Abstract
To develop an effective phytoremediation approach to purify soils polluted by decabromodiphenyl ether (BDE-209) in e-waste recycling area, pot experiments were conducted through greenhouse growth of seven plant species in BDE-209-polluted soils. The hygrocolous rice (Oryza sativa L.) cultivars (XiuS and HuangHZ) and the xerophyte ryegrass (Lolium perenne L.) were found to be as the most effective functional plants for facilitating BDE-209 dissipation, with the removal of 52.9, 41.9, and 38.7 % in field-contaminated soils (collected directly from field, with an average pollution concentration of 394.6 μg BDE-209 kg−1 soil), as well as 21.7, 27.6, and 28.1 % in freshly spiked soils (an average pollution concentration of 4413.57 μg BDE-209 kg−1 soil, with additional BDE-209 added to field-contaminated soils), respectively. Changes in soil phospholipid fatty acid (PLFA) profiles revealed that different selective enrichments of functional microbial groups (e.g., arbuscular mycorrhizal fungi and gram-positive bacteria) were induced due to plant growth under contrasting water management (flooded-drained sequentially, flooded only, and drained only, respectively). The abundance of available electron donors and acceptors and the activities of soil oxido-reductases were also correspondingly modified, with the activity of catalase, and the content of NO3 − and Fe3+ increased generally toward most of the xerophyte treatments, while the activity of dehydrogenase and the content of dissolved organic carbon (DOC) and NH4 + increased toward the hygrophyte treatments. This differentiated dissipation of BDE-209 in soils as function of plant species, pollution doses and time, and water-dependent redox condition. This study illustrates a possibility of phytoremediation for BDE-209-polluted soils by successive cultivation of rice followed by ryegrass coupling with suitable water management, possibly through dissipation pathway of microbial reductive debromination and subsequent aerobic oxidative cleavage of benzene ring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil pollution, occurring in e-waste recycling area, is a problem which has drawn worldwide attention; however, little information is available on restoration of soil polluted by polybrominated diphenyl ethers (PBDEs), a new type flame retardants used worldwide as substitute of polychlorinated biphenyls (PCBs) since PCBs have been banned from usage because of their environmental issue. PBDEs have been frequently detected in various environmental media within typical e-waste recycling area in recent years, including air, water, and soil and sediment, due to their release from the scrap electronic components during improperly recycled processes (Tang et al. 2014). To date, PBDEs have attracted considerable public interest, since some high brominated congeners possess similar physicochemical, bioaccumulative, and toxicological properties to traditional “persistent organic pollutants” (Hassanin et al. 2004). Among all the PBDEs, the highest brominated congener deca-BDE (BDE-209) has the highest portion in total production of PBDEs (over 80 %) (De wit 2002) and caused great concern due to its occurrence in humans and the environment (Huang et al. 2010; Tang et al. 2014).
Environmental removal of pollutants in soil is an important issue. Traditional physical and chemical techniques may have the potential to introduce secondary pollution and are generally expensive, for remediation of polluted soils. Phytoremediation has been highlighted as a green bioremediation technology in which plants are used to remove the organic contaminants (OCs) (Huang et al. 2013). Positive effects of plant growth on the dissipation of OCs, such as PCBs, polycyclic aromatic hydrocarbons (PAHs), chlorinated phenolic compounds, and insecticides, have been verified in many previous studies (He et al. 2007; Ding et al. 2009; Romeh and Hendawi 2013). Mechanisms of phytoremediation are proposed to be the facilitated dissipation of OCs due to the positive effect of the number of degrading microbial population, which is induced by rhizosphere effect following plant growth (He et al. 2005, 2007, 2009). Traditional reviews of phytoremediation emphasize that the rhizosphere effects on facilitated dissipation of OCs are influenced by many factors, including root exudates, root architecture, microbial communities, soil water and nutrient conditions, pollutant stress, and aging effect (Ma et al. 2010).
Microbial degradation of halogenated aromatic OCs can occur either aerobically or anaerobically. Anaerobic reductive dehalogenation is a crucial step for the degradation of highly halogenated compounds such as PBDEs and pentachlorophenol (PCP) (He et al. 2006; Robrock et al. 2008; Xu et al. 2014). For facilitating the degradation process of highly halogenated compounds that have a very stable structure, an initial reductive dehalogenation and subsequent oxidative cleavage of aromatic ring and complete mineralization have been considered as an effective way (Campanella et al. 2002). Removal of halogen atoms decreases hydrophobicity of the highly halogenated compounds and makes their aromatic ring more susceptible to be cleaved, thereby facilitating the subsequent aerobic complete mineralization (Rayne et al. 2003). Soil redox status that is regulated by agricultural management of water directly controls the aerobic and anaerobic metabolisms of soil microorganisms. At present, the multistage treatment processes using sequentially anaerobic and aerobic phases to remit soil pollution are an important and latest frontier for remediation researches (Chen et al. 2010).
Reductive dehalogenation of halogenated aromatic OCs is thought to be coupled to biogeochemical processes driven by electron flow from electron donors such as carbon substrates and reduced minerals (e.g., dissolved organic carbon (DOC), NH4 +, and Fe2+) to halogenated aromatic OCs that serve as an electron acceptor (Xu et al. 2014). However, the co-occurrence of various ion species in soils that are polluted with halogenated aromatic OCs, such as NO3 − and Fe3+, can also serve as terminal electron acceptors during anaerobic redox reactions (Adriaens et al. 1996). Thus, reductive dehalogenation of halogenated aromatic OCs depends on the concentrations of carbon sources that can serve as electron donors and competition between halogenated aromatic OCs and alternative terminal electron-accepting processes. Soil redox enzymes (e.g., catalase and dehydrogenase) may also have impact on this process through catalyzing the redox reaction in soils.
