Abstract
The present study examined the response of zebrafish embryos exposed to different concentrations (10, 20, 30, 40, 50, and 60 mg/L) of monocrotophos under static conditions for 96 h. We found that mortality had occurred within 48 h at all test concentrations, later insignificant mortality was observed. Monocrotophos (MCP) can be rated as moderately toxic to the Zebrafish embryos with a 96-h median lethal concentration (LC50) of 37.44 ± 3.32 mg/L. In contrast, it greatly affected the development of zebrafish embryos by inducing several developmental abnormalities like pericardial edema, altered heart development, spinal and vertebral anomalies in a concentration-dependent manner. A significant percent reduction in length by 9–48 % and heart beats by 18–51 % was observed in hatchlings exposed to LC10 and LC50 concentrations at 96 h when compared to controls. The process of looping formation of heart at embryonic stage was greatly affected by the LC50 concentration of MCP. The neurotoxic potentiality of MCP was assessed by using a marker enzyme, acetylcholinesterase in both in vitro and in vivo experiments. MCP was found to be the most potent inhibitor of AChE in vitro with an IC50 value of 4.3 × 10−4 M. The whole-body AChE enzyme activity in vivo was significantly inhibited during the exposure tenure with the maximum inhibition of 62 % at 24 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Monocrotophos (MCP) is a highly hazardous, systemic, and a less persistent organophosphate insecticide, which is widely used in agriculture and animal husbandry (Gilbert 2009). It controls a range of pests from aphids to caterpillars, mites, moths, stem borers, and locusts on various crops such as cotton, rice, and sugarcane. The use of MCP has resulted in serious consequences that frequently linked to fatal poisoning, both accidental and intentional. The Food and Agriculture Organization (FAO) and WHO have encouraged countries to phase out this highly hazardous pesticide. Monocrotophos is registered for use in approximately 60 countries and currently banned in several countries, and its import is illegal in at least 46 countries (WHO 2013). However, in India, monocrotophos is banned for use on vegetables since 2005 and is under “restricted use” category (Nair and Rathod 2013). Its use on crops such as cotton, paddy, maize, pulses, sugarcane, coconut, and coffee is still allowed to keep in view of its bio-efficacy and cost effectiveness. The demand and production of monocrotophos in India have enormously increased from 4,877 to 7,176 t during 2007 to 2010, respectively (MOSPI 2014). It is accounted for about 4 % of total pesticide and 7 % of production. Monocrotophos is soluble in water and easily gains entry into the wastewater generated during its manufacture. The surface runoff from agricultural fields plus their seepage into groundwater pollutes natural waters and finally enters the aquatic food chain (Shayeghi et al. 2007; Vryzas et al. 2009; Werimo et al. 2009; Arjmandi et al. 2010). Earlier studies on the acute toxicity of monocrotophos on juveniles of Channa marulius and Cyprinus carpio showed that it is moderately toxic with LC50 values of 28.52 and 72.26 mg/L, respectively (Anna Mercy et al. 2001; Davoodi and Abdi 2012). The prolonged exposure of monocrotophos on Clarias gariepinus has a significant reduction effect on weight gain and specific growth rate ratio (Yaji and Auta 2007). Monocrotophos exert toxic effects on the reproductive system of teleost fishes by changing the balance of sex steroid hormones (Wang et al. 2015), modulates the expression of sexual differentiation genes, and causes phenotypic feminization in zebrafish (Zhang et al. 2013a) and disruption of hypothalamic-pituitary-thyroid axis in male goldfish, Carassius auratus (Zhang et al. b).
Organophosphorous (OP) insecticides are known to inhibit acetylcholinesterase, which plays an important role in neurotransmission at cholinergic synapses by rapid hydrolysis of the neurotransmitter acetylcholine to choline and acetate (Kavitha and VenkateswaraRao 2007; Mahaboob and Annappa 2012; Nagaraju and Rathnamma 2013). A number of studies were conducted on the toxicity of MCP on different organisms and found it as a potent neurotoxicant (Qadri et al. 1994; Venkateswararao et al. 2001; VenkateswaraRao 2004).
The zebrafish has been widely used as a prominent vertebrate model organism in different fields because of its small size, low cost, diversified adaptability, short breeding cycle, and high fecundity (Dai et al. 2014). Furthermore, the transparency of the chorionic membrane which allows stepwise developmental visualization of zebrafish makes it ideal for studying both acute and chronic effects of pesticides under standard microscopic analysis. In addition, these embryos have extensively been used in the field of developmental biology to assess the pesticide toxicity (Hill et al. 2005).
Most previous studies have suggested that OP insecticides could induce developmental toxicity in larval zebrafish. Embryos exposed to malathion resulted in significantly shorter body length and eye diameters (Cook et al. 2005), chlorpyrifos, diazinon, and parathion caused acetylcholinesterase inhibition (Yen et al. 2011), behavioral impairments, and developmental abnormalities by DDVP (Sisman 2010), an oxygen analogue of chlorpyrifos disrupted the zebrafish axonal growth and motor behavior (Yang et al. 2011). Acetylcholinesterase (AChE) is critical to the normal development of the zebrafish nervous system and cardiac function (Behra et al. 2002) therefore AChE inhibitors like MCP are particularly relevant for studying vertebrate development. Nonetheless, extensive studies on the toxic effects of monocrotophos on embryonic development of species representing the aquatic system are lacking. Therefore, the present investigation was carried out to find out how significantly the monocrotophos can cause toxicity on developmental parameters like hatching rate, spine, body length, heartbeat, heart formation, and craniofacial and eye formation of the zebrafish upon exposure to its different levels of concentration. In addition, the effects of monocrotophos on the embryonic AChE enzyme activity of developing zebrafish embryos have been analyzed. The occurrence of these malformations induced by MCP was hypothetically correlated with genes or enzymes expression.
Materials and methods
Test chemical and zebrafish maintenance
The test compound used, monocrotophos (CAS No.6923-22-4) was synthesized at the Indian Institute of Chemical Technology and was of 99 % purity. The zebrafish species, Danio rerio (order: Cypriniformes, family: Cyprinidae) were obtained from a local pet store and maintained in glass aquariums (60 × 30 × 30 cm) of 40 L water capacity at laboratory conditions for more than 1 month by using 4–5 days aerated stored water. The water was aerated with a Jumbo-Jet aquarium air pump (Super-8300, made in India). The average values for the culture conditions in aquariums for zebrafish was temperature 28 ± 1 °C, pH 7.10 ± 0.05, and dissolved oxygen 8.15 ± 0.06 mg/L (APHA 1998). The natural photoperiod of 14:10 L:D hours were maintained and the zebrafish were fed with dry flakes twice per day and ad libitum with nauplii of brine shrimp (Artemia salina) once a day. Embryos exposure conditions are maintained by following OECD guidelines (OECD TG 236 2013), temperature 26 ± 1 °C, pH 7.2 ± 0.2, dissolved oxygen 8.15 ± 0.06 mg/L, and a photoperiod of 14:10 light:dark condition.
Egg production
Fertilized eggs were obtained from induced spawning of an equal number of males and females from a glass aquarium containing a breeding trap. Briefly, the day before the collection of eggs, the well-fed male and females (separated by a divider) were transferred to breeding tanks containing marbles at the bottom. On the day of the experiment, the divider was removed just before the light cycle to initiate the breeding activity (starts within 30 min) and fertilized eggs were collected from the bottom of the tank with glass pipette. The eggs were cleaned two to three washes with double distilled water.
Embryo exposure
Fertilized eggs were separated from the non-fertilized ones with a pipette using digital video microscope (HiROXco. Ltd., Japan, Model KH2200 MD2) connected to a computer-assisted video image analysis system, Ethovision-version 2.3 (Noldus Information Technology, Netherlands). Acute toxicity studies of monocrotophos on zebrafish embryos were determined in the laboratory using the static method for up to 96 h, which is similar to the OECD test guidelines (Braunbeck and Lammer 2006). The test concentrations were chosen based on the initial experiments to determine the median lethal concentration (LC50). Test solutions of the selected concentrations (10, 20, 30, 40, 50, and 60 mg/L) were maintained in 5 mL of water in 35-mm-diameter plastic petri dishes (Tarson product Pvt. Ltd., India). Two hundred fertilized embryos (4 hpf, 20 batches of 10 each) were exposed to each concentration separately. As the monocrotophos is completely soluble in water, water without toxicant was used as a control. The percent mortality of the embryos was recorded and dead embryos were removed in each concentration of the toxicant after 24, 48, 72, and 96 h. The data were used to estimate the 24, 48, 72, and 96 h median lethal concentrations (LC50) by means of probit analysis (Finney 1971).
In a separate set of experiments, 4 hpf embryos (n = 200 with 4 replicates of 50 each) were exposed to median lethal concentration (LC50) and a sublethal concentration (LC10). Developmental malformations like bradycardia, angle of curvature (measured by using a protractor) in the spine, and abnormal development of the heart were monitored at 96 hpf through a digital video microscope with a minimum of 20 individuals for each treatment and control. Digital images were used to determine the heart abnormalities and curvature in the body of effected larvae in comparison to controls. The magnification of the snaps was calibrated with the aid of ocular and stage micrometers (ERMA, Tokyo, Japan).
Acetylcholinesterase (AChE EC 3.1.1.7) activity
The whole-body AChE activity of treated (LC10 and LC50) embryos/larvae at 24, 48, 72, and 96 h along with respective controls were used to estimate the in vivo AChE activity. Similarly, the supernatants derived from unexposed larvae (96 hpf) were used to study the in vitro evaluations. A minimum of 50 embryos/larvae were collected randomly from each lot and washed twice with ice-cold PBS (pH 7.5), and were homogenized in ice-cold 0.1 M PBS (pH 7.5) containing 0.2 M NaCl, 1 % (v/v) Triton-X 100 using Potter-Elvehjam homogenizer fitted with a Teflon pestle. The homogenates were centrifuged at 5,000×g for 10 min and the supernatant was further centrifuged at 15,000×g for 10 min at Kubota (Model 6930) refrigerated centrifuge. All enzyme preparations were carried out at 4 °C. The resultant supernatants were used as the enzyme source for the estimation of AChE activity. Protein concentration was estimated by the method of Bradford (1976). AChE assays were performed spectrophotometrically by utilizing the slightly modified method of Ellman et al. (1961). Briefly, the AChE experiments were performed in a 96-well plate consisting 75 μL of 0.1 M phosphate buffer (pH 7.5), 25 μL of 0.4 mM 5,5-dithio-bis (-nitrobenzoic acid) (DTNB) and 25 μL of homogenate (0.3 mg) for each well. The reaction was initiated by adding 25 μL of the substrate, 0.2 mM acetylthiocholine iodide (ATChI) at 28 ± 1 °C, and color development was recorded continuously for 5 min at 412 nm in a spectrophotometer (Molecular Devices, USA; supported by the software, Spectro-max Plus). AChE activity was calculated as nanomoles of acetylcholine hydrolyzed per minute per milligram protein using Origin 6.0 statistical software.
To perform the in vitro AChE activity, 5 μL of each of eight different concentrations (2.24 × 10-4, 2.99 × 10-4, 3.73 × 10-4, 4.48 × 10-4, 5.23 × 10-4, 5.97 × 10-4, 6.72 × 10-4, and 7.47 × 10-4 M) of monocrotophos were mixed with 25 μL of properly diluted control enzyme and measured the AChE activity as above. The median inhibition concentration (IC50) for monocrotophos was calculated based on log-doses vs probit percent inhibition regression.
Data analysis
The median lethal concentrations (LC50) were calculated after linearization of response curves by logarithmic transformation of concentrations, 95 % confidence limits, and slope function to provide a consistent presentation of the toxicity data. The experiments were repeated three times to determine the effects of LC50 and LC10 concentrations of monocrotophos on developmental alterations. Mean and standard errors for all experimental parameters were calculated using BioStat 2008 statistical software. One-way analysis of variance (ANOVA) and the Tukey’s test (honest significant difference—HSD) were carried out to determine whether the treatments were significantly different from the control group (p < 0.01, p < 0.001).
Results
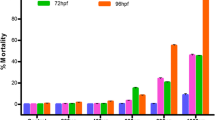
The effects of monocrotophos on zebrafish embryos, D. rerio, was concentration dependent and the percentage survival decreased with increasing concentration of pesticide. Mortality of embryos with the coagulated nuclear material occurred during 6–48 h of exposure in all the test concentrations. Moreover, the results indicated that the embryos, which survived after 48 h of exposure did not show any further mortality. The median lethal concentrations (LC50 values) were determined by probit analysis and are presented in Table 1. It is evident from the results that the monocrotophos can be rated as moderately toxic to embryos with LC50 values of 45.81 ± 4.98, 38.06 ± 3.46, 37.76 ± 3.39, and 37.44 ± 3.32 mg/L for 24, 48, 72, and 96 h, respectively.
The results indicated that the developmental abnormalities were significantly induced in a concentration-dependent manner during the embryonic development of the zebrafish from 24 h onwards and were further intensified when time of exposure progressed. The digital images of developmental abnormalities in zebrafish embryos exposed in vivo to different concentrations of monocrotophos are shown in Fig. 1. About 98 % of control embryos exhibited normal development of hatchlings inside the chorionic membrane and were fully developed within 54 ± 6 h without any deformities (Fig. 1a). However, MCP-exposed embryos exhibited several morphological defects in a concentration-dependent manner. Embryonic malformations associated with signs of fluid accumulation and edema formation in the region of yolk sac and pericardium were found in most of the embryos exposed to 10 and 20 mg/L concentrations (Fig. 1b, c). Enhanced edema observed in >50 % of embryos exposed to 30 and 40 mg/L, which subsequently developed an extraembryonic part on the surface of yolk sac with shorter tails (Fig. 1d, e). Severely underdeveloped hatchlings were observed inside the chorionic membrane in 50 mg/L concentration and showed signs of tail and spinal cord flexure and defective eye formation (Fig. 1f–g). The majority of embryos exposed to 60 mg/L concentration showed cerebral hemorrhages and/or blood clotting problems, tail truncation, and deformed head with microphthalmia (Fig. 1h, i). All the abovementioned characteristics can be easily assessed even in the unhatched embryos of zebrafish.
Malformations in zebrafish embryos during exposure to different concentrations of monocrotophos at 48 h. a Normal development of zebrafish hatchling inside the chorionic membrane. b–c Embryos exposed to 10 and 20 mg/L concentrations exhibited fluid accumulation; edema formation in the region of yolk sac and pericardium. d–e Enhanced edema formation with extraembryonic part on the surface of yolk sac with short tails observed at 30–40 mg/L concentration. f–g Severely under developed hatchlings were observed inside the chorionic membrane in 50 mg/L concentration and showed signs of tail and spinal cord flexure and defective eye formation. h–i Embryos exposed to 60 mg/L concentration displayed cerebral hemorrhage, tail truncation, and deformed head with microphthalmia
Experiments were further extended to study the prolonged morphological aberrations in free-swimming yolk sac larvae (Fig. 2). Control larvae (length 3.43 ± 0.2 mm, n = 20) had transparent bodies with straight tail and spinal cord, darkly pigmented eyes and their anterior part of the yolk sac was bulbous and its posterior part was elongated and cylindrical (Fig. 2a). The embryos exposed to 10 and 20 mg/L of MCP displayed mild pericardial edema, inward bent tail (kyphosis), and impaired lower jaw at 96 hpf (Fig. 2b). Most of the yolk sac larvae hatched from 30 mg/L concentration exhibited similar extended characteristics like kink formation in notochord region, underdeveloped tail fin (Fig. 2c), and pericardial edema with lateral curvature (scoliosis) of body (Fig. 2d, e). Nonetheless, about 32–47 % of hatched larvae from 40 mg/L showed severe pericardial and yolk sac edema, impaired lower jaw, reduced yolk sac tube, and tissue degeneration at yolk sac region after 96 hpf (Fig. 2f). However, the embryos exposed to higher concentrations (beyond 30 mg/L) of MCP exhibited induced malformations with prominent tissue degeneration at tail region; inflated swim bladder, and reduced yolk sac tube (Fig. 2g) at 96 hpf. The majority of them have outward hook-like tail (lordosis), microphthalmia, and irregular tail fin formation (Fig. 2h). These malformations were further intensified in 40 and 50 mg/L exposed embryos with hydrocephalus, severe pericardial and yolk sac edema, tissue damage in the yolk sac region, degenerative swim bladder, and ocular hypertelorism (Fig. 2i, j). All the embryos exposed to 60 mg/L exhibited stunted development, severe pericardial and yolk sac edema, and severely twisted tail with tail truncation (Fig. 2k–l).
Multiple malformations in hatchlings at 96 h exposed to monocrotophos. a Control larvae with transparent bodies with straight tail and spinal card, darkly pigmented eyes. b The embryos exposed to 10 and 20 mg/L of MCP displayed mild pericardial edema, kyphosis, and impaired lower jaw. c–e Kink formation in notochord and under developed tail fin, pericardial edema with scoliosis observed at 30 mg/L concentration. f–j Larvae from 40 and 50 mg/L concentration displayed severe pericardial and yolk sac edema, impaired lower jaw, reduced yolk sac tube, and tissue degeneration at yolk sac and tail region, inflated swim bladder, microphthalmia, reduced yolk sac tube, hydrocephalus, severe pericardial and yolk sac edema, tissue damage in yolk sac region, degenerative swim bladder, and ocular hypertelorism. k–l Embryos exposed to 60 mg/L exhibited stunted development, severe pericardial and yolk sac edema and severely twisted tail with tail truncation
In a separate set of experiments, the effects of LC10 (5.23 mg/L) and LC50 (37.44 mg/L, 96 h) concentrations on hatching percentage, body length, specific curvature, heartbeat, and heart development were assessed by visual examination using a digital video microscope (n = 10). The success of hatching percentage was calculated by using embryos that survived after 48 h exposure only. It is evident from the results that the hatching rate of embryos exposed to LC10 and LC50 concentrations of MCP was similar to controls at 96 h. However, an early hatching was started at 48 h with abnormalities in the LC50 exposed embryos followed by embryos exposed to LC10 and controls. Nevertheless, the hatching was delayed by 5 h in the control group, but at the end of exposure tenure (96 h), there was no significant difference in percent success of hatching between the control, and treated groups.
Initially, the heart of embryonic zebrafish is made up of a thin-walled tube (28–30 h), when the embryo undergoes the transition into juvenile stages, the tube expands its surface area, which circles (33–60 h) to form chambers (i.e., atrium and ventricle) that ensure blood flows in the correct direction on a regular and rhythmic way. Abnormal heart formation in embryos exposed to monocrotophos is presented in Fig. 3. In control embryos, the normal looping process places the ventricle and atrium side by side, so that the two chambers largely overlap each other in lateral view (Fig. 3a). In contrast; the hearts were stretched in the embryos treated with LC10 concentration of monocrotophos with little or practically no overlapping. Besides, the atria were thin and elongated and the ventricles appeared smaller and more condensed than controls (Fig. 3b). The process of looping formation of heart at 60–90 h of embryonic stage was greatly affected by the LC50 concentration of monocrotophos (Fig. 3c). It is evident from the results that monocrotophos exposure leads to structural malformations, altered looping, and decreased size in the heart. The mean heart beat rates in controls were 141 ± 7.6/min. It is evident from the results that both LC10 and LC50-exposed embryos have a significant percent reduction in their heart beats by 17.68 and 51.02 %, respectively (Fig. 3a, b inset). Different degrees of spinal curvature were noticed in both the test concentrations and percent number of effected larvae was counted after 96 h of exposure (Fig. 3c inset). The degree of angle of curvature was measured and divided into four groups, i.e., 0–45°, 46–90°, 91–135°, and 136–180°.
Deformities in heart formation in the hatchlings of zebrafish after 96 h exposure to MCP. a Normal development of heart in controls, b Stretched heart (side by side ventricle and atrium) without overlapping in LC10 exposed hatchlings. c Transformed looping and tube like heart formation in LC50 exposed larvae. Inset: a and b) Heart beats per minute and percent reduction of heart beats at 96 h exposure in control and treated hatchlings. Each value is the mean ± SE of ten independent observations. *** indicate the values are statistically significant at p < 0. 001 level. c Percent spinal curvature of hatchlings with different degrees of curvature exposed to LC10 and LC50 concentrations
The majority of the hatchlings emerged from LC10 exposure exhibited 0–45° angle of curvature (40 %) followed by less percentage of 46–90° (27 %), 91–135° (13 %), and 136–180° (20 %). However, most of the LC50-exposed hatchlings exhibited a gradual increase of the angle of the spine curve from 0–45° to 180°. The exact percentage of differential spine curvature was recorded in all the hatchlings, such as 15 % (0–45°), 25 % (46–90°), 29 % (91–135°), and 31 % (136–180°). The spine/body axis were twisted more in LC50 concentration and sometimes no tail or tail fin formation. It is evident from the results that the MCP prompted different degrees of curvature in the body indicating its teratogenic effects on zebrafish.
There was a significant difference in the length of hatchlings between control and treated groups (p < 0.001). The mean length of control hatchlings is 3.85 ± 0.12 mm, followed by 3.51 ± 0.15 mm in LC10 and 2.84 ± 0.39 mm in LC50 exposed/emerged hatchlings. The percent reduction by 12–14 and 45–48 % was observed in LC10 and LC50 concentrations, respectively, when compared to controls (inset Fig. 4a). About 40 % of hatchlings emerged from LC50 concentration showed severe cerebral hemorrhage (Fig. 4b) and exhibited ocular hypertelorism (Fig. 4c). The whole-body acetylcholinesterase activity in vivo was determined from the control and exposed embryos at an interval of 24, 48, 72, and 96 h by static method. It is evident from Fig. 4d that at 24 h, 29 and 62 % AChE inhibition was observed in LC10 and LC50 concentrations and as the time of exposure increased from 24–96 h, the AChE inhibition reduced to 8.65 and 32.9 %, respectively. The maximum AChE inhibition was observed at 24-h exposure, and gradual recovery was noticed as the exposure time increased.
Multiple malformations in the hatchlings after emerging from monocrotophos exposed embryos. Percent reduction in length of the hatchlings (a). Prominent effects of cerebral hemorrhage and ocular hypertelorism in hatchlings after LC50 exposure (b and c). Percent reduction in the whole-body acetylcholinesterase activity in vivo of control and exposed embryos at 24, 48, 72, and 96 h by static method (d)
The effect of the toxicant on in vitro AChE activity was also studied. The IC50 inhibition studies revealed that 4.03 × 10−4 M concentration of MCP is required to inhibit 50 % of the enzyme activity at 96 h in vitro (Fig. 5).
Discussion
The experimental data of the present study illustrate that the developing zebrafish (D. rerio) embryos are susceptible to monocrotophos. As the concentration of MCP increased, the percent mortality also increased significantly up to 48 hpf and no further mortality thereafter; it may be due to low persistence of MCP and static method of exposure. Earlier studies are also proved that the rate of embryo mortality usually occurs during the gastrulation and segmentation periods [occurring between 5 and 24 hpf (Schilling 2002)], which have previously been termed “critical windows” in the development of fish (Weis and Weis 1991; Chow and Cheng 2003; Johnson et al. 2007).
The estimated median lethal concentration of MCP on zebrafish embryos for 96 h is 37.44 ± 3.32 mg/L, which has found to be slightly toxic. Similar range of toxic concentration was reported previously in zebrafish embryos exposed to another organophosphorous insecticide, dichorovas (Sisman 2010). The LC50 concentration values on embryonic development of zebrafish (24–96 h) are comparatively higher than the 96 h LC50 values of adult Oreochromis mossambicus and Channa punctatus (VenkateswaraRao 2004; Shweta et al. 2006). One possible reason is that fish embryos have a protective envelope called a chorion, which could minimize passing of toxicants into the embryos (Lillicrap 2010) that resulted in high concentration of MCP to cause 50 % mortality in the test population. Fish embryos are relatively resistant to toxins (Ansari and Kumar 1986) than newly hatched larvae exposed to zinc sulfate (Skidmore 1965) and ammonia (Stanley and Robert 1975).
Understanding the mechanisms of OP insecticide developmental toxicity will requires much further studies, but our experiments have shed light on certain developmental abnormalities in developing zebrafish, which exposed to MCP. The responses like pericardial edema, altered heart development, and spinal and vertebral anomalies are considered as toxicity indicators for embryonic development of zebrafish. MCP exposed embryos also exhibited significantly all the above said symptoms at both 36 and 48 hpf, which persisted till the end of the exposure period. Furthermore, MCP-treated embryos showed increased frequency of spontaneous tail flexion and developmental anomalies in comparison to untreated embryos. Tail bending frequency was further enhanced in the hatchlings emerged from treated embryos. Similar abnormalities were noticed when the zebrafish embryos were exposed to nonylphenol (Tamer and Juliette 2011). However, the effects like hydrocephalus, cerebral hemorrhage, and hypertelorism (enhanced distance between the eyes in the hatchlings) (Blechinger et al. 2002; Lefebvre et al. 2004; Incardona et al. 2004) are found to be rare malformations observed during the MCP exposure. Several investigators have shown that high concentrations of organophosphates can penetrate the chorion and cause various teratogenic effects and death of fish embryos (Ansari and Ansari 2011).
The present results clearly indicate that 10 to 50 mg/L concentrations of MCP induced yolk sac and pericardial edema in a concentration-dependent manner. The probable reason for formation of edema in the exposed embryos could be due to failure of the osmoregulatory system associated with pesticide accumulation (Cook et al. 2005) or could be due to inhibition of slc2a10/glut10 or Lrrc10 genes (Willaert et al. 2012; Kim et al. 2007). In addition, MCP has induced deformities in formation of spine by producing different degrees of curvature in the exposed embryos. It may be due to the over expression of growth hormone (Carlos et al. 2010) or inhibition of col27a1a and col27a1b genes expression (Christiansen et al. 2009), or inhibition of lysyl oxidase (Snawder and Chambers 1993).
Hydrocephalus is a condition in which swelling of the head occurs with accumulation of water and this was noticed in the embryos exposed to LC50 concentration of MCP, which could be due to inhibition of CCP1/CCP5 (Lyons et al. 2013) or lgi1b genes (Yong et al. 2011). Microphthalmia and ocular hypertelorism were the other toxic effects induced by the MCP in zebrafish embryos. The retinoic acid deprivation and inhibition of Alx1 gene in developing zebrafish embryos could be the reason for microphthalmia and hypertelorism, respectively (Le et al. 2012; Dee et al. 2013).
It was worth mentioning that the MCP has promoted early hatching than the controls in a time- and concentration-dependent manner. Hatching time is purely depending on the mechanical and structural properties of the protective chorionic membrane that surrounds the embryos. The chorionic membrane is an acellular envelope made of three intercrossing layers, which allows materials (oxygen/carbon dioxide, nutrients, and excretion products) to pass through the embryo via passive diffusion (Berghmans et al. 2008). It appears that the MCP might have passed through the chorionic membrane and triggered the hatching-gland cells to release more amount of matrix metalloproteinase enzyme, which weakens the chorionic membrane and allows the embryo to break free at early stages. Similar effect on hatching was found in embryos exposed with bifenthrin; as the concentration increases, the rate of hatching also increases (Meiqing et al. 2009). In contrast, the lower concentrations of malathion elicited more rapid hatching from the chorion than higher concentrations (Cook et al. 2005).
The heart is the first organ to develop and function in zebrafish (Hill et al. 2005), and to some extent, heart rate has been used as a physiological correlate to predict the metabolic rate (such as oxygen consumption) of fish (Barrionuevo and Burggren 1999; Thorarensen et al. 1996). It is evident from the results that monocrotophos exposure at LC10 and LC50 could alter the development of heart and heart rate significantly (p < 0.0001), compared to controls. Yamauchi et al. (2005) reported that cardiac function could be affected by the malformation of the heart and pericardium, which could result in abnormal heartbeat and blood circulation failure. It is evident from our results that monocrotophos exposure leads to structural malformations, altered looping, and decreased size of the heart (Fig. 3), which resulted in a significant percent reduction in their heart beats by 17.68 and 51.02 % in LC10 and LC50 exposed hatchlings, respectively. Another reason might be that the presence of AChE inhibitors increases the acetylcholine concentration in the synaptic cleft, leading to continuous signals from the acetylcholine receptor and slowing down the heart rate (Lin et al. 2007).
The embryos exposed to MCP displayed reduction in their body lengths (10–48 %) than the controls. It could be due to an increased yolk sac area, which resulted concomitant decrease in the body lengths of hatchlings, which is quite common in metal toxicity tests (Johnson et al. 2007). Similar findings were reported in zebrafish embryos exposed to 3 mg/L concentration of malathion that reduced its body length by 83 % than control body length (Cook et al. 2005).
Apart from teratogenic effects, monocrotophos also has well-known cholinesterase-inhibiting properties. We also demonstrated the inhibitory effect of monocrotophos on AChE in early developing zebrafish embryos, i.e., under in vivo condition from 24 to 96 hpf. Concentration-dependent AChE inhibition was also observed under in vivo conditions. At LC50 concentration, higher rate of inhibition and at LC10 concentration lower rate of inhibition was observed compared to control AChE activity. Generally the percent AChE inhibition will increase with the increase of time of exposure to pesticide treatment, but in this case decreased with increase of time of exposure. The reason may be because of environmentally low persistence of MCP activity. Many experiments conducted in different animal models, including euryhaline fish (O. mossambicus), freshwater fish (C. punctatus), nematode (Caenorhabditis elegans), Daphnia magna, and a fresh water bivalve (Lamellidens marginalis), showed the ability of monocrotophos to inhibit acetylcholinesterase activity (VenkateswaraRao 2004; Shweta et al. 2006; Cole et al. 2004; Lumei et al. 2009; Mundhe and Sangeeta 2014). Under in vitro conditions, concentration-dependent AChE inhibition occurred and the IC50 concentration value is 4.03 × 10−4 M.
It is concluded from the present study that zebrafish and its early life stages are sensitive to low levels of monocrotophos in aquatic environment and significantly affect its populations. Therefore, these pesticides should be used with great caution and in a sustainable way so that it may not be hazardous to aquatic environment and human beings. Further research is warranted to investigate the molecular mechanism of MCP in the embryonic development of zebrafish.
References
Anna Mercy TV, Madhusoodana KB, Korath A, Nair JR (2001) Acute toxicity of organophosphate pesticides on the juveniles of Channa marulius Deraniyagala. Indian J Fish 48(2):227–230
Ansari S, Ansari BA (2011) Embryo and fingerling toxicity of dimethoate and effect on fecundity, viability, hatchability and survival of zebrafish, Danio rerio (Cyprinidae). World J Fish Marine Sci 3(2):167–173
Ansari BA, Kumar K (1986) Malathion toxicity: embryo toxicity and survival of hatchlings of zebrafish (Brachydanio rerio). Acta Hydrochim Hydrobiol 14:567–570
APHA (1998) Standard methods for the examination of water and waste water, 20th edn. American Public Health Association, Washington, DC
Arjmandi R, Tavakol M, Shayeghi M (2010) Determination of organophosphorus insecticide residues in the rice paddies. Int J Environ Sci Technol 7:175–182
Barrionuevo WR, Burggren WW (1999) O2 consumption and heart rate in developing zebrafish (Danio rerio): influence of temperature and ambient O2. Am J Physiol Regul Integr Comp Physiol 276:R505–R513
Behra M, Cousin X, Bertrand C, Vonesch J, Biellmann D, Chatonnet A, Strahle U (2002) Acetylcholinesterase is required forneuronal and muscular development in the zebrafish embryo. Nat Neurosci 5:111–118
Berghmans S, Butler P, Goldsmith P, Waldron G, Gardner I, Golder Z, Richards FM, Kimber G, Roach A, Alderton W, Fleming A (2008) Zebrafish based assays for the assessment of cardiac, visual and gut function—potential safety screens for early drug discovery. J Pharmacol Toxicol Methods 58:59–68
Blechinger SR, Warren JT Jr, Kuwada JY, Krone PH (2002) Developmental toxicology of cadmium in living embryos of a stable transgenic zebrafish line. Environ Health Perspect 110:1041–1046
Bradford MM (1976) Rapid and sensitive method for the quantification of micro quantities of protein utilizing the principle of protein binding. Anal Biochem 72:248–254
Braunbeck T, Lammer E (2006) Background paper on fish embryo toxicity assays. Prepared for German Federal Environment Agency
Carlos ER, Rafael YK, Daniela VA, Carlos FCL, Marcio AF, Aline GD, Duane BF, Luis FM (2010) GH over expression modifies muscle expression of anti-oxidant enzymes and increases spinal curvature of old zebrafish. Exp Gerontol 45:449–456
Chow ESH, Cheng SH (2003) Cadmium affects muscle type development and axon growth in zebrafish embryonic somitogenesis. Toxicol Sci 73:149–159
Christiansen HE, Lang MR, Pace JM, Parichy DM (2009) Critical early roles for col27a1a and col27a1b in Zebrafish notochord morphogenesis, vertebral mineralization and post-embryonic axial growth. PLoS ONE 4(12):e8481, 1-10
Cole RD, Anderson GL, Williams PL (2004) The nematode Caenorhabditis elegans as a model of organophosphorous induced mammalian neurotoxicity. Toxicol Appl Pharmacol 194:248–256
Cook LW, Christopher JP, Barbara L (2005) The pesticide malathion reduces survival and growth in developing zebrafish. Environ Toxicol Chem 24:1745–1750
Dai YJ, Jia YF, Chen N, Bian WP, Li QK, Ma YB, Chen YL, Pei DS (2014) Zebrafish as a model system to study toxicology. Environ Toxicol Chem 33(1):11–17
Davoodi R, Abdi G (2012) Comparative study on the acute toxicity of synthetic pesticides, permethrin 25% and monocrotophos 36%, and neem-based pesticide, neem gold EC 0.03%, to Juvenile Cyprinus carpio Linn. J Biol Environ Sci 6(16):105–108
Dee CT, Szymoniuk CR, Mills PE, Takahashi T (2013) Defective neural crest migration revealed by a Zebrafish model of Alx1-related frontonasal dysplasia. Hum Mol Genet 22(2):239–251
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Finney DJ (1971) Probit analysis. Cambridge University Press, London, pp 68–72
Gilbert SG (2009) A small dose of toxicology: the health effects of common chemicals. CRC Press, Second Edition, p 320
Hill AJ, Teraoka H, Heideman W, Peterson RE (2005) Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 86:6–19
Incardona JP, Collier TK, Scholz NL (2004) Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol 196:191–205
Johnson A, Carew E, Sloman KA (2007) The effects of copper on the morphological and functional development of zebrafish. Aquat Toxicol 84:431–438
Kavitha P, VenkateswaraRao J (2007) Oxidative stress and locomotorbehaviour response as biomarkers for assessing recovery status of mosquito fish, Gambusia affinis after lethal effect of an organophosphate pesticide, monocrotophos. Pestic Biochem Physiol 87:182–188
Kim KH, Antkiewicz DS, Yan L, Eliceiri KW, Heideman W, Peterson RE, Lee Y (2007) Lrrc10 is required for early heart development and function in zebrafish. Dev Biol 308(2):494–506
Le HG, Dowling JE, Cameron DJ (2012) Early retinoic acid deprivation in developing zebrafish results in microphthalmia. Vis Neurosci 29(4–5):219–228
Lefebvre KA, Trainer VL, Scholz NL (2004) Morphological abnormalities and sensorimotor deficits in larval fish exposed to dissolved saxitoxin. Aquat Toxicol 66:159–170
Lillicrap A (2010) Use of zebrafish as an alternative approach for ecotoxicity testing. M.Phil thesis, University of Exeter, UK
Lin CC, Michelle NYH, Cheng SH (2007) Toxicity and cardiac effects of carbaryl in early developing zebrafish (Danio rerio) embryos. Toxicol Appl Pharmacol 222:159–168
Lumei W, Weihong Y, Shanshan Z, Kunde L, Meirong Z, Weiping L (2009) Acute and chronic toxicity of organophosphate monocrotophos to Daphnia magna. J Environ Sci Health B 44:38–43
Lyons PJ, Sapio MR, Fricker LD (2013) Zebrafish cytosolic carboxypeptidases 1 and 5 are essential for embryonic development. J Biol Chem 288:30454–30462
Mahaboob B, Annappa P (2012) Cold stress offered modulation on Chlorpyrifos toxicity in aging Rat central nervous system. Toxicol Int 19:173–181
Meiqing J, Xiaofeng Z, Lijun W, Changjiang H, Ying Z, Meirong Z (2009) Developmental toxicity of bifenthrin in embryo-larval stages of zebrafish. Aquat Toxicol 95:347–354
MOSPI (2014) Ministry of Statistics and Programme Implementation. Production of selected items: monocrotophos (Tonnes)-India. Updated dataset information on 13 January
Mundhe AY, Sangeeta VP (2014) Assessment of toxicity of monocrotophos in fresh water bivalve, Lamellidens marginalis, using different markers. Toxicol Int 21(1):51–56
Nagaraju B, Rathnamma VV (2013) Toxicological effect of profenofos and carbosulfan on acid phosphatase and acetylcholinesterase in freshwater fish Labeo rohita. Adv Agric Sci Eng Res 3:1210–1220
Nair SS, Rathod JL (2013) Monocrotophos induced toxicity and physiological stress on fish Puntius filamentosus. (Val, 1844). IOSR J Environ Sci Toxicol Food Technol 5(5):66–70
OECD TG 236 (2013) OECD guidelines for testing of chemicals; Fish embryos acute toxicity (FET) test
Qadri YH, Swamy AN, Rao JV (1994) Species difference in brain acetylcholinesterase response to monocrotophos in vitro. Ecotoxicol Environ Saf 28:91–98
Schilling TF (2002) The morphology of larval and adult zebrafish. In: Nusslein- Volhard C, Dahm R (eds) Zebrafish, a practical approach. Oxford University Press Inc, New York, pp 59–93
Shayeghi M, Darabi H, Abtahi H, Sadeghi M, Pakbaz F, Golestaneh SR (2007) Assessment of persistence and residue of diazinon and malathion in three Rivers (Mond, Shahpour and Dalaky) of Bushehr province in 2004–2005 years. Iran South Med J 10:54–60
Shweta A, Krishna G, Pandey KC (2006) Biomarkers of monocrotophos in fresh water fish Channa punctatus (Bloch). J Environ Biol 27(2):453–457
Sisman T (2010) Dichlorvos-induced developmental toxicity in Zebrafish. Toxicol Ind Health 26(9):567–573
Skidmore JF (1965) Resistance to zinc sulphate of the zebrafish (Brachydanio rerio Hamilton-Buchanan) at different phases of its life history. Ann Appl Biol 56:47–53
Snawder JE, Chambers JE (1993) Osteolathyrogenic effects of malthion in Xenopus embryos. Toxicol Appl Pharmacol 121:210–216
Stanley DR, Robert MS (1975) Acute toxicity of ammonia to several developmental stages of rainbow trout, Salmo gairdneri. Fish Bull 73:207–211
Tamer SA, Juliette L (2011) Developmental toxicity of nonylphenol in zebrafish (Danio rerio) embryos. Indian J Mar Sci 40(4):509–515
Thorarensen H, Gallaugher PE, Farrell AP (1996) The limitations of heart rate ASA predictor of metabolic rate in fish. J Fish Biol 49:226–236
VenkateswaraRao J (2004) Effects of monocrotophos and its analogs in acetylcholinesterase activity’s inhibition and its pattern of recovery on euryhaline fish, Oreochromis mossambicus. Ecotoxicol Environ Saf 59:217–222
Venkateswararao J, Rajendra JP, Ramakrishna B (2001) Comparative insecticidal activity of profenofos and monocrotophos in relation to in vitro and in vivo acetylcholine esterase activity of the housefly, Musca domestica Linnaeus. Int Pest Cont 43:112–114
Vryzas Z, Vassiliou G, Alexoudis C, Papadopoulou-Mourkidou E (2009) Spatial and temporal distribution of pesticide residues in surface waters in northeastern Greece. Water Res 43:1–10
Wang Z, Zhang X, Tian H, Wang W, Ru S (2015) Effects of monocrotophos pesticide on steroidogenesis and transcription of steroidogenic enzymes in rainbow trout RTG-2 cells involving the protein kinase A signal pathway. Toxicol in Vitro 29(1):155–161
Weis P, Weis JS (1991) The developmental toxicity of metals and metalloids in fish. In: Newman MC, McIntosh AW (eds) Metal ecotoxicology: concepts and applications. Lewis Publishers, NY, pp 145–169
Werimo K, Bergwerff AA, Seinen W (2009) Residue levels of organochlorines and organophosphates in water, fish and sediments from Lake portion. Aquat Ecosyst Health Manag 12:337–341
WHO (2013) Health implications from monocrotophos use: a review of the evidence in India. WHO Publishers, New Delhi, pp 1–60
Willaert A, Sandeep K, Bet LC, Paul JC, Seth DC, Joseph GHL, Elaine CD, Sruti S, Michael T, Anne DP, