent of the azo dye CR were evaluated in two different toxicity tests (each at 48 h). Influent and effluent samples were obtained from the bioremoval study mentioned previously.
Acute toxicity testing in the influent and the effluent were performed using CR concentrations of 5, 10, 15, 20, and 25, and 0.84, 1.85, 3.72, 5.7, and 10.4 mg L−1, respectively. For the test, 33 mL of each influent and effluent concentration was taken, as well as for the control (Bold’s basal mineral medium, Stein 1973). Each experimental condition was done in triplicate. Ten neonates were transferred to each experimental unit and were placed in a bioclimatic chamber at 24 ± 1 °C, with a photoperiod of 16:8 h (light/dark). The neonates were not fed during the experiment. The number of affected and/or dead animals was registered at 24 and 48 h. The lack of mobility and the absence of a cardiac pulse were used as criteria for mortality (USEPA 2002; Mexican Standard NMX-AA-087-SCFI 2010).
Analytic methodology
The determination of the residual concentration of CR in each sample was conducted by measuring the absorbance of cell-free supernatant at 494 nm using a UV-vis (Genesys 10 UV, Thermo Electron Corporation) spectrophotometer. All determinations were performed in triplicate.
Bioremoval efficiency and dye biosorption
Efficiency was expressed as follows:
where C o and C f correspond to the initial and final concentrations of CR (mg L−1).
Next, dye adsorption capacity was determined from experimental biosorption data (q t, mg dye g−1 biosorbent). The adsorption capacity is defined as the amount of dye removed per mass unit of adsorbent material at various contact times and was calculated using the following equation (Guendouz et al. 2013):
Here, C o is the initial concentration of the dye (mg L−1), C f is the concentration of CR at time t (mg L−1), V is the volume of dye solution used (L), and m is the mass of the biosorbent added (g). When the reaction reaches equilibrium, C f is the concentration of CR at equilibrium (C eq) and q t is the capacity for removal of CR at equilibrium q eq.
Biosorption isotherm
To describe the adsorption isotherm in equilibrium, several models with two (Langmuir, Freundlich, Temkin, Dubinin-Radushkevich, and Halsey) or three parameters (Sips, Radke-Prausnitz, and Toth) were applied.
Langmuir isotherm
The Langmuir model is expressed as follows (Vijayaraghavan et al. 2006):
where q e is the CR biosorption capacity at equilibrium (mg g−1), q max is the maximum CR biosorption capacity (mg g−1), C e is the liquid phase concentration of CR at equilibrium (mg L−1), and b is the biosorption equilibrium constant (L mg−1). The essential features of a Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor or intensity of biosorption, R L, which is expressed in Eq. 4 (Michalak and Chojnacka 2010):
where C o is the initial CR concentration (mg L−1). Another important parameter is the surface coverage (θ, Eq. 5), which indicates the fraction of the biosorption sites occupied by the dye at equilibrium.
Freundlich isotherm
This isotherm describes the reversible adsorption on a heterogeneous surface and a multilayer adsorption. It is expressed by the following equation (Vijayaraghavan et al. 2006):
Here, C e is the concentration of the sorbate in the liquid phase at equilibrium (mg L−1), K F is the Freundlich constant (mg g−1) (mg L−1)−1/n, and n (dimensionless) is an empirical parameter associated with biosorption intensity, which varies according to the heterogeneity of the material.
Temkin isotherm
The Temkin model (Vijayaraghavan et al. 2006) assumes that the heat of adsorption of molecules in the layer decreases linearly when the coverage of the adsorbent surface increases. Its equation is defined as follows:
where R is the universal gas constant (8.314 J mol K−1), T is the absolute temperature (K), b T is the Temkin constant related to sorption heat (J mol−1), and a T (L mg−1) is another constant of the Temkin model.
