Abstract
The acidity of Ultisols (pH <5) is detrimental to crop production. Technologies should be explored to promote base saturation and liming effect for amelioration of Ultisol pH. Column leaching experiments were conducted to investigate the amelioration effects of canola straw (CS) and peanut straw (PS) in single treatment and in combination whether with alkaline slag (AS) or with lime on Ultisol profile acidity. The treatment without liming materials was set as control, and the AS and lime in single treatment are set for comparison. Results indicated that all the liming materials increase soil profile pH and soil exchangeable base cations at the 0–40-cm depth, except that the lime had amelioration effect just on 0 to 15-cm profile. The amelioration effect of the liming materials on surface soil acidity was mainly dependent on the ash alkalinity in organic materials or acid neutralization capacity of inorganic materials. Specific adsorption of sulfate (SO4 2−) or organic anions, decarboxylation of organic acids/anions, and the association of H+ with organic anions induced a “liming effect” of crop residues and AS on subsoil acidity. Moreover, SO4 2− and chloride (Cl−) in PS, CS, and AS primarily induced base cations to move downward to subsoil and exchange with exchangeable aluminum (Al3+) and protons (H+). These anions also promoted the exchangeable Al to leach out of the soil profile. The CS was more effective than PS in decreasing soil acidity in the subsoil, which mainly resulted from higher sulfur (S) and Cl content in CS compared to PS. The CS combined with AS was the better amendment choice in practical agricultural systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid Ultisol is a kind of highly weathered clayey soils and distributes widely in the tropical and subtropical regions around the world (Qafoku et al. 2004). In the Ultisol profile, toxicity of Al and Mn to plants and deficiencies of Ca, Mg, and K can severely limit root elongation and crop growth and reduce crop yield (Farina et al. 2000; Tang et al. 2003; Brown et al. 2008; Fageria and Baligar 2008). Surface soil acidity is easily corrected by liming, but incorporating lime (CaCO3) into topsoil has very limited effects on subsoil acidity in the short term due to low solubility of lime and slow downward movement of Ca2+ through the soil profile (Carvalho and van Raij 1997; Tang et al. 2003; Fageria and Baligar 2008). Incorporation of lime into the subsoil is effective (Sumner et al. 1986; Shainberg et al. 1989) but economically unfeasible. Thus, alternative technologies should be explored for amelioration of subsoil acidity. Surface-applied amendments are considered practical and cost-effective methods to ameliorate subsoil acidity (Sumner et al. 1986; Farina et al. 2000; Liu and Hue 2001). The amelioration effect depends on transport of bases from the surface horizon and the reaction of these bases with acid in the subsoil horizons. Gypsum and phosphogypsum have been proposed as effective amendments for subsoil acidity when surface-applied, since they are more soluble than lime and enable calcium (Ca2+) to move through the soil with SO4 2− in a larger quantity and at greater speed compared with applied lime (Sumner et al. 1986; Shainberg et al. 1989; McLay et al. 1994). SO4 2− leached into subsoil can effectively reduce subsoil aluminum (Al) toxicity by self-liming, formation of nontoxic AlSO4 +, or precipitation of Al–OH–SO4 minerals (Alva et al. 1990; Sposito 1996; Carvalho and van Raij 1997; Sun et al. 2000). Moreover, SO4 2− promotes base cations to leach downward to the subsoil and decreases the Al3+ activity in the soil solution through an increase in ionic strength and induces displacement of Al3+ off soil colloids by Ca2+ and subsequently leaching of Al–SO4 complexes from the soil (Oates and Caldwell 1985; McLay et al. 1994; Carvalho and van Raij 1997). But, their widespread use can be limited by availability, cost, and even environmental risk. Anions derived from organic materials are expected to produce results analogous to those of gypsum or phosphogypsum in ameliorating acid subsoils. The decomposition of organic materials can release soluble humic molecules and low-molecular-weight organic anions as well as inorganic anions, such as SO4 2− and Cl− (Haynes and Mokolobate 2001). In addition to SO4 2−, organic anions complex with Al in soil solution and induce Al to become nonphytotoxic species (Sposito 1996; Haynes and Mokolobate 2001). Specific adsorption of soluble humic molecules and low-molecular-weight organic anions to Al and iron oxide surfaces in soils can also displace OH− off soil surfaces and thus confer a greater negative charge on the soils, which tends to increase the Ca2+ adsorption capacity (Xu et al. 2003). Decarboxylation of organic acids/anions and the association of H+ with organic anions also consume H+ in the subsoil (Butterly et al. 2011, 2013; Rukshana et al. 2011). Moreover, high mobility of most complexed Ca species means that they are also easily leached downward to the subsoil. For example, citrate and acetate promoted the movement of approximately 44 and 55 % of surface-applied Ca to the subsoil layers of 10–15 cm, respectively (Inoue et al. 2001). Hue and Licudine (1999) suggested that manure-derived organic molecules facilitated the downward movement of Ca as Ca complexes. Along their downward path, these complexes reacted with Al, releasing Ca that would become plant-available and forming complexed Al that may be nonphytotoxic. The organic acids/anions and SO4 2− released from the decomposition of organic materials all contributed to ameliorating subsoil acidity, but the ameliorating effect depends on properties of organic materials.
Crop residues are very important organic materials to cropland to improve soil nutrient and maintain soil quality (Haynes and Mokolobate 2001; Bending et al. 2002; Ogbodo 2011; Yuan et al. 2011). Moreover, crop residues have also proved to be effective amendments for soil acidity in surface soil due to the high content of ash alkalinity (Yan et al. 1996; Xu et al. 2006; Wang et al. 2009, 2012; Butterly et al. 2011). Use of crop residues is specifically interesting due to huge biomass production of main crops in the agricultural systems. Crop residues with low content of base cations should be coupled with lime materials to increase their ameliorating effects on soil acidity. Many industrial by-products containing calcium and sulfate can act as alternative amendments (Fageria and Baligar 2008; Li et al. 2010). Recently, AS, an industrial by-product produced from ammonia–alkali production of sodium carbonate, has proved very effective in correcting surface soil acidity in indoor incubation and field experiments (Li et al. 2010, 2014; Wang et al. 2012; Masud et al. 2014, 2015). The AS contains calcium carbonate as well as calcium sulfate, which would be beneficial for correcting soil acidity similar to lime combined with gypsum (Li et al. 2010, 2014; Masud et al. 2014, 2015). In order to develop low-input methods for ameliorating subsoil acidity, the current study investigated the ameliorating effect of crop residues combined with AS on soil acidity in the profile of an Ultisol using a column leaching experiment. Lime was used for comparison.
Materials and methods
Soils and amendments
An acid Ultisol (US Soil Taxonomy; Haplic Acrisol in the WRB taxonomy) used in the study was collected from Langxi, Anhui Province, China (31° 6′ N, 119° 8′ E) and derived from quaternary red earth. The soil samples were collected at two different depths, with topsoil collected from 0 to 20 cm and subsoil from 20 to 60 cm. The collected soil was air-dried and passed through a 2-mm sieve. Chemical properties of the original soil are presented in Table 1.
In our previous study, peanut straw (PS) and canola straw (CS) were found to be very effective amendments among the leguminous and nonleguminous crop residues, respectively (Wang et al. 2009). Thus, four kinds of liming materials were selected for this experiment: lime, alkaline slag (AS), PS and CS. Using these materials, nine treatments were established: (1) control without liming materials, (2) lime, (3) AS, (4) PS, (5) PS combined with lime (PS + lime), (6) PS combined with AS (PS + AS), (7) CS, (8) CS combined with lime (CS + lime), and (9) CS combined with AS (CS + AS), each with three replications. Ca2+ and magnesium (Mg2+) are two important cations for amendments to exchange with H+ and Al3+ and thus ameliorate subsoil acidity. Thus, the application rates of lime, AS, and PS were based on similar quantities of 25 mmolc kg−1 of Ca2+ or the sum of Ca2+ and Mg2+ together applied in the surface soil, and this application rate of lime was chosen in order to increase the soil pH to about 6.0 based on the preliminary study. The application rate of CS was equal to that of PS. The detailed application rates of amendments (all g kg−1) were as follows: (2) lime 1.25, (3) AS 1.6, (4) PS 20, (5) PS + lime 20 + 1.25, (6) PS + AS 20 + 1.6, (7) CS 20, (8) CS + lime 20 + 1.25, and (9) CS + AS 20 + 1.6. AS was obtained from ammonia–alkali production of sodium carbonate, with major chemical components being calcium sulfate and calcium carbonate. PS and CS were obtained locally in Langxi County, oven-dried at 80 °C, and ground to pass a 1-mm sieve. Lime was the analytic agent CaCO3.
Chemical analysis for amendments
The acid neutralization capacity of lime and AS was determined by titrating their suspensions to pH 5.0 with 0.1 M H2SO4 (Wong et al. 1998). AS was digested by alkaline fusion (Li2CO3–H3BO3) to determine the contents of major elements. Potassium (K), sodium (Na), calcium (Ca), magnesium (Mg ), phosphorus (P), and sulfur (S) were measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES, VISTA-MPX, Varian, USA) and Cl− by potentiometric titration with silver nitrate (Li et al. 2010). The ash alkalinity of the plant materials was determined using a modified titration method (Slattery et al. 1991). Two grams of the ground plant material was heated at 200 °C for 1 h and then at 500 °C for 4 h in a muffle furnace. The ash of the sample was dissolved in 25 mL of 1.0 M HNO3. One part of the solution was titrated against a standardized solution of 0.25 M NaOH to obtain the ash alkalinity. Another part of the solution was used to measure Ca, Mg, K, Na, P, and S with ICP-AES and Cl with potentiometric titration as for the methods provided above. Total carbon (C) and nitrogen (N) of the crop residues were determined using the Leco CN-2000 analyzer (Leco Corp., St. Joseph, Mich., USA) at 1300 °C (Wright and Bailey 2011). The chemical properties of lime, AS, and crop straws are presented in Table 2.
