Abstract
This study (i) investigated the concentration levels of nine phthalates and bisphenol A (BPA) in sludge samples originating from a French wastewater treatment plant (WWTP), (ii) studied the distribution of target compounds according to soil depth and calculated their half-lives, and (iii) compared the contamination level of the agricultural soil with those of soils with other land uses. The sludge contamination levels varied from a few hundred nanograms per gram dry weight (dw) for diethyl phthalate (DEP), di-iso-butyl phthalate (DiBP), di-n-butyl phthalate (DnBP), and butyl-benzyl phthalate (BBP) to a few micrograms per gram dw for diethylhexyl phthalate (DEHP), di-iso-nonyl phthalate (DiNP), and di-iso-decyl phthalate (DiDP). After sludge application, an 8-fold increase for DEHP level and a 3-fold increase for BPA level occurred in the surface horizon of the soil. The mean distribution of phthalates according to the depth showed a positive gradient for the low molecular weight compounds and inversely, a negative gradient for the highest ones. The half-lives in the 0–20-cm soil horizon were 64 days for DEHP and 36 days for BPA. A predictive environmental concentration (PEC) of 0.3 μg g−1 dw was estimated for DEHP, while the experimental value was 0.16 μg g−1 dw, suggesting degradation processes in soil and/or formation of non-extractable residues. Comparisons of contamination levels for soils from different origins (urban, rural, agricultural, and forest) showed that the urban soil remained the most contaminated one, prior to the agricultural soil after treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The continuous contamination of the environment arising from man-induced activities leads to a chronic exposure of the biota to a number of xenobiotic molecules. Among them, phthalates and bisphenol A (BPA), commonly used as plasticizers, are believed to exert endocrine-disrupting effects.
Indeed, phthalates have numerous applications in everyday life, such as automotive manufacturing, coatings for floors and walls, medical equipment, medicines, food packaging, and cosmetics (German Federal Environment Agency 2011). The diethylhexyl phthalate (DEHP) may reach up to 50 % in polyvynil chloride (PVC) composition, and as it is not chemically bound to the polymer, it can be easily released in the environment during PVC production as well as end-product use, storage, or degradation (Heudorf et al. 2007). BPA is an essential ingredient for polycarbonate production, and contrary to phthalates, it is chemically bound to the polymer. However, that polymer displays limited resistance: hydrolysis occurs at high temperatures (over 500 °C) or under basic conditions (pH over 8) (Biedermann-Brem and Grob 2009).
Phthalates and BPA are known to compete with endogenous hormones through binding to their specific receptors or damaging their synthesis and metabolism (Craig et al. 2011). Exposure to these compounds was associated with altered hormone levels, reproductive adverse effects (particularly male fertility), precocious puberty, increased incidence of chronic disease, and possible role in cancer development (Meeker 2010).
The transfer and the fate of phthalates and of BPA are determined by their physicochemical characteristics. Indeed, volatility and solubility in water decrease significantly with increasing length of the phthalate alkyl chains. The low water solubility and the high K ow values for high molecular weight phthalates might lead to their strong adsorption upon organic matter and particles (Cousins et al. 2003; Fernández et al. 2012). These compounds are frequently detected at high levels in wastewaters from municipal and industrial sources as well as in final sludge from wastewater treatment plant (WWTP) in many countries such as Austria, Canada, and Spain (Fürhacker et al. 2000; Lee et al. 2004; Höhne and Püttmann 2008; Sanchez-Avila et al. 2009).
The land application of treated sewage sludge is the option favored internationally for sludge management as it contributes positively to the recycling of nutrients. The current regulation for agricultural application of sewage sludge in France (Decree 97-1133) authorizes a maximum of 30 t of dry matter per hectare every 10 years. A limit value for the DEHP concentration of 100 mg kg−1 in sludge for use on land was proposed by a working committee of the European Union (Working document on sludge, third draft, 27 April 2000).
In France, a predictive environmental concentration (PEC) in agricultural soils after sewage sludge application, of 2.6 mg kg−1 dry weight for DEHP, was estimated from the EUSES model (EUR 23384 EN/2 2008), but no PEC data are available for BPA. However, the BPA behavior after addition of biosolids to soil under laboratory conditions or land application was investigated in Australia, showing that the use of laboratory experiments to predict its field persistence may greatly overestimate the degradation rate and inaccurately predict its dissipation (Langdon et al. 2011a, 2012). Thus, the fates of phthalates and BPA in soil after sludge application need to be monitored under field environmental conditions because of their potential migration toward surface water and groundwater or uptake by plants.
Our purpose was first to determine contamination levels for phthalates and BPA, in sewage sludge from a WWTP receiving wastewaters from domestic and hospital origins. Next, we investigated the fate of phthalates and BPA with time and soil depth, after the sewage sludge application upon an agricultural soil. Last, we compared the contamination of the agricultural amended soil with those of soils from different origins: urban, rural, and forestry.