Dubinin-Radushkevich isotherm
This model is based on the Polanyi-Dubinin potential theory of the adsorption and assumes that there is a multilayer character of sorption potential where free energy of sorption is related to degree of pore filling (Vijayaraghavan et al. 2006). The model is expressed as follows:
where q max is the maximum adsorption capacity at equilibrium (mg g−1), B D is the activity coefficient related to adsorption energy (mol2 J−2), and ε is the Polanyi potential.
Halsey isotherm
This model allows the description of biosorption performed in multilayers. A good fit of the model to the results suggests that the pores of the biosorbent are heterogeneous (Vijayaraghavan et al. 2006). Its equation is as follows:
where K H and n are Halsey’s constant and exponent, respectively.
Sips isotherm
Sips isotherm is a combination of the Langmuir and Freundlich isotherms (Vijayaraghavan et al. 2006) and is described as follows:
where q max is the maximum biosorption capacity (mg g−1), K s is the Sips constant (mg L−1)−1/ns, and n is a parameter of heterogeneity.
Radke-Prausnitz isotherm
The Radke-Prausnitz isotherm (Vijayaraghavan et al. 2006) can be represented as follows:
where a R (L g−1) and r R (L mg−1) are constants of the Radke-Prausnitz model and β r is the exponent of the model.
Toth isotherm
This isotherm is derived from the potential theory and assumes an asymmetrical quasi-Gaussian energy distribution, in which most of the sites have adsorption energy below the mean energy of adsorption. This isotherm is described (Vijayaraghavan et al. 2006) as follows:
where q max is the maximum biosorption capacity (mg g−1), b T (L mg−1) is a Toth constant, and n T is a parameter of heterogeneity.
Data analysis
The results were statistically analyzed by one-way ANOVA (P < 0.05) and Tukey’s test for multiple comparisons using Sigma Plot, version 11.0.
All parameters of the isotherm models were evaluated by nonlinear regression analysis using GraphPad Prism, version 6.0. The optimization procedure required an error function defined to evaluate the fit of mathematical models to experimental data. The coefficient of determination (r 2), the residual error or the sum of squared errors (SSE), and the standard error or root-mean-square error (RMSE) were chosen as selection criteria to measure the goodness of fit of the isotherm models.
The average effective concentration (CE50) was determined with a probit model using academic software (Stephan 1997).
Results and discussion
Bioremoval of CR
Figure 1a shows that the quantity of residual dye decreased after exposure of live Chlorella vulgaris cells to CR dye for 96 h. Furthermore, the residual dye decreased compared to the initial concentrations of CR. Using initial CR concentrations of 5, 10, 15, 20, and 25 mg L−1, the residual concentrations after 96 h were 0.84, 1.88, 3.73, 5.7, and 10.4 mg L−1, respectively. This result shows that the colorant is bioremoved by the microalgae. The highest values of bioremoval percentage (Fig. 1b) were found for the lowest initial dye concentrations of 5 and 10 mg L−1. After 96 h of exposure, the bioremoval percentages were 83 and 81 %, respectively, but these percentages decreased to 75, 72, and 58 % with higher concentrations of the dye (15, 20, and 25 mg L−1).
However, Hanan (2008) showed that the microalga Chlorella vulgaris was able to bioremove the azo dye Tartrazine by 48, 43, and 20 % from initial concentrations of 5, 10, and 15 mg L−1, respectively. Other authors such as Acuner and Dilek (2004) demonstrated that the bioremoval ability of Chlorella vulgaris for the Yellow dye Tectilon G decreases when the dye concentration increases. They observed 69, 66, and 63 % reductions by bioremoval of the concentrations 50, 200, and 400 mg L−1, respectively. However, El-Sheekh et al. (2009) reported that Chlorella vulgaris was unable to bioremove Methyl Red dye at the concentration of 20 mg L−1.
Based on our findings and those reported in the literature for Chlorella vulgaris, it can be inferred that the capacity of this microalga for azo dye bioremoval depends mainly on the structure and concentration of the dye and on the experimental conditions.