Column preparation
Polyvinyl chloride (PVC) columns (diameter 7.8 cm and height 50 cm) were used in the leaching experiment (Fig. 1). The PVC columns consisted of two equal halves taped together with a PVC plate used as the bottom. A hole of 4-mm internal diameter in the middle of the bottom plate was used to collect leachates from the columns in 1-L plastic bottles that stood on the ground below the columns. A small hole was made in each bottle cap with size just suitable for the tube, which connected bottles and columns (to minimize water evaporation during collection). Paraffin wax was applied on the soil–tube interface to avoid preferential flow between the PVC cylinder and the soil. A filter paper and a plastic mesh were attached at the bottom of each column to avoid soil losses. Then, 2-cm depth at the bottom of the columns was filled with acid-washed SiO2, and a plastic mesh was placed above this layer. Next, untreated subsoil was packed over the SiO2 layer to a bulk density of 1.25 g cm−3 in three increments of 10 cm, totaling 30 cm (1795 g of air-dried). Above this subsoil layer, topsoil (574 g air-dried) with amended materials was packed and compressed to 10-cm thickness and a bulk density of 1.2 g cm−3. The treated amendments were thoroughly mixed with topsoil (10 cm). Each layer (10 cm) was respectively slowly wetted to adjust to 80 % of the soil water-holding capacity at the filling time. The top part of column was filled with acid-washed SiO2 in a 2-cm layer and a filter paper placed over it to induce uniform water distribution and avoid water evaporation loss.
Irrigation and leachate analysis
To simulate amelioration effect in the season under actual field conditions, all soil columns were allowed 15 days for reaction of amended materials with soil before the first leaching. The leaching experiment was arranged in a laboratory with summer air temperature (average day 35 °C and night 25 °C in Nanjing, China) in a completely random design with three replications. During the experiment, water was applied to the top of the columns based on the average annual rainfall for the district in the previous 10 years. During the period of leaching, each column received a total of 1632 mm of simulated rainfall divided into ten events in 3 months. Ten milliliters of deionized water was added to each column in 1 h with a peristaltic pump, and the water flow rate was set enough fast but ensured that no water retention occurred on the surface of soil column based on the preliminary experiment. Each leaching event included 78 h of irrigation (780 mL water) and a 9-day interval. The leachates were collected each event and split into two samples. Subsample of leachate was used to measure electrical conductivity (EC) and pH immediately after collection, and the remaining sample was kept in a freezer (−20 °C) until subsequent laboratory analyses for the measurements of Ca, Mg, K, Na, Al, SO4-S, Cl, ammonium (NH4 +)-N, (NO3 - + NO2 -)3-N, and total organic carbon (TOC). Concentration of Ca and Mg in the leachates was analyzed by atomic adsorption spectrometry (AAS, novAA 350, Analytik Jena AG, Germany) and the K and Na by flame photometry (FP640, Aopu, Shanghai, China). (NH4 +)-N, (NO3 − + NO2 −)-N, and Cl− were analyzed by UV spectrophotometer, Al and SO4-S were measured by ICP-AES, and TOC was analyzed by TOC analyzer (Shimadzu, TOC-500).
Soil sampling and analysis
At the end of the leaching experiment, soil columns were separated into two parts from the taped places and were sectioned immediately to seven layers (0–10, 10–15, 15–20, 20–25, 25–30, 30–35, and 35–40 cm) by cutting. The soil samples were air-dried, ground, and passed through 60-mesh sieve for subsequent analysis. Soil pH was measured in a 1:2.5 soil/water suspension. Soil exchangeable base cations were extracted with 1 M NH4OAc. Ca2+ and Mg2+ in the extractants were measured by AAS and K+ and Na+ by flame photometry. Soil exchangeable acidity was extracted with 1 M KC1 and then titrated by 0.01 M NaOH to pH 7.0 (Pansu and Gautheyrou 2006).
Statistical analyses
SPSS 15.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. One-way analysis of variance was used to determine differences of soil chemical properties among treatments. Least significant difference test was performed to determine significant differences of each soil property among treatments at every depth level.
Results and discussion
Chemical composition of leachates
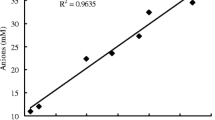
The chemical compositions of leachates in different treatments are presented in Table 3. The surface application of amendments significantly increased the leaching of cations and anions along the soil profile. The magnitudes of amendments in promoting the leaching of ions were in the following order: CS > PS ≈ AS > lime in the sole amendment treatments and CS + AS > PS + AS > CS + lime > PS + lime in the combined treatments. Therefore, CS was more effective than PS in promoting the leaching of base cations and Al3+ along the soil profile, whether in sole application or in combination with either lime or AS. Sole applied PS, CS, and AS were less effective than their respective combined amendments in promoting the leaching of base cations and Al3+ along the soil profile. The cation leaching pattern can be explained by the principle of electrical neutrality; a cation cannot move down the soil profile unless accompanied by an equivalent anion (Pavan et al. 1984; Cahn et al. 1993). Little phosphate was detected in the leachates due to the strongly specific adsorption of this anion by the Ultisol (Gimsing et al. 2007). The total amounts of inorganic anions (SO4 2−, Cl−, and NO3 −) and cations (Ca2+, Mg2+, K+, Na+, NH4 +, and Al3+) in the leachates showed a good linear relationship, with correlation coefficient (R 2) of 0.9256 (Fig. 2), suggesting that these inorganic anions were primarily responsible for the leaching of cations along the soil profile. Among the anions leached out, SO4 2− made the greatest contribution to the leaching of cations, followed by Cl−, and NO3 − showed the least contribution in each treatment (Table 3). Liu and Hue (2001) also found that downward Ca movement was mainly assisted by SO4 2− when surface-applied compost was used to ameliorate subsoil acidity. The amounts of SO4 2− and Cl− leached out followed the same order as the quantities of these anions applied in different amendment treatments, suggesting that amendments with higher contents of SO4 2− and Cl− were better for ameliorating subsoil acidity.
The highest ECs of the leachates (Fig. 3) were in the second event, generally followed by the fourth event in different treatments. Since EC primarily represents the concentration of ions in solution, this indicates that high concentrations of cations and anions were leached out mainly in the second and fourth leaching events. The relationships between total cations and total inorganic anions or TOC in the second and fourth leachates (Fig. 4) indicated that total cations were correlated well with inorganic anions (R 2 = 0.8652) but correlated poorly with TOC (R 2 = 0.2176). The results demonstrated that inorganic anions (SO4 2−, Cl−, and NO3 −) were predominant in promoting the movement and leaching of cations out of the soil profile, while organic anions played minor roles in the moving and leaching of cations. This demonstrated that when crop straws were surface applied to soil, most organic anions released from the decomposition of crop residues were primarily either adsorbed by the Ultisol in the column or completely decomposed into carbon dioxide before leaching out of the soil profile. These results differ from those obtained with crop residues continuously leached in a few days or with fulvate and humate (Liu and Hue 2001; Peiris et al. 2002). When surface-applied crop residues were subjected to continuous leaching over a few days, crop residues were difficult to completely decompose. Fulvate and humate were also not easily decomposed. Thus, organic anions in the previous studies contributed greatly to the leaching of cations along the soil profiles (Liu and Hue 2001; Peiris et al. 2002).
Soil pH in the profile
Figure 5 shows the effect of surface application of sole PS, CS, AS, and lime and their combined application on soil pH in the Ultisol profile. The amendments increased the soil pH in the Ultisol profile to different degrees, and the pH increments decreased from surface horizon to 40-cm soil depth. Sole application of amendments significantly increased the soil pH from 5.05 for control to 5.50 for PS, 5.29 for CS, 5.67 for AS, and 5.74 for lime in the layer of 0–10 cm (P < 0.05). The magnitude of amendments in increasing soil pH followed the order: lime > AS > PS > CS, which was generally in agreement with the alkalinity added in the treatments. The amount of alkalinity (calculated from the application rate and acid neutralization capacity for lime and AS, and ash alkalinity for crop straws) added in the amendments was 14.36, 7.55, 10.62, and 6.31 mmol for lime, AS, PS, and CS, respectively. These results suggested that alkalinity in the amendments was the major factor determining the efficiency of crop straws and inorganic amendments in ameliorating surface soil acidity (Sakala et al. 2004; Wang et al. 2009). One exception was that AS was more effective than PS in increasing soil pH in the layer of 0–10 cm, even though less alkalinity was added in AS than PS. Increases in soil pH induced by crop residues are mainly attributed to the decarboxylation of organic acids/anions and the association of H+ with organic anions and other negatively charged chemical functional groups in the crop straws (Yan et al. 1996; Butterly et al. 2011; Rukshana et al. 2011). Specific adsorption of organic anions and SO4 2− also benefits the pH increase due to the ligand exchange between OH− and these anions (Barrow 1986; Yu 1997). The ash alkalinity for plant material is defined as the sum of cations minus the sum of inorganic anions, and the excess positive charge is balanced by organic anions and is used to estimate the liming potential of a residue. Thus, the ash alkalinity in crop straws only represents the potential alkalinity to neutralize soil acidity but is not available under all conditions (Sakala et al. 2004). Their ameliorating effects are markedly affected by initial soil pH (Xu et al. 2006).
In the layer of the 10–20-cm depth, surface application of PS, CS, and AS also significantly increased soil pH (P < 0.05), while lime significantly increased soil pH only at 10–15 cm (P < 0.05). For example, the soil pH was 4.71, 4.78, 4.88, 4.87, and 4.88 in the control, lime, PS, CS, and AS treatments at the 15–20-cm depth, respectively. Lime had little effect in increasing soil pH at 20–40 cm compared to controls. Crop straws and AS also increased the soil pH at 20–40 cm, and CS was the most effective among the different treatments (P < 0.05). These results suggested that the amelioration effect of lime had little effect on subsoil below 15 cm. This is due to the lime-released OH− and HCO3 − ions being quickly neutralized by topsoil acidity (Hue and Licudine 1999; Liu and Hue 2001; Brown et al. 2008). In the PS and CS treatments, SO4 2− and organic anions, released from crop residue decomposition, could move into the subsoil and be specifically adsorbed by the Ultisol, which replaced OH− in neutralizing soil acidity (Barrow 1986; Sumner et al. 1986; Alva et al. 1990). Decarboxylation of organic acids/anions and the association of H+ with organic anions were beneficial for pH increase (Yan et al. 1996; Butterly et al. 2011, 2013; Rukshana et al. 2011). SO4 2− released from dissolution of CaSO4 in AS also had such a “liming effect” on subsoil acidity (Li et al. 2010).
When crop straws were surface applied in combination with lime or AS, they had more effect than the respective sole applications on soil pH in the Ultisol profiles, especially at the 0–20-cm depth (P < 0.05). Since higher content of alkalinity was added in combined amendment treatments than the respective sole treatments, this led to larger pH increases in surface and subsurface soils in the combined amendment treatments. The CS was more effective than PS in increasing soil pH at the 20–40-cm depth when these two crop straws were surface applied in combination either with lime or AS (P < 0.05). These results suggested that the property differences between CS and PS were responsible for the pH variations. Higher contents of S and Cl in the CS might have promoted more base cations to move downward to the subsoil, which exchanged with the exchangeable H+ and Al3+, and also induced more Al to leach out of the soil profile. This was supported by the observed results presented in Tables 2 and 3 and Figs. 6 and 7. Higher contents of S in the CS benefited the more specific adsorption of SO4 2− and displaced more OH− in the subsoil (Sumner et al. 1986; Alva et al. 1990). Both mechanisms were responsible for the greater pH increase in subsoil in CS treatments compared with PS treatments.
Soil exchangeable acidity in the profile
Surface-applied amendments decreased the exchangeable acidity in the soil profile to different degrees, and the amelioration effect was reduced with increasing soil depth (Fig. 6). The effect of amendments in decreasing exchangeable acidity was generally consistent with their effect in increasing soil pH. The CS showed the least effect in decreasing exchangeable acidity in soil at the 0–10-cm depth since CS had the lowest alkalinity among the different treatments. Low alkalinity resulted in a weak ability of CS in neutralizing soil acidity. However, at the 15–40-cm depth, CS was the most effective in decreasing soil exchangeable acidity, followed by PS and AS, and lime had little effect. For example, the soil exchangeable acidity was 52.6, 46.3, 48.2, 47.9, and 51.6 mmolc kg−1 in the control, CS, PS, AS, and lime treatments at the 20–25-cm depth, respectively. When crop straws were surface applied in combination with lime or AS, all the combined amendments significantly decreased the soil exchangeable acidity in the soil profile (P < 0.05). All the combined amendments had a similar effect in decreasing the exchangeable acidity at the 0–25-cm depth, but PS + lime was less effective than PS + AS, CS + lime, and CS + AS (P < 0.05). CS was also more effective than PS in significantly decreasing the exchangeable acidity at the 25–40-cm depth when these two crop straws were surface applied in combination with either lime or AS (P < 0.05). The amendments acted mainly through specific adsorption of SO4 2− and organic anions to release OH−, which induced neutralization of exchangeable acidity and the precipitation of exchangeable Al in the subsoil (Sumner et al. 1986; Alva et al. 1990; Li et al. 2010). The SO4 2−, Cl−, and even organic anions in the amendments induced base cations to leach downward to subsoil and exchange with exchangeable Al3+ and H+. These anions also promoted the exchangeable Al to leach out of the soil profile (Table 3). There were good relationships between SO4 2− (or Cl−) and Ca, Mg, K, Na, or Al (all with R 2 > 0.75), which supported the hypothesis that SO4 2− and Cl− made a significant contribution in ameliorating subsoil acidity. Therefore, AS was more effective than lime in ameliorating subsoil acidity due to higher contents of SO4 2− and Cl− in AS compared to lime. The CS treatment contained highest content of SO4 2− and Cl− and induced the most effective amelioration effect on subsoil acidity among the sole amendment treatments. For the same reason, there were greater amelioration effects of the combined amendments as compared to respective sole amendment treatments.
Soil exchangeable base cations in the profile
The surface application of lime significantly increased the exchangeable base cations only at the 0–15-cm depth (P < 0.05) but decreased the exchangeable base cations at the 30–40-cm depth (Fig. 7). All the amendments increased the exchangeable base cations at the 0–10-cm depth in the following order: PS > lime > AS > CS. This was generally in agreement with the quantities of base cations added in each treatment. The quantity of base cations added in each column was 31.9, 25.0, 25.6, and 20.7 mmolc kg−1 for PS, lime, AS, and CS, respectively. However, although the smallest quantity of base cations was supplied in the CS treatment, CS was more effective than PS and AS in increasing the exchangeable base cations at the 15–40-cm depth (P < 0.05). When crop straws were surface applied in combination with lime or AS, they had more effect than the respective sole applications on soil exchangeable base cations in the Ultisol profiles. Moreover, at the 15–40-cm depth, CS + AS was more effective than the other combined treatments in increasing exchangeable base cations in the subsoil, which was mainly due to the higher contents of SO4 2− and Cl− in CS + AS treatment. The high contents of anions promoted the moving and leaching of base cations into the subsoil (Table 3) and a subsequent exchange with the exchangeable acidity (Carvalho and van Raij 1997; Farina et al. 2000; Liu and Hue 2001).
The variation of exchangeable Ca2+, Mg2+, and K+ in the profile was dependent not only on absolute amounts and relative ratios of these cations in the amendments, but also on amounts and properties of accompanying anions. Surface-applied amendments significantly increased the exchangeable Ca in the soil profile, except lime which only significantly (P < 0.05) increased the exchangeable Ca2+ at the 0–15-cm depth (Table 4). SO4 2−, Cl−, and even organic anions promoted the Ca2+ in the amendments to move downward to the subsoil in treatments of crop straw or AS, while the effect of lime was confined to the surface and subsurface soils, as no stable anions could accompany the Ca2+ (Carvalho and van Raij 1997; Sun et al. 2000). Combined use of the crop straws with lime or AS induced more increase in soil exchangeable Ca2+ at the 0–30-cm depth, because more SO4 2−, Cl−, and even organic anions in the combined treatments also promoted more Ca2+ to move downward to the subsoil (Table 3). There was no evident difference between the combined amendment treatments (Table 4).
The surface application of lime had little effect on the exchangeable Mg2+ in the soil profile (Table 4). Surface-applied PS, CS, and AS increased the exchangeable Mg2+ at the 0–10-cm depth. PS and AS induced little increase in exchangeable Mg2+ at the 15–40-cm depth, while CS gradually increased the exchangeable Mg2+ as the depth increased (15–40 cm). There was a similar effect on Mg2+ distribution in the Ultisol profile for the CS and CS + lime treatments. The CS and lime both contained little Mg but had high contents of Ca. Ca2+ released from CS or lime would exchange with soil exchangeable Mg2+ and promoted the downward movement of exchangeable Mg2+ to deeper layers (Pavan et al. 1984; Liu and Hue 2001). Surface application of CS + AS, PS + lime, and PS + AS significantly increased the exchangeable Mg in the entire Ultisol profile (P < 0.05). PS + AS was more effective than CS + AS and PS + lime in increasing the exchangeable Mg2+ at the 0–15-cm depth, since more Mg was supplied in PS + AS. The difference in exchangeable Mg2+ among the combined amendment treatments was not significant at the 15–40-cm depth.
Surface application of PS, CS, lime, and AS also induced variations of exchangeable K+ in the Ultisol profile (Table 4). Surface application of lime decreased the exchangeable K+ at the 0–25-cm depth because the Ca2+ released from lime exchanged with soil exchangeable K+ and promoted the leaching of K+ from topsoil downward to the subsoil (Mclay et al. 1994; Sun et al. 2000). AS had little effect on exchangeable K+ in the Ultisol profile. PS and CS significantly increased the exchangeable K+ at the 0–25-cm depth but had little effect at the 25–40-cm depth. Crop residues combined with lime and AS increased the exchangeable K+ at the 0–25-cm depth for CS + AS treatment and at the 0–20-cm depth for the other combined treatments. However, the PS + lime, PS + AS, and CS + lime treatments induced significant decreases in exchangeable K+ at the 30–40-cm depth because high and unbalanced ratio of Ca2+ and K+ supplied in these treatments promoted the loss of K+ in the subsoil (Mclay et al. 1994; Sun et al. 2000). Therefore, the combined application of CS + AS appears the best with optimal/balanced quantities of exchangeable Ca2+, Mg2+, and K+ levels and improved soil fertility in the Ultisol profile.
Conclusions
Increased pH and exchangeable base cations and decreased exchangeable acidity in the whole soil profile were observed when crop straws and AS were used to ameliorate soil acidity in an Ultisol profile. However, lime had little amelioration effect on subsoil acidity. Specific adsorption of SO4 2− or organic anions, decarboxylation of organic acids/anions, and the association of H+ with organic anions induced a liming effect of crop residues and AS on subsoil acidity. SO4 2− and Cl− were the major anions of amendments that promote base cations to move downward to subsoil and exchange with exchangeable Al3+ and H+. These anions also promoted some exchangeable Al3+ leaching out of the soil profile. CS combined with AS is suggested as a potential amendment to correct soil acidity and improve soil fertility in the whole Ultisol profile.
References
Alva AK, Sumner ME, Miller WP (1990) Reactions of gypsum or phosphogypsum in highly weathered acid subsoils. Soil Sci Soc Am J 54:993–998
Barrow NJ (1986) Reaction of anions and cations with variable charge soils. Adv Agron 38:183–230
Bending GD, Turner MK, Jones JE (2002) Interactions between crop residue and soil organic matter quality and the functional diversity of soil microbial communities. Soil Biol Biochem 34:1073–1082
Brown TT, Koenig RT, Huggins DR, Harsh JB, Rossi RE (2008) Lime effects on soil acidity, crop yield, and aluminum chemistry in direct-seeded cropping systems. Soil Sci Soc Am J 72:634–640
Butterly CR, Kaudal BB, Baldock JA, Tang C (2011) Contribution of soluble and insoluble fractions of agricultural residues to short-term pH changes. Eur J Soil Sci 62:718–727
Butterly CR, Baldock JA, Tang C (2013) The contribution of crop residues to changes in soil pH under field conditions. Plant Soil 366:185–198
Cahn MD, Bouldin DR, Cravo MS (1993) Amelioration of subsoil acidity in an Oxisol of the humid tropics. Biol Fertil Soils 15:153–159
Carvalho MCS, van Raij B (1997) Calcium sulphate, phosphogypsum and calcium carbonate in the amelioration of acid subsoils for root growth. Plant Soil 192:37–48
Fageria NK, Baligar VC (2008) Ameliorating soil acidity of tropical oxisols by liming for sustainable crop production. Adv Agron 99:345–399
Farina MPW, Thibaud GR, Channon PA (2000) A comparison of strategies for ameliorating subsoil acidity. II. Long-term soil effects. Soil Sci Soc Am J 64:652–658
Gimsing AL, Szilas C, Borggaard OK (2007) Sorption of glyphosate and phosphate by variable-charge tropical soils from Tanzania. Geoderma 138:127–132
Haynes RJ, Mokolobate MS (2001) Amelioration of Al toxicity and P deficiency in acid soils by additions of organic residues: a critical review of the phenomenon and the mechanisms involved. Nutr Cycl Agroecosyst 59:47–63
Hue NV, Licudine DL (1999) Amelioration of subsoil acidity through surface application of organic manure. J Environ Qual 28:623–632
Inoue K, Kondo S, Tamano Y, Yokota H (2001) Amelioration of subsoil acidity in a nonallophanic andosol by surface application of organic calcium salts. Soil Sci Plant Nutr 47:113–122
Li JY, Wang N, Xu RK, Tiwari D (2010) Potential of industrial byproducts in ameliorating acidity and aluminum toxicity of soils under tea plantation. Pedosphere 20:645–654
Li JY, Liu ZD, Zhao AZ, Xu RK (2014) Microbial and enzymatic properties in response to amelioration of an acidic Ultisol by industrial and agricultural by-products. J Soil Sediment 14:441–450
Liu J, Hue NV (2001) Amending subsoil acidity by surface applications of gypsum, lime, and composts. Commun Soil Sci Plant Anal 32:2117–2132
Masud MM, Li JY, Xu RK (2014) Use of alkaline slag and crop residue biochars to promote base saturation and reduce acidity of an acidic Ultisol. Pedosphere 24:791–798
Masud MM, Li JY, Xu RK (2015) Application of alkaline slag and phosphogypsum for alleviating soil acidity in an Ultisol profile: a short-term leaching experiment. J Soil Sediment 15:365–373
McLay CDA, Ritchie GSP, Porter WM, Cruse A (1994) Amelioration of subsurface acidity in sandy soils in low rainfall regions. II. Changes to soil solution composition following the surface application of gypsum and lime. Aust J Soil Res 32:847–865
Oates KM, Caldwell AG (1985) Use of by-product gypsum to alleviate soil acidity. Soil Sci Soc Am J 49:915–918
Ogbodo EN (2011) Effect of crop residue on soil chemical properties and rice yield on an Ultisol at Abakaliki, Southeastern Nigeria. World J Agric Sci 7:13–18
Pansu M, Gautheyrou J (2006) Handbook of soil analysis-Mineralogical, organic and inorganic methods. Springer-Verlag, Berlin
Pavan MA, Bingham FT, Pratt PF (1984) Redistribution of exchangeable calcium, magnesium, aluminum following lime or gypsum applications to a Brazilian Oxisol. Soil Sci Soc Am J 48:33–38
Peiris D, Patti AF, Jackson WR, Marshall M, Smith CJ (2002) The use of Ca-modified, brown-coal-derived humates and fulvates for treatment of soil acidity. Aust J Soil Res 40:1171–1186
Qafoku NP, Van Ranst E, Noble A, Baert G (2004) Variable charge soils: their mineralogy, chemistry and management. Adv Agron 84:159–215
Rukshana F, Butterly CR, Baldock JA, Tang C (2011) Model organic compounds differ in their effects on pH changes of two soils differing in initial pH. Biol Fertil Soils 47:51–62
Sakala GM, Rowell DL, Pilbeam CJ (2004) Acid–base reactions between an acidic soil and plant residues. Geoderma 123:219–232
Shainberg I, Sumner ME, Miller WP, Farina MWP, Pavan MA, Fey MV (1989) Use of gypsum on soils: a review. Adv Soil Sci 9:1–111
Slattery WJ, Ridley AM, Windsor SM (1991) Ash alkalinity of animal and plant products. Aust J Exp Agric 31:321–324
Sposito G (1996) The environmental chemistry of aluminum, 2nd edn. CRC Press, Boca Raton
Sumner ME, Shahandeh H, Bouton J, Hammel J (1986) Amelioration of an acid soil profile through deep liming and surface application of gypsum. Soil Sci Soc Am J 50:1254–1258
Sun B, Poss R, Moreau R, Aventurier A, Fallavier P (2000) Effect of slacked lime and gypsum on acidity alleviation and nutrient leaching in an acid soil from southern China. Nutr Cycl Agroecosyst 57:215–222
Tang C, Rengel Z, Diatloff E, Gazey C (2003) Responses of wheat and barley to liming on a sandy soil with subsoil acidity. Field Crops Res 80:235–244
Wang N, Li JY, Xu RK (2009) Use of various agricultural by-products to study the pH effects in an acid tea garden soil. Soil Use Manag 25:128–132
Wang L, Butterly CR, Yang XL, Wang Y, Herath HMSK, Jiang X (2012) Use of crop residues with alkaline slag to ameliorate soil acidity in an Ultisol. Soil Use Manag 28:148–156
Wong MTF, Nortcliff S, Swift RS (1998) Method for determining the acid ameliorating capacity of plant residue compost, urban waste compost, farmyard manure, and peat applied to tropical soils. Commun Soil Sci Plant Anal 29:2927–2937
Wright AF, Bailey JS (2011) Organic carbon, total carbon, and total nitrogen determinations in soils of variable calcium carbonate contents using a Leco CN-2000 dry combustion analyzer. Commun Soil Sci Plant Anal 32:3243–3258
Xu RK, Zhao AZ, Ji GL (2003) Effect of low-molecular-weight organic anions on surface charge of variable charge soils. J Colloid Interface Sci 264:322–326
Xu JM, Tang C, Chen ZL (2006) The role of plant residues in pH change of acid soils differing in initial pH. Soil Biol Biochem 38:709–719
Yan F, Schubert S, Mengel K (1996) Soil pH increase due to biological decarboxylation of organic anions. Soil Biol Biochem 28:617–624
Yu TR (1997) Chemistry of variable charge soils. Oxford University Press, New York
Yuan JH, Xu RK, Qian W, Wang RH (2011) Comparison of the ameliorating effects on an acidic Ultisol between four crop straws and their biochars. J Soil Sediment 11:741–750
Acknowledgments
This study was supported by the National Key Basic Research Program of China (2014CB441003 and 2014CB441001) and the National Natural Science Foundation of China (41271010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Jiu-yu Li and M. M. Masud contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, Jy., Masud, M.M., Li, Zy. et al. Amelioration of an Ultisol profile acidity using crop straws combined with alkaline slag. Environ Sci Pollut Res 22, 9965–9975 (2015). https://doi.org/10.1007/s11356-015-4176-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4176-5