Material and methods
Sampling location
The agricultural plot studied (X 48° 56′ 58.57″, Y 2° 9′ 00.4″) was located in a rural area close to Fontenay-les-Briis (Essonne, France) (Appendix 1). The WWTP of Fontenay-les-Briis treated wastewaters of domestic (60 %) and hospital (40 %) origins. The plant equipped with a combined tank (decantation and activated sludge) treated 157,000 m3 of wastewaters per year, corresponding to 5000 equivalent inhabitants, by biological processes and produced 32 t of sludge in 2010. The treatment capacity was 350 kg day−1 for the suspended matter, 300 kg day−1 for the DBO5, 75 kg day−1 for the reduced nitrogen (NK), corresponding to wastewater flow of 1000 m3 day−1. The hydraulic retention time (HRT) was of 0.835 day−1. The wastewater flow entering the WWTP ranged from 270 to 532 m3 day−1 for the 2010 to 2011 period and never exceeded the maximal treatment capabilities. The mean annual decrease between inputs and output for biological oxygen demand (BOD5), chemical oxygen demand (COD), NK, total nitrogen, and phosphorus were of 84, 95, 88, 83, and 65 %, respectively.
Sludge was formed by settling processes of suspended particles, the removal efficiency for the suspended matter ranging from 82 to 96 %. Sludge was then stored in anaerobic conditions, into a silo of 83 m3, then dried on sand beds (6 × 100 m2) and stored under cover for up to a year before spreading on agricultural plots near the WWTP. Sludge samplings were carried out from the WWTP storage area and kept at −18 °C.
Soil samples were collected from the agricultural plot before spreading to determine the background contamination level. Then, four successive sampling campaigns were conducted in 2010–2011 after a single sludge application, according to the sampling timing presented in Appendix 2. The last campaign was realized post ploughing. Four soil horizons (0–20, 20–40, 40–60, and 60–80 cm) were sampled with a ground auger. The maximum depth was limited to 80 cm, due to the presence of a surface aquifer beyond this depth. The samples were stored at −18 °C. A mean pooled sample was made from four samples distributed throughout the agricultural plot to be more representative of the soil quality. The agricultural work corresponded to ploughing until about 25 cm from the surface.
A second series of sampling was conducted in 2012 from the surface horizon (0–20 cm) only, to compare agricultural with non-agricultural soils: forest (Fontenay-les-Briis), urban (Paris 5th and Paris 13th, 20,000 inhabitants km−2), and rural (Doue, Seine-et-Marne, 1029 inhabitants, 50.3 inhabitants km−2) (Appendix 1).
Chemicals and reagents
Solvents for cleaning and extraction purposes were supplied by Merck-Chimie (Fontenay-sous-Bois, France). Superclean LC-Florisil (6 mL/1 g) cartridges for solid-phase extraction (SPE) cleanup were bought from Sigma-Aldrich (Saint-Quentin Fallavier, France). Mobile phase for GC/MS, helium and nitrogen (99.999 %), were purchased from Air-Liquide (Paris, France). Mobile phase for LC/MS/MS was prepared in the laboratory with methanol and ultrapure water from a Milli-Q system.
A standard solution of six phthalates in isooctane (dimethyl phthalate (DMP), diethyl phthalate (DEP), di-n-butyl phthalate (DnBP), butyl-benzyl phthalate (BBP), diethylhexyl phthalate (DEHP), di-n-octyl phthalate (DnOP)) was purchased from Supelco (via Sigma-Aldrich). Di-iso-butyl phthalate (DiBP) and di-iso-nonyl phthalate (DiNP) were added to the mixture of six standards at the final concentration of 8 μg mL−1 in isooctane, and di-iso-decyl phthalate (DiDP) at the final concentration of 4 μg mL−1 in isooctane. Dipentyl phthalate (DPP) (Supelco) was used as internal standard (IS) and benzyl benzoate (Supelco) as surrogate standard (SrS).
BPA (98 % purity assay) was obtained from Sigma-Aldrich, and a solution of 2 μg mL−1 was prepared in methanol. BPA D16 from Supelco was used as IS for BPA determination.
Sample extraction
Soil and sludge samples were freeze-dried for 3 days. Then, they were homogenized and sieved (1 mm) and aliquots of 2 g were weighted. The samples were spiked with ISs (BPA-D16 1.15 μg; DPP 4 μg in isooctane) in 40 mL centrifuge glass tubes and then extracted with 15 mL of a mixture of hexane 50 vol/acetone 50 vol in a Bransonic 2510 ultra-sonic bath (VWR) for 20 min. Extracts were centrifuged (2 min at 900 g), and supernatants were collected. This procedure was performed two times, and both extracts were combined before concentration to 500 μL under nitrogen stream.
Laboratory blanks were realized for each series (one blank for six samples) with 20 mL of hexane 50 vol/acetone 50 vol in centrifuge glass tubes that followed the same procedure steps as samples.
Fractionation and cleanup procedures
The extracts were passed through Florisil cartridges for phthalates and BPA fractionation and for cleaning. Solvent mixtures with increasing polarity were used: hexane 80 vol/diethyl ether 20 vol (2 × 5 mL) for the phthalate fraction, followed by methylene chloride 95 vol/methanol 5 vol (2 × 5 mL) for the BPA fraction.