Table 1 shows the azo reductase activity at different initial concentrations of CR (0–25 mg L−1). The maximum value measured was 20.4 ± 2.4 μg substrate g−1 dry weight h−1, which corresponds to the highest dye concentration of 25 mg L−1. In general, it was observed that the activity of the azo reductase increases when the initial concentration of dye in the culture medium increases. This phenomenon was reported by Jinqi and Houtian (1992) for the same microalga. They suggested that Chlorella vulgaris azo reductase is responsible for degrading azo dyes into aromatic amines by breaking the azo linkage and that the enzyme is inducible.
Hanan (2008) reported a Chlorella vulgaris azo reductase activity of 12 μg substrate g−1 dry weight h−1 for the Tartrazine dye and 9 μg substrate g−1 dry weight h−1 for Ponceau dye. Both dyes were tested at 20 mg L−1. The azo reductase activity obtained in the present work at a 25 mg L−1 initial CR dye concentration was 77 and 122.22 % higher than that obtained with Tartrazine and Ponceau dye, respectively.
Biosorption experiments
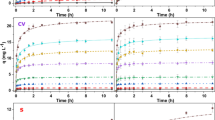
The residual concentrations of CR at various contact times and the various tested biomass concentrations of inactive cells (51, 73, 108, and 146 mg L−1) are shown in Fig. 2. With the various cell concentrations tested (Fig. 2a–d), the residual dye concentration decreased rapidly during the first 10 h of treatment. Subsequently, the residual concentration decreased slowly.
In general, we can see from Fig. 2 that when the content of inactive cell biomass increased from 51 mg L−1 (Fig. 2a) to 146 mg L−1 (Fig. 2d) at different initial concentrations of CR, there was an increase in the amount of dye adsorbed. Therefore, the amount of residual dye decreased significantly.
Figure 3 shows that when the dye concentration increased from 5 to 25 mg L−1, the percentage of biosorption of the dye decreased from 67 to 32 % for a concentration of biosorbent of 51 mg L−1, from 73 to 44 % for a biosorbent concentration of 73 mg L−1, from 79 to 65 % for 108 mg L−1, and from 87 to 74 % for 146 mg L−1. In general, the adsorption percentage of the dye decreased when the initial concentration of the dye increased and increased with the biosorbent concentration. The decrease of biosorption efficiency with the increase of dye concentration may be because the dye molecules present in solution at low concentration interact more easily with biosorbent binding sites, thus facilitating biosorption. However, all adsorbents have a limited number of binding sites and become saturated at certain concentrations (Deniz and Saygideger 2011). Therefore, at high dye concentrations, the amount of non-adsorbed CR dye molecules increases due to the saturation of binding sites. This phenomenon results in the decrease of removal percentage and therefore in the increase in the residual dye concentration.
Here, it is likely that CR is adsorbed by the cell wall of Chlorella vulgaris. The biosorption process is performed by the interactions between the dye molecule and the functional groups of the cell wall. Biosorption studies using the microalgae Chlorella vulgaris performed by Aksu and Tezer (2005) show that the cell wall of green algae contains various functional groups such as amino, carboxyl, hydroxyl, sulfate, and other charged groups, to which dyes can bind.