Phospholipid fatty acids (PLFAs) are specific components of cell membranes that only exist in living cells (He et al. 2013). Analysis of PLFAs can be used to detect the responses of soil microbial communities during the phytoremediation processes, thereby illustrating the microbial mechanisms underpinning the facilitated dissipation of OCs. Certain PLFAs can be extracted from soils as indicatives of major microbial populations to indicate relative amounts of certain functional groups of organisms. The total PLFAs can estimate soil total microbial biomass (McKinley et al. 2005). Through profiling of soil PLFAs, exact plant-microbe interactions accounting for phytoremediation of polluted soil can be clarified.
The objectives of this study were to develop an effective phytoremediation approach to purify soils polluted by BDE-209 in e-waste recycling area and to illustrate the possible mechanism involved. Variation among plant species (xerophyte vs. hygrophyte), pollution doses (high vs. low pollution level), and time (freshly spiked vs. field-contaminated), along with contrasting water management for regulation of soil redox status (flooded-drained sequentially vs. flooded vs. drained), was simultaneously considered in the pot experiment. It was hypothesized that the exact promoting impact of plants on BDE-209 dissipation depends on variation of plant-microbe interactions in rhizosphere of different plant species and polluted soil habitats. The interest was directed toward accelerating the rhizosphere process of BDE-209 removal, by screening functional plant species with high removal efficiency, coupled with the suitable management practice for soil redox status control during their growth period.
Material and methods
Chemicals, soils, and plants
BDE-209 was Acros standard with the purity of 99 % (Acros Organics, Belgium). Acetone and n-hexane (analytically pure grade) were obtained from Changqing Chemical Industry (Changchun, China) and redistilled before use.
Soils were surface horizon (0–20 cm) of uncultivated soils collected from Taizhou district of China, one of the biggest typical e-waste recycling areas all over the world. Soil sampling was conducted at Mukeng, the most seriously polluted village in Taizhou and Wenling joint area of Taizhou district. The soils contained 8.86 % clay, 29.18 % silt, and 62.14 % sand. Available K, available P, organic carbon, CEC, and pH (water/soil = 1:2.5) were 202 mg kg−1, 15.6 mg L−1, 20.2 g kg−1, 11.0 cmol kg−1, and 6.3, respectively. Soil samples were air-dried and sieved <2 mm before use. The initial concentration of BDE-209 in this soil was 394.6 μg kg−1.
Seven indigenous plant species were selected as the test plants, including five xerophytes of pumpkin (Cucurbita pepo L.), tall fescue (Festuca arundinacea), milk vetch (Astragalus sinicus), alfalfa (Medicago sativa L.), and ryegrass (Lolium perenne L.) and two hygrocolous rice (Oryza sativa L.) cultivars Huanghuazhan (HuangHZ) and Xiushui 134 (XiuS). Especially, HuangHZ was testified as the most tolerant rice cultivars under pollution stress of BDE-209 in our preliminary experiment among four representative rice cultivars commonly planted in the study area (data not shown), while XiuS was the staple cultivars popularized by local government, which was expected to have natural resistance since it has been suffered from pollution stress of PBDEs over a long plantation period around e-waste recycling area.
Experimental design
A rhizobag pot experiment was conducted following a modification of the methods of Xu et al. (2009). Details for rhizobag can be found in the supplementary information (SI). Soils were used to fill the inside (as rhizosphere) and enclose the outside (as nonrhizosphere) of the rhizobag. Each pot received 4 kg of polluted soil, with BDE-209-free soil (65 g) covered on the upper 0.5–1.0 cm as a buffer layer to minimize the loss of BDE-209 due to volatilization and photolysis (Huang et al. 2010).
Two pollution groups of BDE-209 with different pollution dose and time were designed, giving an average soil concentration of 394.6 and 4413.57 μg BDE-209 kg−1 soil (dry weight), respectively. One is the field-contaminated soils with low pollution (a) that were collected directly from the field, and the other is freshly spiked soils with high pollution (f) that were amended with additional BDE-209. Preparation for the freshly spiked soil, bringing the soil to a final BDE-209 concentration close to the highest environmental rates reported in soil (ca. 5000 μg kg−1) (Leung et al. 2007), was basically described by Huang et al. (2010). Details can be found in SI. Soil samples of both pollution groups were amended with mineral nutrients at rates of 300 mg N (urea), 100 mg P (ground phosphate rock), and 200 mg K kg−1 (muriate of potash) as basal fertilizers before seeding with plants.
Seeds of plants were separately sterilized in 10 % H2O2 solution for 15 min, followed by thoroughly rinsing with deionized water, and then soaked in a 3-mmol solution of Ca(NO3)2 for 6 h in the dark. Each clean culture dish that received 20 pre-germinated seeds was put in biochemical incubator to germinate on two-layer filter paper in the dark. Then, the seeds were incubated at 25 °C and 80 % of relative humidity in the dark till germination. Uniformly well seedlings after germinated (two for rice, one for pumpkin, and ten for others, respectively) were selected and transplanted into the root bag within each pot. Pots were arranged in a randomized design and then grown under greenhouse conditions (a photoperiod of 14 h day−1 and a relative humidity of 70 %) for 90 days, with their position rotated every 2 days to ensure uniform conditions. Soil moisture contents for pots of xerophytes were adjusted to 70 % of soil water holding capacity (WHC), to produce xerophytes required for aerobic conditions, by watering to weight as required with deionized water. For pots of rice cultivars, fluctuating flood and drought soil moisture conditions were made, with a 2-cm water layer covering the soil surface, to produce flooded state in the initial 76 days, followed by a dry state through flushing out the redundant water and withholding from watering until the moisture contents decreased below 70 % WHC. Two corresponding sets of pots without plants were included as controls, that is, aerobically dry set (CK-D) and anaerobic-aerobic sequentially wetting-drying cycling set (CK-WD). Another set of flooded pots without plants was also conducted as anaerobic wet control (CK-W). Each treatment was conducted separately for field-contaminated and freshly spiked soil groups, with three replicates. When harvest, the BDE-209-free soil in the upper layer was removed, and the rhizosphere and the bulk soil were then harvested separately from each pot. Soil samples for analysis of enzyme activities were stored at 4 °C, and those for PLFAs and soil BDE-209 residue were freeze-dried before measurement.