Phthalate fractions were concentrated under nitrogen stream to a volume of 400 μL and then transferred into vials for analysis.
BPA fractions were collected and evaporated to dryness under a nitrogen stream. The residues were dissolved into 400 μL of the mobile phase methanol/water (40 vol/60 vol) and filtered through centrifugal filters (0.2 μm, 500 μL—VWR, USA).
Analytical conditions
BPA was quantified by LC/MS/MS with an Agilent 1200 LC device MS/MS Series G6410B equipped with an Agilent Zorbax Eclipse XDB-C18 column (L 50 mm, Ø 4.6 mm, porosity 1.8 μm) under a water/methanol mobile phase (Appendix 3). The source was in ESI negative mode (N2 350 °C; gas flow 660 L/h; capillary 4000 V), and the MS parameters are presented in Appendix 3.
Phthalates were analyzed by GC/MS with a 7890 A GC coupled to a 5975 A MS (Agilent Technologies, Massy, France). The specific conditions were as follows: column ZB-7HG, 30 m, 250 μm ID × 0.25 μm film thickness; detector, electronic impact, 70 eV; vector gas helium 1 mL min−1 (IC quality, Air Liquide). The injector heated at 290 °C was in split less mode and the injection volume was 1 μL. The oven program was as follows: 50 °C for 1 min, then 30 °C/min to 280 °C, then 15 °C/min to 310 °C for 4 min. Benzyl benzoate was used as syringe compound. MS parameters are presented in Appendix 4.
Quantification was carried out by calculating a response factor for each compound relative to its corresponding IS and concentrations were obtained using a linear regression analysis of relative responses versus relative concentrations. The calibration curve was linear inside the range 20 to 20,000 pg (r > 0.997) for BPA and 31 to 8,000 pg (r > 0.998) for phthalates.
The limits of detection (LODs) were considered, from a standard solution of phthalates (125 ng mL−1) and of BPA (20 ng mL−1), as quantities with a signal/noise ratio of 3 peak to peak (Appendix 5). The limits of quantification (LOQs) were determined with 2 g of soil samples spiked with 1000 ng of phthalates and 100 ng of BPA (n = 3). The LOQs corresponded to concentrations of a signal/noise ratio of 9 peak to peak (Appendix 5). Recoveries from the spiked soil matrix ranged from 55 to 160 % depending on the compound. Sample measurements were corrected by the concomitant procedural blank values (Appendix 5).
Results were expressed as micrograms per gram dry weight for phthalates and nanograms per gram dry weight for BPA. Phthalates were given as individual compounds or Σ9 phthalates.
DEHP and BPA half-lives were estimated from the linear regression of their concentrations in the 0–20 cm soil horizon for the period from March 2011 (after sludge spreading) to July 2011.
Results and discussion
Sludge contamination
Phthalate and BPA contents in the sewage sludge varied depending on their physicochemical properties and the characteristics of the wastewater contamination. BPA and phthalates were found except DMP because of its high water solubility (Fig. 1) and less uses as compared to phthalates of high molecular weight. DEHP, DiNP, and DiDP were strongly trapped in the sludge related to their high log K ow. The phthalate levels varied from a few hundred nanograms per gram dw for DEP, DiBP, DnBP, and BBP to a few micrograms per gram dw for DEHP, DiNP, and DiDP, corresponding to relatively low contamination levels of sludge compared to WWTP from other countries. Indeed, in a town from UK (11,000 inhabitants), Oliver et al. (2005) found higher DEP and DEHP levels of, respectively, 1.6 and 30 μg g−1 dw, in sludge from a combined primary sedimentation tank and humus tank that were thickened in a sludge settlement tank. Also, in Finland, Marttinen et al. (2003) reported for a WWTP treating 74,000 m3 day−1 by sedimentation combined to biological processes, DEHP contents in the treated sludge of 163 μg g−1 dw. The DEHP removal efficiency was of 94 %, the main removal process being sorption to primary and secondary sludge. Last, in WWTPs using conventional activated sludge in China, concentration values in final sludge ranged from 0.004 to 2 μg g−1 dw for DMP, from 0.01 to 11 μg g−1 dw for DEP, from 0.006 to 3.7 μg g−1 dw for DnBP, from 0.023 to 35 μg g−1 dw for BBP, from 4 to 108 μg g−1 dw for DEHP, and from < LQ to 6.6 μg g−1 dw for DnOP (Cai et al. 2007).
We found very low BPA contents (<1 ng g−1 dw) in sludge, which is related to poor particle adsorption and high biodegradation of this compound in the WWTP (Tran et al. 2015). In the same way as phthalates, BPA values were in the lower range of concentrations from 0.1 to 3.2 × 107 ng g−1 dw encountered at global scale (Flint et al. 2012). Langdon et al. (2011b) reported that BPA concentrations were significantly lower in sludge from WWTPs using predominantly aerobic treatment than anaerobic treatment. Mass balance experiments in a pilot plant showed that more than 99 % of BPA were removed by aerobic biodegradation at steady state (Press-Kristensen et al. 2008). BPA degradation by aerobic activated sludge could be described by a first-order reaction equation with a constant degradation rate of 0.80 h−1 at 20 °C (Zhao et al. 2008).
Agricultural soil contamination
Fate of the plasticizers over time
Mean contents of DEHP and BPA in agricultural soils are shown on Fig. 2. Contaminant levels in soil before sludge spreading were very low with a maximum value of 29 μg kg−1 dw for DEHP and under LOQ for BPA. The phthalates distribution was dominated by the low molecular weight compounds like DEP, DiBP, and DnBP (Fig. 3). Plasticizer inputs to soils could occur mainly from bulk atmospheric deposition and surface runoff. After spreading, the mean phthalate profiles in soil and sludge were similar, with a clear enrichment in high molecular weight phthalates in soil (Figs. 2 and 3). The main compound was DEHP, with the following sequence: DEHP > DiNP > DiDP > DiBP > DnBP. In sludge-amended soils from Denmark, DEHP and DiNP were the most abundant phthalates and with concentrations similar to ours (VikelsØe et al. 2002). In agricultural soils from the Czech Republic, higher levels of DBP and DEHP, respectively, of 0.3 and 10.3 mg kg−1 dw were reported (Zorníková et al. 2011).
An 8-fold increase of DEHP (from 29 to 242 µg kg−1 dw) was found in the surface horizon of the soil after sludge spreading. A time variation, corresponding to a 2-fold decrease was observed from spring to summer. Similarly, a sharp DEHP increase (10-fold) was observed in experimental maize crop fields from France during the period of sewage sludge spreading (Patureau et al. 2007).
Very low BPA levels were found in all samples, ranging from < LOQ to 79 ng kg−1 dw. A 79-fold increase of BPA content was observed in the surface horizon after sludge spreading (from < LOQ to 79 ng kg−1 dw). In the surface horizon (0–10 cm) of agricultural soil collected in Spain, higher BPA levels, ranging from 0.7 to 4.6 μg kg−1 dw, were found (Sánchez-Brunete et al. 2009).
In September 2014, while sampling was performed after soil tillage, we observed in the surface horizon a 55-fold increase of BPA contents and a 3.7-fold increase of phthalate contents (Fig. 2). In the same agricultural soil, the behavior of antibiotics showed a similar phenomenon, i.e., the oldest amended plots displayed the highest antibiotic levels (Dinh 2012).
The homogenization of agricultural soil by ploughing could lead to a release of bound residues resulting in an increase of plasticizer contents in the different soil horizons. The mean content taking in account the four horizons was of 134 μg kg−1 dw after spreading against 162 μg kg−1 dw after ploughing for DEHP. On the contrary, for BPA, the content was higher after spreading (25.5 ng kg−1 dw) than after ploughing (16.0 ng kg−1 dw) corresponding to a lower stability of this compound in the agricultural soil, compared to DEHP. A rapid dissipation of 14C-BPA was observed in various soils tested in laboratory experiments, suggesting that soil microorganisms capable of transforming BPA were ubiquitous in the terrestrial environment (Fent et al. 2003).
The formation of bound residues, classically described for pesticides, is usually associated with organic matter and biological activity but very few information about their remobilization are available (Barriuso et al. 2008). Patureau et al. (2007) hypothesized the formation of bound residues of DEHP to explain its persistence in the soil. Laboratory soil experiments conducted with 14C-BPA showed a strong affinity of BPA to the soil matrix mainly by rapid formation of bound residues (Fent et al. 2003). Thus, fragmentation and aeration of agricultural soil by tillage could lead to release bound residues of BPA and phthalates adsorbed to clay sheets or bacteria as already described for pesticides (Barriuso et al. 2008). Further parameters such as soil hygrometry can play a part during the compound release. Heavy rains during the period previous the post tillage sampling (Appendix 2) could contribute to BPA desorption because of its high aqueous solubility (300 mg L−1). Adsorption of BPA is generally reversible, and desorption occurs quickly and is completed after few desorption steps (Loffredo and Senesi 2002).
Distribution of the plasticizers relative to depth
The phthalate distribution into the different horizons of the soil (Fig. 4) showed that for the high molecular weight compounds, the highest contents were in the surface horizon (0–20 cm). Similar profiles were observed for DEHP and DnBP in low sludge-amended soil and meadow soil of Denmark (VikelsØe et al. 2002). Phthalate compounds with high K ow presented low mobility through the soil due to their trend to adsorb upon particles particularly in the surface horizon enriched in organic matter after sludge spreading. Moreover, the log K oc of phthalates, ranging from 5.5 to 6.8, was in favor of their trap to the organic matter. In contrast, the low molecular weight phthalates (DMP, DEP, DiBP, and DnBP) were also the most mobile and consequently, these compounds were more abundant in depth.