Figure 4 shows the adsorption capacity (q) of various concentrations of inactive cell biomass versus time. Figure 4a–d shows that biosorption curves are continuous and lead to saturation of the biosorbent. Furthermore, these figures show that adsorption occurs mainly in two stages, an initial rapid stage during the first 10 h, followed by a slower secondary stage, with a horizontal plateau due to the equilibration of adsorption after 72 h. The process of biosorption is heavily influenced by the initial concentration of the adsorbate (Saha et al. 2012). Figure 4a–d indicates that the biosorption capacity q (mg g−1) of the inactive cells of Chlorella vulgaris increases with the initial concentration of CR dye in the biosorption system. For example, when an inactive cell biomass concentration of 51 mg L−1 (Fig. 4a) was in contact for 96 h with CR solution at the concentrations of 5 and 25 mg L−1, the adsorption capacities were 52.17 and 144.67 mg g−1, respectively. This increase may be because the higher initial concentration of adsorbate caused an increased concentration gradient which provided a higher thermodynamic driving force to overcome all mass transfer resistances of the dye from the aqueous solution to the biosorbent (Chowdhury and Saha 2010). The biosorption capacities discussed above are higher than those reported by Annadurai et al. (2002), who used banana peel as biosorbent and CR as the adsorbate in a range of initial concentrations from 10 to 120 mg L−1. Aksu and Tezer (2005) evaluated the adsorption ability of Chlorella vulgaris cells when exposed separately to Remazol Black B and Remazol Red. When the dye concentration increased from 20 to 800 mg L−1, the adsorption capacity of Chlorella vulgaris increased from 15.9 to 419.5 mg g−1 for the dye Remazol Black B and from 4.2 to 181.9 mg g−1 for Remazol Red.
From the results of this study and from previous reports in the literature, it is clear that the adsorption capacity depends on the adsorbent and adsorbate used as well as on the experimental conditions of the adsorption process (e.g., pH, temperature, concentration).
Biosorption isotherm
In this study, the biosorption isotherm was generated by varying the initial concentration of CR dye (5–25 mg L−1) and the biosorbent while maintaining the pH (7.0) and temperature (25 °C) at constant levels. The experimental biosorption isotherm shown in Fig. 5a, b is regular and concave relative to the concentration axis. This graph shows that by increasing the concentration of residual CR at equilibrium, the adsorption capacity at equilibrium also increases.
The shape of the isotherm indicates that as biosorption sites of the biosorbent are occupied, it becomes more difficult for dye molecules to find available biosorption sites. This result suggests a progressive saturation of the biosorbent. The CR biosorption isotherm is a type L isotherm according to the classification of Giles et al. (1974). It indicates a high affinity between the cells of Chlorella vulgaris and CR dye and that no strong competition exists between the solvent and the dye molecules to occupy the biosorption sites.
In order to describe the experimental biosorption isotherm of CR (Fig. 5a, b), several two-parameter (Langmuir, Freundlich, Temkin, Dubinin-Radushkevich, and Halsey) and three-parameter (Sips, Radke-Prausnitz, and Toth) models were used.
The values of the parameters of each model with a confidence interval of 95 %, coefficient of determination (r 2), residual error (SSE), and standard error (RMSE) are shown in Table 2. This table shows that among the two-parameter models, the Langmuir model manifested the best fit to the experimental data of biosorption, with a coefficient of determination (r 2) of 0.994 and predicted a maximum biosorption capacity (202.9 mg g−1) similar to that found experimentally (200 mg g−1). The value for constant n in the Freundlich model was 2.053 (Table 2), which is within the interval of n values (n = 1–10) which indicate favorable biosorption (Basha and Murthy 2007). However, the Freundlich model gave a determination coefficient (r 2 = 0.9646) that was lower, and RMSE and SSE values that were higher, than those obtained when applying the Langmuir model.
The Temkin and Halsey models yielded lower coefficients of determination and higher RMSE and SSE values than the Langmuir model, and consequently, these models are not suitable for describing the CR experimental isotherm. Among the two-parameter models tested, the poorest fit to experimental values at equilibrium was obtained using the Dubinin-Radushkevich model, which presented the lowest coefficient of determination (r 2 = 0.9205) and the highest SSE and RMSE values, compared to those obtained using the Langmuir, Freundlich, Temkin, and Halsey models. Besides, the Dubinin-Radushkevich model predicted a q max approximately 32 % below the experimental value.