Analytical methods
The concentration of BDE-209 in soils was determined by a method based on ultrasonic extraction, subsequent solid phase enrichment followed by GC-μECD analysis, as a modification from Huang et al. (2010). In brief, each 2-g soil sample weighted accurately was extracted by ultrasonic agitation for 30 min at 30 °C, with a mixture of acetone and hexane (1:1, v/v) added with a certain amount of anhydrous sodium sulfate to remove moisture and 1-g activated copper granules to remove elemental sulfur and pigment. After centrifugation at 2500 rpm for 15 min, the supernatant was decanted and collected, and the soil residue was then extracted twice more followed by centrifugation. The supernatant extracts were combined and concentrated to approximately 1 ml in a rotary evaporator (Büchi vacuum V-500) equipped with Büchi vacuum controller V-800 (Büchi Labortechnik). Concentrated extracts were then flushed with isooctane three times and transferred thoroughly through a Florisil column (6 ml, 1 g) which had been added a certain amount of anhydrous sodium sulfate and activated by isooctane. The volume was then eluted with 5 ml isooctane three times, and glass tubes were then dried under a stream of N2. The final volume was made by 1 ml hexane and eddied for 2 min prior to analysis. The GC analysis was done using an Agilent 6890II GC system equipped with DB-5HT capillary column (15 m × 0.25 mm × 0.10 μm) and microelectron capture detector (μECD) (Agilent, USA). The temperature program was as follows: oven temperature was maintained at 110 °C for 2 min, increased at 15 to 310 °C/min, and held for 15 min. Injector and detector temperatures were 300 and 320 °C, respectively. A 2-μl amount of the extracted sample was injected to the GC column. Procedural blanks were determined by going through the same extraction and cleanup procedures for each series of samples. The limit of detection of the method, a signal of three times the noise level, was 25 ng g−1. The average recovery of BDE-209 reached 93.4 ± 1.68 %.
PLFAs were extracted with Bligh-Dyer extracting solution from freeze-dried soil samples of adequately blended mixture of three duplicates of each treatment and then identified with a MIDI Sherlock® microbial identification system (Version 4.5, MIDI, USA), according to the method of He et al. (2009). The analytic procedures were described in detail in SI. Note that it is usually not a good strategy to mix the three replicates of each treatment for PLFA analysis. The reason for use of the mixture of three duplicates for extract PLFAs in this study was that a lot of soil samples were consumed for the analyses of the BDE-209 residues and soil redox parameters, and the rest was not enough for conducting a separate extract of PLFAs for each replicate samples. Thus, all the PLFA analyses were conducted two times, and the PLFA data presented were the average of two analyses.
Soil DOC was extracted with Milli-Q water, and the supernatant was measured for DOC concentration using an automated total organic carbon analyzer (Multi N/C 3100, Analytik Jena AG, Jena, Germany). Other soil redox relevant biochemical indexes, including the activities of catalase (Stepniewska et al. 2009) and dehydrogenase (Liu et al. 2011), as well as the content of NH4 +/NO3 + (Zhou et al. 2014) and Fe2+/Fe3+ (Hayat et al. 2011), were also analyzed according to the previous work. Details for analytical methods can be found in SI.
Statistical analysis
Statistical analysis was conducted with the SPSS 17.0 software package CORRELATE and FACTOR procedures. Treatment effects as a function of plant species and water management, as well as rhizosphere and non-rhizosphere, were tested by analysis of variance (ANOVA), performed on mean values using least significant difference test at p < 0.05 significant and 0.01 highly significant levels. The PLFA data were subject to principal component analysis (PCA) to examine patterns of microbial group combinations among different plant species (plus rhizosphere and non-rhizosphere) and water management treatments. The potential dependence of BDE-209 removal rate and soil redox biochemical indexes on the PLFA-based microbial community structure was further identified using correlation tests of significant principal component scores of PCA. Redundancy analysis (RDA) was also applied to visualize the relationships among the environmental variables (soil redox biochemical indexes), the response variable values (PLFAs), and the samples of different treatments (McKinley et al. 2005), with results graphed using R version 3.0.1 and OriginPro 8.6.
Results
Differences in BDE-209 concentration among different treatments
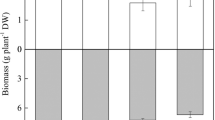
The concentrations of BDE-209 in plant roots after harvest were under the limit of detection of the analytic method (25 ng g−1) and not detected in planted treatments of both field-contaminated and freshly spiked. The BDE-209 residual concentration in soil after 90-day cultivation is illustrated in Fig. 1. Details for BDE-209 removal amounts and dissipation ratio (as percentage removal of the initial amounts) in different treatments are given in Table 1. The residual BDE-209 in soils declined naturally even without plant growth, but the extent of depletion varied among different water managements. The general trend was CK-WD > CK-W > CK-D (Fig. 1). In field-contaminated group, the BDE-209 percent removal was 26.2, 22.6, and 18.3 % for CK-WD, CK-W, and CK-D, respectively, higher than that of the corresponding treatments in freshly spiked group (13.5, 8.90, and 2.06 %, respectively).