BPA was also encountered in the surface horizon with a higher level than in the depth, which might be partly explained by its high log K oc ranging from 2.7 to 3.1 (Ivashechkin et al. 2004). Indeed, laboratory experiments upon the BPA adsorption in soils indicated a low mobility of that compound (Fent et al. 2003). Moreover, BPA was not detected in the soil during the summer period (July 2011) probably related to a higher biodegradation under warm temperatures.
The agricultural soil characteristics, determined for the 0–30-cm horizon and the 50–80-cm horizon, showed a general clay loam nature (Table 1). The surface horizon was characterized by the highest levels of organic matter as well as organic carbon related to sludge application (Table 1). Also, sands and coarse silts were higher in the surface horizon, while clays displayed an inverse distribution.
Sorption processes may occur on the solid phase of soils (Von Oepen et al. 1991) and also on the dissolved organic matter (Spark and Swift 2002), the compound retention resulting to adsorption mechanisms at the surface of particles and migration processes inside aggregates (Pignatello 1999) by diffusion through nanopores (Wauchope et al. 2002). The structure (particle size), the nature (clay, silt), and the organic matter content of soil influence the transfer of organic compounds. VikelsØe et al. (2002) observed a maximal DEHP retention in soils with visible clay in the upper layers. Moreover, the intensity and the nature of interactions with the organic fraction varied, depending on its composition and on the chemical properties of the molecules. The mineral fraction in clays also contributes to that retention (Vischetti et al. 2010). The top horizon of agricultural soil enriched in organic matter during sewage sludge spreading may thus have a retention capacity greater than that of the deep horizon, the potential of phthalate adsorption for a soil being correlated to its organic matter content (Rhind et al. 2013; Niu et al. 2014).
Thus, despite the fact that adsorbent materials like clays or silts with small particle size were more abundant in the depth, the proportion of organic matter at the soil surface appeared to be the major factor controlling the contaminant migration through the soil.
Plasticizer half-life estimations in the agricultural soil
A DEHP half-life of 64 days (r = 0.998) from its disappearance in the 0–20-cm soil horizon was estimated. This is consistent with the range from 42 to 126 days provided in the literature for 90 % degradation time of DEHP adsorbed to humus or clay (Erhardt and Prüeß 2001). DEHP was recalcitrant in soil, because of a poor bioavailability only 10 % degraded by 70 days at 20 °C (Cartwright et al. 2000).
A value of 36 days (r = 0.88) for BPA confirmed our observation of higher biodegradability for this compound than for phthalates. Langdon et al. (2011a) found BPA half-fives ranging from 18 to 102 days in soil under laboratory conditions. Ying and Kookana (2005) showed that although BPA degraded rapidly in the soil within 7 days under aerobic conditions, little or no degradation was noted under anaerobic conditions, during a study of 70 days. Moreover, BPA removal rates of only 35 % were found during sludge anaerobic digestion (Samaras et al. 2013). However, in this study, in the surface horizon corresponding to aerobic conditions, the presence of a recalcitrant fraction due to non-reversible sorption of BPA following the application of sludge could explain our half-life result. Indeed, Langdon et al. (2012) estimated the formation of a recalcitrant BPA fraction of 23 % of its initial concentration.
Plasticizers predictive environmental concentrations in the agricultural soil
Regulation relative to the spreading of sewage sludge in France (Decree 97-1133) authorizes a maximum application rate of 30 t of dry matter per hectare every 10 years. From this value, a predictive environmental concentration in the agricultural soil (PEC soil) was calculated from the DEHP level determined in the sewage sludge.
- C1 :
-
DEHP concentration in the sewage sludge as micrograms per gram dry weight (C1 = 33 μg g−1 dw)
- Q1 :
-
Amount of sludge brought to the agricultural soil as grams per square meter (Q1 = 300 g m−2)
- V :
-
Volume of dispersion in the surface horizon (0–20 cm) of the soil corresponding to an area of 1 m2 (V = 0.02 m3)
- D :
-
Density of the surface horizon soil (D = 1.67 × 106 as g m−3, Table 1)
Therefore, the PEC for DEHP was 0.3 μg g−1 dw. On the other hand, the experimental value measured in the amended soil was 0.16 μg g−1 dw, suggesting the occurrence of a significant DEHP degradation or the formation of non-extractable residues as related for pesticides by Barriuso et al. (2008).
Our values remained lower than the PEC of 2.6 μg g−1 dw given by the UESES model (European Union Risk Assessment Report CAS No. 117-81-7) for agricultural soils amended with sewage sludge (EUR 23384 EN/2, 2008). This PEC was estimated from the model EUSES for DEHP in agricultural soils on the basis of the following contributions: 2.5 % by the production, 2.5 % by industrial employment, 32 % by employment of finished products, and 63 % by waste treatment.
Comparison between agricultural and non-agricultural soils (urban, rural, and forestry)
We investigated four different soils characterized by contrasted land uses: urban, agricultural, and forest (Appendix 1).