Generally, the three-parameter models (Sips, Radke-Prausnitz, and Toth) fitted the experimental data well (Fig. 5). Table 2 shows that the three-parameter models had coefficients of determination, as well as RMSE and SSE values, similar to those of the Langmuir model. However, the parameters of the Radke-Prausnitz model had a confidence interval of 95 %, which is very high compared to the other models. This model was therefore not suitable for describing the experimental isotherm. Similarly, although the Sips and Toth models rendered a high determination coefficient value (r 2 = 0.99) and low RMSE and SSE values, the predicted q max values were lower than the experimental value. Therefore, the Toth and Sips models did not properly describe the biosorption equilibrium of CR by the microalga. Based on these results, the Langmuir model concurs best with the experimental data for CR biosorption by Chlorella vulgaris. This model has great practical importance because it is a simpler two-parameter model that can be applied and interpreted more easily, and may therefore be especially useful for scaling and engineering biosorption processes (Vijayaraghavan et al. 2006).
Table 2 shows that the predicted Langmuir q max of 202.9 mg g−1 approximates the experimental value of the maximum biosorption capacity of CR (q max = 200 mg g−1). Comparing the q max results of this work with those reported in the literature for CR dye using other types of adsorbents (Table 3), we found that the q max obtained in this work was higher by up to 29.5 % compared with the q max reported for magnetically modified Chlorella vulgaris (Safarikoval et al. 2008). This comparison also showed that the q max of Chlorella vulgaris was 34.2, 12.07, 8.92, 13.86, 3.91, and 2.83 times higher than banana pith, commercial activated carbon, wheat bran, rice bran, Trametes versicolor biomass, and Porphyra yezoensis Ueda biomass, respectively.
The affinity constant of the Langmuir model can be used to determine whether the biosorption process is favorable or unfavorable by calculating the separation factor R L (Michalak and Chojnacka 2010). Table 4 shows the adsorption intensity determined for each of the initial concentrations of CR tested.
Table 4 also shows that the separation factor tends to decrease significantly as the starting concentration of CR increases, which indicates that CR biosorption is enhanced as the initial concentration of the dye in solution increases. This behavior is due to the increased probability that the dye contacts the biosorbent when the amount of the dye in the solution increases. Therefore, the interaction between the colorant and the biosorbent increases along with the dye biosorption capacity. It is known that when the separation factor is 0, the biosorption process is irreversible. If the value is between 0 and 1, the biosorption is favorable, and if the R L value is equal to 1, the isotherm is a linear biosorption. If the value is greater than 1, the separation factor value indicates that biosorption is unfavorable (McKay et al. 1987). In this work, all R L values obtained were between 0 and 1, indicating that the CR biosorption by inactive Chlorella vulgaris biomass was favorable.
Table 4 shows the increase of the θ value as the initial concentration of CR increases. This phenomenon occurs because the biosorbent surface was nearly fully covered with a monomolecular layer at the higher dye concentrations, achieving up to 85 % of active site occupancy at the initial concentration of 25 mg L−1. Furthermore, it can be observed that the surface coverage varied significantly in all concentrations of the dye.
Acute toxicity bioassays before and after the bioremoval process
Figure 6a shows the survival percentages of D. magna subjected to five concentrations of CR. In general, survival percentages in the influent decreased significantly with increasing concentrations of CR. Furthermore, the survival percentage was higher in the effluent compared to the influent, indicating that the toxicity of the dye was significantly decreased after treatment.
Furthermore, Fig. 6a shows that with the initial effluent concentrations of 5 to 20 mg L−1, survival was greater than or equal to 90 %. Therefore, it is expected that this effluent does not produce significant acute toxic effects in the environment. At these concentrations, mortality was less than or equal to 10 %, which is the maximum value allowed for acceptance in acute toxicity bioassays with D. magna (USEPA 2002; Mexican Standard NMX-AA-087-SCFI 2010). However, at the highest concentration tested (25 mg L−1), the mortality in the treated effluent was greater than 10 %, which is likely due to residual dye concentrations high enough to generate lethal responses during the time of exposure (48 h). Furthermore, the metabolites could have accumulated if this reaction was a biodegradation process. Golka et al. (2004) reported that the major metabolites generated by CR dye are aromatic amines, which are highly toxic compounds.