The concentration of BDE-209 in soils of various sampling treatments as a function of plant species and water management. a Field-contaminated soils. b Freshly-spiked soils. Data of treatments with plants are shown as the average values of rhizosphere and non-rhizosphere. Bars are the standard error of means of three replicates. Different letters indicate significant differences among treatments at the p < 0.05 level
Compared with the plant-free controls, growth of XiuS (52.9 %), HuangHZ (41.9 %), ryegrass (38.7 %), and pumpkin (33.8 %) significantly enhanced the removal of BDE-209 in field-contaminated soils, while alfalfa (28.3 %), ryegrass (28.1 %), HuangHZ (27.6 %), and XiuS (21.7 %) were significantly more effective in freshly spiked soils (p < 0.05) (Fig. 1, with the concentration of BDE-209 in planted treatments calculated as the average values of rhizosphere and non-rhizosphere). Irrespective of plant species, the percent removal of BDE-209 was higher in field-contaminated (range 13.2–52.9 %) than in freshly spiked (range 9.49–28.3 %) soil groups.
The dissipation of BDE-209 in non-rhizosphere was almost consistent within plant species under both polluted groups (Fig. 2). In general, the non-rhizosphere removed an average of 12.5 and 3.24 % of the initial BDE-209 in 90 days from field-contaminated and freshly spiked soils respectively, much smaller than the rhizosphere removed (an average of 33.4 and 16.9 %, respectively). Differences between rhizosphere and non-rhizosphere were significant for both polluted groups (p < 0.05), only except milk vetch in field-contaminated soils and pumpkin in freshly spiked soils with reasons unclear.
Changes of soil microbial community structure and other soil biochemical indexes
Changes of soil microbial community structures were investigated based on PLFA analysis. Twenty-two PLFAs with chain lengths from C12 to C20 were identified. Thirteen of them have been identified as biomarkers of specific microbial groups in soils (Table S1) (He et al. 2013). Relative to those in non-rhizosphere, the total PLFAs in rhizosphere increased in both pollution groups after plant growth, irrespective of plant species (Table 2). And, there were large increases in percentages observed in field-contaminated rhizosphere under low pollution stress (+3.3 to +23.5 %, an average of +17.4 %), as compared to those in freshly spiked rhizosphere under high pollution stress (+1.6 to +15.8 %, an average of +8.0 %). Additionally, the total PLFAs in control soils without plant were also affected by different water management modes, enhanced in water flooding treatments (e.g., CK-W/CK-WD vs. CK-D). The PCA analysis of selected rhizosphere biomarker PLFAs demonstrated the treatment effect in microbial composition (Fig. 3). The first and second principal components (PC1 and PC2, accounted for 53.4 % of the total variation) separated the treatments of contrasting plant species, pollution doses and time, and water managements. All water flooding treatments gathered to the negative axis of PC1 (including CK-W, CK-WD, XiuS and HuangHZ), with the others to the right (including CK-D and treatments planted with five xerophytes) (Fig. 3), indicating that most of the variation in PC1 was induced by water-dependent soil redox status. There was selective enrichment of i17:0, 10Me18:0, 10Me17:0, and cy19:0ω8c, with their loading values greater than 0.8 on PC1 and the highest weight achieved by i17:0 (0.928). The 15:0, 16:1ω5c, i15:0, and 14:0 were also enriched on PC2 with loading values greater than 0.6. Among them, the highest weight was achieved by 15:0 (0.798), followed by 16:1ω5c (0.765) (Fig. 3).
Effect of cultivation of different plants coupling with water management on the first two principal components of phospholipid fatty acids (PLFAs) data set. a Results present principal component (PC) scores for PC1 and PC2. Plot of samples where “field” and “freshly” indicate field-contaminated and freshly spiked treatments, respectively. b Results present the component matrix loadings of the individual PLFA for PC1 and PC2
The activity of catalase and the content of NO3 − and Fe3+ increased generally toward most of the xerophyte treatments, while the activity of dehydrogenase and the content of DOC and NH4 + increased toward the hygrophyte treatments (Table 3). The reduced state NH4 + and Fe2+ showed significant negative correlations with the oxidized state NO3 − and Fe3+, respectively (Table 4). This was expected, as catalase is an indicator enzyme for aerobic organisms (prevalent in rhizosphere of xerophyte), while dehydrogenase activity generally increases in anaerobic conditions. The reason was same for NH4 +/NO3 − and Fe2+/Fe3+. Additionally, the activity of catalase and the content of NO3 − were positively dependent on the scores of PC1 (p < 0.01), while the activity of dehydrogenase and the content of NH4 +, DOC, and Fe2+ were negatively dependent on the scores of PC1 (p < 0.01), respectively. The significantly negative relationship upon the scores of PC2 only occurred with the activity of catalase (p < 0.05) (Table 4).
Relationship between BDE-209 removal ratios and soil biochemical indexes
The specific relevance of BDE-209 depletion among different treatments was identified by correlating BDE-209 removal ratios to PC scores of PCA and soil biochemical indexes, respectively (Table 4). The BDE-209 percent removal related positively and significantly (r = 0.535, p < 0.05) to PC2 scores. It was also correlated highly significantly with the fatty acids 15:0 and 18:1ω7c (p < 0.01) and significantly with the fatty acids 12:0, i14:0, i15:0, a15:0, and 16:1ω5c (p < 0.05) (Table S1). It also had a positive correlation with the activity of dehydrogenase (p < 0.01) and the concentrations of NH4 + and DOC (p < 0.05) and a negative correlation with the activity of catalase and the concentration of NO3 − (Table 4).