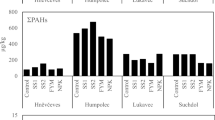
Phthalate and BPA contents in the soils are presented in Table 2. The urban soil displayed the highest contamination level before the agricultural soil even after the sewage sludge application, the sequence being as follows: urban > agricultural > rural > forest. The total phthalate contents in the urban soil (1089 ng g−1 dw) were 2.7 times higher than in the agricultural one (407 ng g−1 dw), 7 times than in the rural one (154 ng g−1 dw), and 18 times higher than in the forest one (60 ng g−1 dw). Indeed, phthalate in soils might have several origins, mainly local sources for urban soils as described by Zeng et al. (2009) and sewage sludge application or agricultural uses of plastic films for agricultural soils (Cai et al. 2008). The low levels encountered in rural and forest soils can be explained by land use and remoteness of the sources.
Phthalate distribution showed that DEHP was predominant and that DMP and DnOP were the less abundant compounds, whatever the soil characteristics. Very few literature data for European soils were available. In soils collected throughout Scotland over a 3-year period, DEHP levels ranged from 25 to 1596 ng g−1 dw but with no consistent effects of soil or vegetation or distance from urban centers and possibly related to atmospheric deposition and to soil organic content (Rhind et al. 2013). An increasing gradient of phthalate levels was shown between rural and urban soils in China, but with one to two orders of magnitude higher than our contents (Hongjun et al. 2013).
BPA was found at lower levels as compared to phthalates (<10 ng g−1 dw) but with the same sequence of soil contamination (rural > agricultural > rural > forest). No data were available for BPA at global scale for comparison of contamination levels between soils with different land uses because that compound was considered as poorly persistent in the environment.
Conclusion
An important route for plasticizer entry to terrestrial environments is via application of sewage sludge as fertilizers. This study that deals with the simultaneous investigation of two current plasticizers in sewage sludge and soils from different origins in France brings new information about their behavior in different soil horizons which depend of degradation, mineralization, and adsorption-bound residue formation processes. High discrepancies of phthalate and BPA levels, depending on land uses and agricultural practices, were underlined.
Our field experiments indicate that the compound distributions, in the amended soil, follow the same trend as in the sludge. The variations of contaminant levels depend on their physicochemical properties, which control their adsorption and their transfer toward soil depth.
Considering their contents in sludge and soil, it appears that whatever the degradability range of the target compounds, they might have long-term effects in soil if high amounts were spread. The global production levels for those two types of plasticizers are similar: about five million tons per year. However, the risk to dispersion in the environment seems to be lower for BPA chemically bound to polymer, contrary to DEHP.
The low levels of BPA in sludge related to its water solubility and potential biodegradation in WWTP limit its transfer into the environment. However, the question of plasticizer transfers from amended soil toward the atmospheric compartment via volatilization and toward the surface water via runoff or the groundwater via percolation remains of current concern, especially considering the potential release of bound residues.
In that context, the monitoring of plasticizers in sludge and agricultural soil might participate to improvements of the regulation that recommends maximum levels of contaminants in sludge for land application.
References
Barriuso E, Benoit P, Dubus I (2008) Formation of pesticide nonextractable (bound) residues in soil: magnitude, controlling factors and reversibility. Environ Sci Technol 42:1845–1854. doi:10.1021/es7021736
Biedermann-Brem S, Grob K (2009) Release of bisphenol A from polycarbonate baby bottles: water hardness as the most relevant factor. Eur Food Res Technol 228:679–684. doi:10.1007/s00217-008-0978-8
Cai QY, Mo CH, Wu QT, Zeng GY, Katsoyiannis A (2007) Occurrence of organic contaminants in sewage sludges from eleven wastewater treatment plants in China. Chemosphere 68:1751–1762. doi:10.1016/j.chemosphere.2007.03.041
Cai QY, Mob CH, Wua QT, Katsoyiannisc A, Zenga QY (2008) The status of soil contamination by semivolatile organic chemicals (SVOCs) in China: a review. Sci Total Environ 389:209–224. doi:10.1016/j.scitotenv.2007.08.026
Cartwright CD, Thompson IP, Burns RG (2000) Degradation and impact of phthalate plasticizers on soil microbial communities. Environ Toxicol Chem 19:1253–1261. doi:10.1002/etc.5620190506
Cousins IT, Mackay D, Pakerton TF (2003) Physical-chemical properties and evaluative fate modeling of phthalate esters. The handbook of environmental chemistry. Springer, Berlin, vol 3, part Q. 57–84. doi:10.1007/b11463
Craig ZR, Wang W, Flaws JA (2011) Endocrine-disrupting chemicals in ovarian function: effects onsteroidogenesis, metabolism and nuclear receptor signaling. Reproduction 142:633–646
Decree 97-1133: http://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000000570287
Dinh Q-T (2012) Transferts et comportements d’antibiotiques à l’échelle du bassin versant élémentaire. Thèse: Systèmes intégrés, environnement et biodiversité. Ecole Pratique des Hautes Etudes, Paris, 204 p. (EPHE/Université Paris VI)
Erhardt W, Prüeß A (2001) Organic contaminants in sewage sludge for agricultural use. http://ec.europa.eu/environment/archives/waste/sludge/report6.htm. Accessed 13 March 2015
EUR 23384 EN/2 (2008) European Union Risk Assessment Report bis (2-ethylhexyl) phthalate (DEHP), Volume 80. Edi - rs: S. Pakalin, K. Aschberger, O. Cosgrove, B-O. Lund, A. Paya-Perez, S. Vegro. Luxembourg: Office for Official Publications of the European Communities – VIII pp., 22 pp EUR – Scientific and Technical Research series – ISSN 1018-5593
Fent G, Hein WJ, Moendel MJ, Kubiak R (2003) Fate of 14C-bisphenol A in soils. Chemosphere 51:735–746. doi:10.1016/S0045-6535(03)00100-0
Fernández MA, Gómara B, González MJ (2012) Occurrence of phthalates and their metabolites in environment, and human health implications. In: Emerging organic contaminants and human health. The Handbook of Environmental Chemistry 307–336
Flint S, Markle T, Thompson S, Wallace E (2012) Bisphenol A exposure effects and policy: a wildlife perspective. J Environ Manag 104:19–34. doi:10.1016/j.jenvman.2012.03.021
Fürhacker M, Scharf S, Weber H (2000) BPA: emission from point sources. Chemosphere 41:751–756
German Federal Environment Agency (2011) Bundesgesundheitsbl-Gesundheitsforsch-Gesundheitsschutz 54(6):770–785
Heudorf U, Mersch-Sundermann V, Angerer J (2007) Phthalates: toxicology and exposure. Int J Hyg Environ Health 210:623–634. doi:10.1016/j.ijheh.2007.07.011
Höhne C, Püttmann W (2008) Occurrence and temporal variations of the xenoestrogensbisphenol A, 4-tert-octylphenol, and tech. 4-nonylphenol in two German wastewater treatment plants. Environ Sci Pollut Res 15:405–416. doi:10.1007/s11356-008-0007-2
Hongjun Y, Wenjun X, Qing L, Jingtao L, Hongwen Y, Zhaohua L (2013) Distribution of phthalate esters in topsoil: a case study in the Yellow River Delta, China. Environ Monit Assess 185:8489–8500. doi:10.1007/s10661-013-3190-7
Ivashechkin P, Corvini FX, Dohmann M (2004) Behaviour of endocrine disrupting chemicals during the treatment of municipal sewage sludge. Water Sci Technol 50:133–151
Langdon KA, Warne M, St J, Smernik RJ, Shareef A, Kookana RS (2011a) Degradation of 4-nonylphenol, 4-t-octylphenol, bisphenol A and triclosan followingbiosolids addition to soil under laboratory conditions. Chemosphere 84:1556–1562
Langdon KA, Warne M, St J, Smernik RJ, Shareef A, Kookana RS (2011b) Selected personal care products and endocrine disruptors in biosolids: an Australia-wide survey. Sci Total Environ 409:1075–1081
Langdon KA, Warne M, St J, Smernik RJ, Shareef A, Kookana RS (2012) Field dissipation of 4-nonylphenol, 4-t-octylphenol, triclosan and bisphenol A following land application of biosolids. Chemosphere 86:1050–1058
Lee HB, Peart TE, Chan J, Gris G (2004) Occurrence of endocrine-disrupting chemicals in sewage and sludge samples in Toronto, Canada. Water Qual Res J Can 39:57–63
Loffredo E, Senesi N (2002) Developments in Soil Science, volume 28A pp 143–159. In: Violante A, Huang PM, Bollag JM, Gianfreda L (eds) Elsevier Science B.V.
Marttinen S, Kettunen R, Sormunen KM, Rintala J (2003) Removal of bis(2-ethylhexyl)phthalate at a sewage treatment plant. Water Res 37:1385–1393
Meeker JD (2010) Exposure to environmental endocrine disrupting compounds and men’s health. Maturitas 66:236–241. doi:10.1016/j.maturitas.2010.03.001
Niu L, Xu Y, Xu C, Yun L, Liu W (2014) Status of phthalate esters contamination in agricultural soils across China and associated health risks. Environ Pollut 195:16–23. doi:10.1016/j.envpol.2014.08.014
Oliver R, May E, Williams J (2005) The occurrence and removal of phthalates in a trickle filter STW. Water Res 39:4436–4444. doi:10.1016/j.watres.2005.08.011
Patureau D, Laforie M, Lichtfouse E, Caria G, Denaix L, Schmidt JE (2007) Fate of organic pollutants after sewage sludge spreading on agricultural soils: a 30 years field-scale recording. Water Pract Technol 2:1–10. doi:10.2166/wpt.2007.0008
Pignatello JJ (1999) The measurement and interpretation of sorption and desorption rates for organic compounds in soil media. Adv Agron 69(69):1–73. doi:10.1016/S0065-2113(08)60946-3
Press-Kristensen K, Lindblom E, Schmidt JE, Henze M (2008) Examining the biodegradation of endocrine disrupting bisphenol A and nonylphenol in WWTPs. Water Sci Technol 57:1253–1258. doi:10.2166/wst.2008.229
Rhind SM, Kyle CE, Kerr C, Osprey M, Zhang ZL, Duff EI, Lilly A, Nolan A, Hudson G, Towers W, Bell J, Coull M, McKenzie C (2013) Concentrations and geographic distribution of selected organic pollutants in Scottish surface soils. Environ Pollut 182:15–27. doi:10.1016/j.envpol.2013.06.041
Samaras VG, Stasinakis AS, Mamais D, Thomaidis NS, Lekkas TD (2013) Fate of selected pharmaceuticals and synthetic endocrine disrupting compounds during wastewater treatment and sludge anaerobic digestion. J Hazard Mater 244–245:259–267. doi:10.1016/j.jhazmat.2012.11.039
Sanchez-Avila J, Bonet J, Velasco G, Lacorte S (2009) Determination and occurrence of phthatlates, alkylphenols, BPA, PBDEs, PCBs, and PHAs in an industrial sewage grid discharging to a Municipal Wastewater Treatment Plant. Sci Total Environ 407:157–4167. doi:10.1016/j.scitotenv.2009.03.016
Sánchez-Brunete C, Miguel E, TadeoJ L (2009) Determination of tetrabromobisphenol-A, tetrachlorobisphenol-A and bisphenol-A in soil by ultrasonic assisted extraction and gas chromatography–mass spectrometry. J Chromatogr A 1216:5497–5503. doi:10.1016/j.chroma.2009.05.065
Spark K, Swift R (2002) Effect of soil composition and dissolved organic matter on pesticide sorption. Sci Total Environ 298:147–161. doi:10.1016/S0048-9697(02)00213-9
Tran BC, Teil M, Blanchard M, Alliot F, Chevreuil M (2015) BPA and phthalate fate in a sewage network and an elementary river of France. Influence of hydroclimatic conditions. Chemosphere 119:3–51. doi:10.1016/j.chemosphere.2014.04.036
VikelsØe J, Thomsen M, Carlsen L (2002) Phthalates and nonylphenols in profiles of differently dressed soils. Sci Total Environ 296:105–116
Vischetti C, Corti G, Monaci E, Cocco S, Coppola L, Agnelli A (2010) Pesticide adsorption and degradation in fine earth and rock fragments of two soils of different origin. Geoderma 154(3–4):348–352. doi:10.1016/j.geoderma.2009.11.006
Von Oepen B, Kördel W, Klein W (1991) Sorption of nonpolar and polar compounds to soils: processes, measurements and experience with the applicability of the modified OECD-Guideline 106. Chemosphere 22:285–304. doi:10.1016/0045-6535(91)90318-8
Wauchope RD, Yeh S, Linders J, Kloskowski R, Tanaka K, Rubin B, Katamaya A, Kördel W, Gerstl Z, Lane M, Unsworth JB (2002) Pesticide soil sorption parameters: theory, measurement, uses, limitations and reliability. Pest Manag Sci 58:419–445. doi:10.1002/ps.489
Working document on sludge, third draft, 27 April 2000. http://www.ewa-online.eu/comments.html. Accessed 13 March 2015
Ying GG, Kookana RS (2005) Sorption and degradation of estrogen-like-endocrine disrupting chemicals in soil. Environ Toxicol Chem 24:2640–2645. doi:10.1897/05-074R.1
Zeng F, Cui K, Xie Z, Wu L, Luo D, Chen L, Lin Y, Liu M, Sun G (2009) Distribution of phthalate esters in urban soils of subtropical city, Guangzhou, China. J Hazard Mater 164:1171–1178
Zhao J, Li Y, Zhang C, Zeng Q, Zhou Q (2008) Sorption and degradation of bisphenol A by aerobic activated sludge. J Hazard Mater 155:305–311. doi:10.1016/j.jhazmat.2007.11.075
Zorníková G, Jarošová A, Hřivna L (2011) Distribution of phthalic acid esters in agricultural plants and soils. Acta Univ Agric Silvic Mendelianae Brun 59:233–238. doi:10.11118/actaun201159030233
Acknowledgments
This work was financially supported by the PIREN-SEINE Research Programme and by the “Laboratoire Hydrologie et Environnement” (EPHE).We wish to thank Catherine Bourges and Annie Desportes for their technical assistance. The authors are grateful to Météo-France for providing the meteorological data and also to Monsieur Long who allowed us to access to the WWTP of Fontenay-les-Briis settlement and helped us for sample collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Leif Kronberg
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1
(DOCX 348 kb)
Appendix 2
(DOCX 46 kb)
Appendix 3
(DOCX 47 kb)
Appendix 4
(DOCX 46 kb)
Appendix 5
(DOCX 47 kb)
Appendix 6
(DOCX 2601 kb)
Rights and permissions
About this article
Cite this article
Tran, B.C., Teil, MJ., Blanchard, M. et al. Fate of phthalates and BPA in agricultural and non-agricultural soils of the Paris area (France). Environ Sci Pollut Res 22, 11118–11126 (2015). https://doi.org/10.1007/s11356-015-4178-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4178-3