The survival percentages of Ceriodaphnia dubia in the influent and effluent of CR dye are shown in Fig. 6b. In general, it appears that these percentages decreased significantly compared to the control for all concentrations tested. Additionally, these percentages were higher in the effluent compared to the influent. The lowest (5 mg L−1) and highest (25 mg L−1) concentrations of CR had survival rates in the influent of 30 and 0 %, respectively. However, these percentages were 88.5 and 62.3 % in the treated effluent. This result indicates that dye toxicity for this species was significantly reduced by treatment with Chlorella vulgaris.
The sensitivity of cladocerans to the dye was also evaluated by determining the EC50 (48 h). For D. magna, the maximum value was 17.0 mg L−1 (limits of 15.60 and 18.84 mg L−1). However, the value was 3.32 mg L−1 for Ceriodaphnia dubia, and the upper and lower limits of the confidence interval were 1.24 and 4.91 mg L−1, respectively. Therefore, we can conclude that Ceriodaphnia dubia was approximately five times more sensitive than D. magna to CR.
Versteeg et al. (1997) suggested that among freshwater invertebrates, Ceriodaphnia dubia is a better test organism in ecotoxicity studies due to its high sensitivity and its short life cycle. However, there are no published reports showing the sensitivity of this cladoceran to azo dyes.
In contrast, Meric et al. (2005) conducted toxicity bioassays in D. magna exposed to the dye Remazol Black B and reported an EC50 value of 75 mg L−1. Bae and Freeman (2007) studied the toxicity of four Direct dyes and one Direct metallic dye (Direct Black 281) and found that the four Direct dyes, which had no metal in their structure, were non-toxic (LC50 > 100 mg L−1), whereas the Direct Black 281 dye molecules, which contain two copper atoms in their structure, were highly toxic (LC50 6.0 mg L−1 at 24 h, LC50 3.6–6.0 mg L−1 at 48 h). This suggests that the copper in the structure of the dye increases the acute toxicity. The CR dye does not contain any metallic chemical compound in its structure and is mainly based on benzidine, a compound reported to act as a carcinogen by generating metabolites such as aromatic amines (Sponza and Isik 2005).
To date, no studies have reported toxic effects of CR in these species of cladocerans. However, the present results show that toxicity caused by the influent on the survival of cladocerans is sufficient to indicate the potential damage that this type of dye would cause if discharged into the aquatic ecosystem without remedial pretreatment.
Conclusion
The present work clearly showed that the green microalga Chlorella vulgaris can be efficiently used for CR bioremoval. Based upon the results, the CR bioremoval mechanism may be attributed to the process of biosorption and biodegradation. These results imply the presence of the azo reductase enzyme in the microalga and its participation in the CR biodegradation process. The inactive biomass of Chlorella vulgaris was shown to be potentially useful as a biosorbent for the dye at low concentrations, and it may be considered as an alternative to more costly materials such as activated carbon. Isotherm studies showed that the biosorption of CR onto Chlorella vulgaris closely follows the Langmuir model, with an experimental maximum biosorption capacity of 200 mg g−1. The cladocerans D. magna and Ceriodaphnia dubia can be used as organism models for extrapolating the toxicological implications of effluents containing azo dyes and to monitor the efficiency of degradation by observing toxicity reduction in treated effluents.
Further research is required to evaluate the performance of Chlorella vulgaris using real textile effluents, as these effluents may contain several toxic dyes and other harmful contaminants such as heavy metals and these may affect the biodegradation and/or biosorption capabilities of the microalga. It is also of interest to elucidate the nature of the metabolic intermediates formed during the CR biodegradation process and define whether other enzymes, apart from azo reductase, are involved in the biodegradation of CR. This information will increase knowledge concerning the mechanisms employed by Chlorella vulgaris for biodegrading dyes.
References
Acuner A, Dilek FB (2004) Treatment of Tectilon Yellow 2G by Chlorella vulgaris. Process Biochem 39:623–631
Aksu Z, Tezer S (2005) Biosorption of reactive dyes on the green algae Chlorella vulgaris. Process Biochem 40:1347–1361
Annadurai G, Juang RS, Lee DJ (2002) Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J Hazard Mater B92:263–274
Bae JS, Freeman HS (2007) Aquatic toxicity evaluation of new Direct dyes to the Daphnia magna. Dyes Pigments 73:81–85
Basha S, Murthy ZVP (2007) Kinetic and equilibrium models for biosorption of Cr (VI) on chemically modified seaweed, Cystoseira indica. Process Biochem 42:1521–1529
Binupriya AR, Sathishkumar M, Swaminathan K, Kuz CS, Yun SE (2008) Comparative studies on removal of Congo Red by native and modified mycelial pellets of Trametes versicolor in various reactor modes. Bioresour Technol 99:1080–1088
Blaise C, Férard JF (2005) Small-scale freshwater toxicity investigations. Vol. 1–Toxicity test methods. Springer, Berlin, 422 p
Chowdhury S, Saha P (2010) Sea shell power as a new adsorbent to remove Basic Green 4 (Malachite Green) from aqueous solutions: equilibrium, kinetic and thermodynamic studies. Chem Eng J 164(1):168–177
Deniz F, Saygideger SD (2011) Removal of a hazardous azo dye (Basic Red 46) from aqueous solution by princess tree leaf. Desalination 268:6–11
El-Sheekh MM, Gharieb MM, Abou-El-Souod GW (2009) Biodegradation of dyes by some green algae and cyanobacteria. Int Biodeterior Biodegrad 63:699–704
Fu Y, Viraraghavan T (2002) Removal of Congo Red from an aqueous solution by fungus Aspergillus niger. Adv Environ Res 7:239–247
Giles CH, Smith D, Huitso A (1974) A general treatment and classification of the solute adsorption isotherms. I Theoretical. J Colloid Interface Sci 47:755–765
Golka K, Kopps S, Myslak WZ (2004) Carcinogenicity of azo colorants: influence of solubility and bioavailability. Toxicol Lett 15:203–210
Guendouz S, Khellaf N, Zerdaoui M, Ouchefoun M (2013) Biosorption of synthetic dyes (Direct Red 89 and Reactive Green 12) as an ecological refining step in textile effluent treatment. Environ Sci Pollut Res 20:3822–3829
Hanan HO (2008) Algal decolorization and degradation of monoazo and diazo dyes. Pak J Biol Sci 11(10):1310–1316
Hazrat A (2010) Biodegradation of synthetic dyes—a review. Water Air Soil Pollut 213:251–273
Hernández-Zamora M, Perales-Vela HV, Flores-Ortiz CM, Cañizares-Villanueva RO (2014) Physiological and biochemical responses of Chlorella vulgaris to Congo Red. Ecotoxicol Environ Saf 108:72–77
Jinqi L, Houtian L (1992) Degradation of azo dyes by algae. Environ Pollut 75:273–278
Kumar R, Ahmad R (2011) Biosorption of hazardous crystal violet dye from aqueous solution onto treated ginger waste (TGW). Desalination 265:112–118
Martínez-Jerónimo F, Espinosa-Chávez F, Villaseñor-Córdova R (2000) Effect of culture volume and adult density on the neonate production of Daphnia magna, as test organisms for aquatic toxicity test. Environ Toxicol 15:155–159
McKay G, El-Geundi MS, Nassar MM (1987) Equilibrium studies during the removal of dyestuffs from aqueous solutions using bagasse pith. Water Res 21:1523–1530
Meric S, Selçuk H, Belgiorno V (2005) Acute toxicity removal in textile finishing wastewater by Fenton’s oxidation, ozone and coagulation-floculation processes. Water Res 39(6):1147–1153
Mexican Standard NMX-AA-087-SCFI (2010) ANÁLISIS DE AGUA-Evaluación de Toxicidad Aguda con Daphnia magna Straus (Crustacea Cladocera)-Método de prueba, 44 pp
Michalak I, Chojnacka K (2010) The new application of biosorption properties of Enteromorpha prolifera. Appl Biochem Biotechnol 160:1540–1556
Namasivayam C, Prabha D, Kumar M (1998) Removal of Direct Red and Acid Brilliant Blue by adsorption on to banana pith. Bioresour Technol 64:77–79
Ong S, Uchiyama K, Inadama D, Ishida Y, Yamagiwa K (2010) Treatment of azo dye Acid Orange 7 containing wastewater using up-flow constructed wetland with and without supplementary aeration. Bioresour Technol 101:9049–9057
Pandey A, Singh P, Iyengar L (2007) Bacterial decolorization and degradation of azo dyes. Int Biodeterior Biodegrad 59:73–84
Safarikoval M, Pona BMR, Mosiniewicz-Szablewska E, Weyda F, Safarik I (2008) Dyes adsorption on magnetically modified Chlorella vulgaris cells. Fresenius Environ Bull 17:486–492
Saha PD, Chowdhury S, Mondal M, Sinha K (2012) Biosorption of Direct Red 28 (Congo Red) from aqueous solutions by eggshells: batch and column studies. Sep Sci Technol 47:112–123
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42:138–157
Sponza DT, Isik M (2005) Toxicity and intermediates of C.I. Direct Red 28 dye through sequential anaerobic/aerobic treatment. Process Biochem 40:2735–2744
Stein JR (1973) Handbook of phycological methods. Culture methods and growth measurements. Cambridge University Press, London, pp 7–24
Stephan CE (1997) Methods for calculating an LC50. In: Mayer FL, Hamelink JL (ed) Aquatic toxicology and hazard evaluation. American Society for Testing and Materials (ASTM), Philadelphia Pennsylvania, pp. 65–84, 534.
Sudha M, Saranya A, Selvakumar G, Sivakumar N (2014) Microbial degradation of azo dyes: a review. Int J Curr Microbiol Appl Sci 3(2):670–690
USEPA (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 5ª ed. US Environmental Protection Agency, Office of Water (4303 T). N.W. Washington, DC 20460. EPA-821-R-02-012, 266 p
Versteeg DJ, Stalmans M, Dyer SD, Janssen C (1997) Ceriodaphnia and Daphnia: a comparison of their sensitivity to xenobiotics and utility as a test species. Chemosphere 34:869–892
Vijayaraghavan K, Padmesh TVN, Palanivelu K, Velan M (2006) Biosorption of nickel (II) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models. J Hazard Mater B133:304–308
Wang XS, Chen JP (2009a) Biosorption of Congo Red from aqueous solution using wheat bran and rice bran: batch studies. Sep Sci Technol 44:1452–1466
Wang XS, Chen JP (2009b) Removal of the azo dye Congo Red from aqueous solutions by the marine alga Porphyra yezoensis Ueda. Clean 37(10):793–798
Acknowledgments
The authors thank the Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional for the support to develop this investigation. Hernández-Zamora M. received a post-graduate scholarship from Consejo Nacional de Ciencia y Tecnología (Grant no. 204491) for doctoral studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Hernández-Zamora, M., Cristiani-Urbina, E., Martínez-Jerónimo, F. et al. Bioremoval of the azo dye Congo Red by the microalga Chlorella vulgaris . Environ Sci Pollut Res 22, 10811–10823 (2015). https://doi.org/10.1007/s11356-015-4277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4277-1