The RDA ordination plot shows the soil samples of different treatments and the environmental gradient arrows for the PLFA data (Fig. 4). The NO3 − and catalase have the reversed orientations with NH4 + and dehydrogenase, respectively, indicating significantly negative correlations between NO3 − and NH4 and between dehydrogenase and catalase, respectively. This was well consistent with the result of Pearson correlation in Table 4. Regardless of field-contaminated or freshly spiked, both the sequentially anaerobic-aerobic and anaerobic treatments were plotted to the left, and most of the other aerobic treatments were to the right, coincident with the results of PCA (Fig. 3). Furthermore, the plot of RDA also suggested that BDE-209 percent removal was positively correlated with the activity of dehydrogenase and the content of electron donors DOC, Fe2+, and NH4 +, while negatively correlated with the activity of catalase and the content of electron acceptors NO3 − and Fe3+.
Discussion
Dissipation of BDE-209 in soils
Environmental dissipation of PBDEs in soils can be mainly summarized to the contributions of plant uptake (only for planted treatments), volatilization, chemical sequestration (e.g., sorption and formation of non-extractable bound residue), and microbial degradation which involves hydroxylation, reductive debromination, and photodegradation (Huang et al. 2011). In this experiment, each pot had a buffer layer to minimize photolysis and volatilization of BDE-209; thus, the dissipation content via photodegradation might be ignored as evidenced by Huang et al (2010). Concentrations of BDE-209 in plant roots were also not detected. As a consequence, BDE-209 dissipated concentrations in all pots could mainly result from microbial degradation and chemical sequestration. Since all the treatments used the same soil with identical physicochemical properties, chemical sequestration was speculated to occur at a same rate. Therefore, any difference in BDE-209 removal among different treatments could be mainly regulated by microbial degradation.
Differences of BDE-209 dissipation between rhizosphere and non-rhizosphere, and among different plant species, suggested that plant growth enhanced the depletion of BDE-209. However, the interaction between root growth and dissipation of BDE-209 varied among plant species, pollution doses, and time. Among the species tested, the staple rice cultivar XiuS had the highest percent removal in field-contaminated low pollution soils; the tolerant rice cultivar HuangHZ and xerophyte ryegrass were consistently qualified in both field-contaminated and freshly spiked soils, with their ability in dissipating BDE-209 more effective under high pollution stress, while the ability of alfalfa exhibited contradictory, dependent on soil pollution conditions (Fig. 1).
Enhanced dissipation of OCs resulting from plant rhizosphere effect has been demonstrated by several previous studies (He et al. 2006; Mueller et al. 2006; Huang et al. 2010). In this study, the percent removal of BDE-209 was higher in field-contaminated than in freshly spiked soils, regardless of plant growth (Fig. 1 and Table 1). This might be due to the following three possible reasons: (i) indigenous microbes have already adapted for BDE-209 in field-contaminated soils, while they might be inhibitory in freshly spiked soils, (ii) the greater inhibition for microbial growth caused by high pollution stress, as suggested by the smaller total PLFAs of control groups in freshly spiked than in field-contaminated soils (Table 2), and (iii) the rapid degradation rate by soil microorganisms and assimilation proportion by plants under low pollution stress. Note that higher absolute removal of BDE-209 was found in soils with additional BDE-209 load (e.g., with an average of 118.5 and 715.5 μg BDE-209 kg−1 for field-contaminated and freshly spiked treatments, respectively) (Table 1). This might be due to a larger absolute retention amount caused by chemical sequestration in soils with additional BDE-209 load.
Besides the influence of plant growth, soil redox status was evidenced as an important factor affected the microbial communities (Fig. 3) and thereby the BDE-209 removal (Table 1). A higher removal ratio of BDE-209 was achieved by CK-WD as compared with CK-D (with difference as p < 0.05) and CK-W. The same tendency occurred with the two hygrophytes (XiuS and HuangHZ) cultivated via sequential wet-dry alternation, compared to the other five xerophytes cultivated under 70 % WHC aerobically. These consequences suggested that a sequentially anaerobic-aerobic environment, rather than mono-aerobic or mono-anaerobic environment, could be more competent for accelerating BDE-209 removal from soils. Since it was unable to simultaneously identify the intermediates during BDE-209 dissipation due to the limited quantity of rhizosphere soils that were sampled from rhizobag, it was speculated that the possible reason might lie on the dissipation pathway of BDE-209 under sequentially anaerobic-aerobic condition, through anaerobically reductive debromination to lesser brominated congeners firstly, followed by benzene ring cleavage aerobically. This speculation needs to be verified in further experiment by detecting lower brominated PBDEs as the debrominated products of BDE-209.
Biochemical mechanisms underpinning the facilitated BDE-209 dissipation
Release of OCs into soil must incur responses from soil microbes to this environmental stress. The increases of total PLFAs in rhizosphere soils were accompanied by the enhanced dissipation of BDE-209, compared with the plant-free controls and bulk soils (Table 2 and Figs. 1 and 2). This demonstrated that suppressed soil indigenous microorganisms could be stimulated by root exudates following plant growth, thereby supporting for the facilitated removal of BDE-209.
The PLFA 16:1ω5c, as a biomarker for arbuscular mycorrhizal fungi (AMF) (He et al. 2013), was selectively enriched with a high weight on PC2 that exhibited a highly significant correlation with BDE-209 dissipation (Fig. 3). A positive correlation was also found between them (r = 0.502, p < 0.05) (Table S1). Therefore, it was inferred that AMF was crucial in degrading BDE-209. Many previous studies have verified the important roles of AMF played on dissipation of PAHs, PCBs, PCP, and other numerous xenobiotics in soils (Campanella et al. 2002; He et al. 2007, 2009; Ding et al. 2009; Qin et al. 2014; Wu et al. 2014). Furthermore, PLFAs i15:0, i14:0, and a15:0, which are commonly used as biomarkers for gram-positive bacteria (G+) (He et al. 2013), had relatively positive higher weight on PC2 (Fig. 3). They are also significantly correlated with BDE-209 percent removal (r > 0.505, p < 0.05) (Table S1). This might indicate that G+ may be selectively enriched in soils once suffered from PBDE pollution, so as to be naturalized for degrading BDE-209 efficiently. The degradation of PCBs by G+ has already been well testified in previous studies, and the reason was suggested as that G+ could exhibit an amount of catabolic activities for facilitating dissipation of OCs (Asturias and Timmis 1993). Since very few researches have addressed PBDE degradation by G+, the above speculation still needs to be verified in further experiments. Another interesting finding was that among the biomarkers of gram-negative bacteria (G−) (He et al. 2013), only 18:1ω7c was significantly positively correlated with BDE-209 removal ratio (r = 0.656, p < 0.01) (Table S1). However, Qin et al. (2014) showed that G− was significantly correlated with PCB removal. Therefore, the function of G− in the BDE-209 dissipation of halogenated aromatic OCs (in this case, BDE-209) cannot be confirmed in the study and also needs more investigation.
The anaerobic transformation of halogenated OCs in anaerobic environments is largely regulated by coordination and competition of the available electron donors and acceptors (Zhang et al. 2010; Payne et al. 2011; Xu et al. 2014). The bioavailable DOC, NH4 +, and Fe2+ usually act as electron donors, while their oxidized ionic species, such as NO3 − and Fe3+, usually act as competitive electron acceptors during microbial dehalogenated respiration (Kotik et al. 2013). Our results indicated that both NO3 − and Fe3+ inhibited BDE-209 dissipation in either rhizosphere or non-rhizosphere, of which NO3 − affected more significantly than Fe3+ (Table 4 and Fig. 4). Actually, reductive debromination has been described for a variety of PBDEs under anaerobic conditions in the past two decades (Gerecke et al. 2005; Robrock et al. 2008). Although there is limited direct evidence that reported the influence of environmental electron acceptors/donors on reductive debromination of PBDEs as they are new type pollutants, D’Angelo and Reddy (2000)) found that PCP transformation was inhibited by NO3 − and Fe3+. The reason was ascribed to the competitive consumption of available electron donors by NO3 − and Fe3+ that should be used in reductive dechlorination for PCP. In addition, striking similarity between PBDEs and other halogenated compounds (e.g., PCBs) about dehalogenation studies was found in previous studies (Gerecke et al. 2005; He et al. 2006; Robrock et al. 2008). Therefore, diversion of electrons from reductive debromination of BDE-209 to the successive reduction of NO3 − and Fe3+ might be the mechanism involved in this study. On another note, it can also explain why DOC, NH4 +, and Fe2+ showed the abilities to enhance dissipation of BDE-209, with the influence of DOC and NH4 + reached significant levels (p < 0.05) (Fig. 4 and Table 4), because they may act as electron donors to increase the transport of electrons to BDE-209 and thus to facilitate its reductive debromination. The above results illustrated that the availability of electron acceptors and donors was significantly related to the ratios of BDE-209 dissipation in the aerobic-anaerobic interfaces.
Catalase was usually found in aerobic bacteria and most facultative anaerobes and absent in obligate anaerobes (Shiyin et al. 2004). It was correlated negatively with BDE-209 removal ratio and PC2 (p < 0.05) and positively with PC1 (p < 0.01) (Table 4), consistent with the results reflected by RDA (Fig. 4). The lowest weight on PC2 and the highest weight on PC1 were almost simultaneously enriched by the PLFAs 10Me17:0 and 10Me18:0 (Fig. 3), which occur almost exclusively in actinomycetes (He et al. 2013). This indicated a potential relationship between actinomycetes and catalase. Positively correlation between actinomycetes and catalase was also found in several studies (Dong et al. 2012; Fu et al. 2012). It was thus speculated that actinomycetes might inhibit the dissipation of BDE-209 through catalase production under aerobic condition. Dehydrogenase is an enzyme capable of illustrating soil’s microbial activity (García-Orenes et al. 2010). Both RDA and simple Pearson correlation analysis suggested that the increase in activity of dehydrogenase was coincident with the increased dissipation of BDE-209 (Table 4 and Fig. 4). It was also negatively correlated with PC1 in a significant level (p < 0.05). Therefore, the activity of dehydrogenase might be stimulated under anaerobic condition which had been confirmed before (Wolińska and Stępniewska 2012), thereby facilitating the reductive debromination process of BDE-209.
Conclusion
The present study demonstrated that BDE-209 dissipation in soils was simultaneously influenced by plant species, pollution doses and time, and water management practices. The sequential wetting-drying management created an anaerobic-aerobic alternate habitat in plant rhizosphere and modified the interaction between root and functional microbial groups. This induced the most facilitated removal of BDE-209 from soils, possibly through initial microbial reductive debromination to lesser brominated congeners followed by aerobic oxidative cleavage of benzene ring. By screening the most functional plant species for BDE-209 removal and regulating soil redox status during plant growth, this study recommends a cost-effective pattern for purifying soils polluted by BDE-209 around e-waste recycling area, through successive cultivation of rice followed by ryegrass coupling with suitable water management. Especially, the rice cultivar XiuS is more applicable for low pollution soils while HuangHZ for high pollution soils, respectively.
The results from this pot experiment may not consist with field environment, and further verification in field was necessary, with more effort directly toward identification of lower brominated congeners or metabolites in the soil-plant system transformed from BDE-209, such as penta- and tetra-BDEs, since they may be more toxic and bioavailable than BDE-209 (Gandhi et al. 2011). Meanwhile, given that rice is a widely cultivated food crops, it is necessary to further concern the human exposure risk during field verification of recommending rice cultivars XiuS and HuangHZ as a potential plant for removing BDE-209.
References
Adriaens P, Chang P, Barkovskii A (1996) Dechlorination of PCDD/F by organic and inorganic electron transfer molecules in reduced environments. Chemosphere 32:433–441
Asturias JA, Timmis KN (1993) Three different 2, 3-dihydroxybiphenyl-1, 2-dioxygenase genes in the gram-positive polychlorobiphenyl-degrading bacterium Rhodococcus globerulus P6. J Bacteriol 175:4631–4640
Campanella BF, Bock C, Schröder P (2002) Phytoremediation to increase the degradation of PCBs and PCDD/Fs. Environ Sci Pollut Res 9:73–85
Chen Y, Lin CJ, Lan H, Fu S, Zhan H (2010) Changes in pentachlorophenol (PCP) metabolism and physicochemical characteristics by granules responding to different oxygen availability. Environ Progr Sustain Energy 29:307–312
D'Angelo EM, Reddy KR (2000) Aerobic and anaerobic transformations of pentachlorophenol in wetland soils. Soil Sci Soc Am J 64:933–943
De Wit CA (2002) An overview of brominated flame retardants in the environment. Chemosphere 46:583–624
Ding N, Guo HC, Hayat T, Wu YP, Xu JM (2009) Microbial community structure changes during Aroclor 1242 degradation in the rhizosphere of ryegrass (Lolium multiflorum L.). FEMS Microbiol Ecol 70:305–314
Dong LL, Hao ZP, Zuo YM, Li XL, Wang Q, Christie P (2012) Effect of garlic bulb aqueous extract on cucumber seedlings, soil microbial counts, and enzyme activities. Soil Sci Plant Anal 43:2888–2896
Fu QL, Liu C, Ding NF, Lin YC, Guo B, Luo JF, Wang HL (2012) Soil microbial communities and enzyme activities in a reclaimed coastal soil chromosequence under rice-barley cropping. J Soils Sediment 13:1134–1144
Gandhi N, Bhavsar SP, Gewurtz SB, Tomy GT (2011) Can biotransformation of BDE-209 in lake trout cause bioaccumulation of more toxic, lower-brominated PBDEs (BDE-47,-99) over the long term? Environ Int 37(1):170–177
García-Orenes F, Guerrero C, Roldán A, Mataix-Solera J, Cerdà A, Campoy M, Bárcenas G, Caravaca F (2010) Soil microbial biomass and activity under different agricultural management systems in a semiarid Mediterranean agroecosystem. Soil Tillage Res 109(2):110–115
Gerecke AC, Hartmann PC, Heeb NV, Kohler HP, Giger W, Schmid P, Zennegg M, Kohler M (2005) Anaerobic degradation of decabromodiphenyl ether. Environ Sci Technol 39:1078–1083
Hassanin A, Breivik K, Meijer SN, Steinnes E, Thomas GO, Jones KC (2004) PBDEs in European background soils: levels and factors controlling their distribution. Environ Sci Technol 38:738–745
Hayat T, Ding N, Ma B, He Y, Shi JC, Xu JM (2011) Dissipation of pentachlorophenol in the aerobic-anaerobic interfaces established by the rhizosphere of rice (Oryza sativa L.) root. J Environ Qual 40:1722–1729
He Y, Xu JM, Tang CX, Wu YP (2005) Facilitation of pentachlorophenol degradation in the rhizosphere of ryegrass (Lolium perenne L.). Soil Biol Biochem 37:2017–2024
He J, Robrock KR, Alvarez-Cohen L (2006) Microbial reductive debromination of polybrominated diphenyl ethers (PBDEs). Environ Sci Technol 40:4429–4434
He Y, Xu JM, Ma ZH, Wang HZ, Wu YP (2007) Profiling of PLFA: implications for nonlinear spatial gradient of PCP degradation in the vicinity of Lolium perenne L. roots. Soil Biol Biochem 39:1121–1129
He Y, Xu JM, Lv XF, Ma ZH, Wu JJ, Shi JC (2009) Does the depletion of pentachlorophenol in root–soil interface follow a simple linear dependence on the distance to root surfaces? Soil Biol Biochem 41(9):1807–1813
He Y, Ding N, Shi JC, Wu M, Liao H, Xu JM (2013) Profiling of microbial PLFAs: implications for interspecific interactions due to intercropping which increase phosphorus uptake in phosphorus limited acidic soils. Soil Biol Biochem 57:625–634
Huang HL, Zhang SZ, Christie P, Wang S, Xie M (2010) Behavior of decabromodiphenyl ether (BDE-209) in the soil-plant system: uptake, translocation and metabolism in plants and dissipation in soil. Environ Sci Technol 44:663–667
Huang HL, Zhang SZ, Christie P (2011) Plant uptake and dissipation of PBDEs in the soils of electronic waste recycling sites. Environ Pollut 159:238–243
Huang LF, Zhuo JF, Guo WD, Spencer RG, Zhang ZY, Xu J (2013) Tracing organic matter removal in polluted coastal waters via floating bed phytoremediation. Mar Pollut Bull 71:74–82
Kotik M, Davidová A, Voříšková J, Baldrian P (2013) Bacterial communities in tetrachloroethene-polluted groundwaters: a case study. Sci Total Environ 454–455:517–527
Leung AOW, Luksemburg WJ, Wong AS (2007) Spatial distribution of polybrominated diphenyl ethers and polychlorinated dibenzo-p-dioxins and dibenzofurans in soil and combusted residue at Guiyu, an electronic waste recycling site in southeast China. Environ Sci Technol 41:2730–2737
Liu X, Wang Z, Zhang X, Wang J, Xu G, Cao Z, Zhong C, Su P (2011) Degradation of diesel-originated pollutants in wetlands by Scirpus triqueter and microorganisms. Ecotoxicol Environ Saf 74:1967–1972
Ma B, He Y, Xu JM, Rengel Z (2010) Dissipation of polycyclic aromatic hydrocarbons (PAHs) in the rhizosphere: synthesis through meta-analysis. Environ Pollut 158:855–861
McKinley VL, Peacock AD, White DC (2005) Microbial community PLFA and PHB responses to ecosystem restoration in tallgrass prairie soils. Soil Biol Biochem 37:1946–1958
Mueller KE, Mueller-Spitz SR, Henry HF, Vonderheide AP, Soman RS, Kinkle BK, Shann JR (2006) Fate of pentabrominated diphenyl ethers in soil: abiotic sorption, plant uptake, and the impact of interspecific plant interactions. Environ Sci Technol 40:6662–6667
Payne RB, May HD, Sowers KR (2011) Enhanced reductive dechlorination of polychlorinated biphenyl impacted sediment by bioaugmentation with a dehalorespiring bacterium. Environ Sci Technol 45:8772–8779
Qin H, Brookes PC, Xu J (2014) Cucurbita spp. and Cucumis sativus enhance the dissipation of polychlorinated biphenyl congeners by stimulating soil microbial community development. Environ Pollut 184:306–312
Rayne S, Ikonomou MG, Whale MD (2003) Anaerobic microbial and photochemical degradation of 4, 4′-dibromodiphenyl ether. Water Res 37:551–560
Robrock KR, Korytár P, Alvarez-Cohen L (2008) Pathways for the anaerobic microbial debromination of polybrominated diphenyl ethers. Environ Sci Technol 42:2845–2852
Romeh AA, Hendawi MY (2013) Chlorpyrifos insecticide uptake by plantain from polluted water and soil. Environ Chem Lett 11:163–170
Shiyin L, Lixiao N, Panying P, Cheng S, Liansheng W (2004) Effects of pesticides and their hydrolysates on catalase activity in soil. Bull Environ Contam Toxicol 73:600–606
Stepniewska Z, Wolińska A, Ziomek J (2009) Response of soil catalase activity to chromium contamination. J Environ Sci 21:1142–1147
Tang ZW, Huang QF, Cheng JL, Yang YF, Yang J, Guo W, Nie ZQ, Zeng N, Jin L (2014) Polybrominated diphenyl ethers in soils, sediments, and human hair in a plastic waste recycling area: a neglected heavily polluted area. Environ Sci Technol 48(3):1508–1516
Wolińska A, Stępniewska Z (2012) Dehydrogenase activity in the soil environment. Biochem Genet Mol Biol 14:183–210
Wu F, Yu X, Wu S, Wong M (2014) Effects of Inoculation of PAH-degrading bacteria and arbuscular mycorrhizal fungi on responses of ryegrass to phenanthrene and pyrene. Int J Phytoremediat 16:109–122
Xu DF, Xu JM, He Y, Huang PM (2009) Effect of iron plaque formation on phosphorus accumulation and availability in the rhizosphere of wetland plants. Water Air Soil Pollut 200:79–87
Xu Y, He Y, Feng XL, Liang LY, Xu JM, Brookes PC, Wu JJ (2014) Enhanced abiotic and biotic contributions to dechlorination of pentachlorophenol during Fe(III) reduction by an iron-reducing bacterium Clostridium beijerinckii Z. Sci Total Environ 473:215–223
Zhang H, Ziv-El M, Rittmann BE, Krajmalnik-Brown R (2010) Effect of dechlorination and sulfate reduction on the microbial community structure in denitrifying membrane-biofilm reactors. Environ Sci Technol 44:5159–5164
Zhou J, Xia F, Liu XM, He Y, Xu JM, Brookes PC (2014) Effects of nitrogen fertilizer on the acidification of two typical acid soils in South China. J Soils Sediments 14:415–422
Acknowledgments
This research was financially supported by the National High Technology Research and Development Program of China (863 Program, No. 2012AA06A203), the National Natural Science Foundation of China (41322006, 41090284), the Fundamental Research Funds for the Central Universities, and Zhejiang University K.P. Chao’s High Technology Development Foundation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Leif Kronberg
Electronic supplementary material
Details for the design of rhizobag, preparation of the freshly BDE-209 spiked soil, and analytical methods of soil PLFAs, DOC, and other biochemical indexes; Table S1 (The 22 identified phospholipid fatty acids (PLFAs) and their attributive microbial species as well as their correlations with BDE-209 percent removal). (DOC 164 kb)
ESM 1
(DOC 164 kb)
Rights and permissions
About this article
Cite this article
He, Y., Li, X., Shen, X. et al. Plant-assisted rhizoremediation of decabromodiphenyl ether for e-waste recycling area soil of Taizhou, China. Environ Sci Pollut Res 22, 9976–9988 (2015). https://doi.org/10.1007/s11356-015-4179-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4179-2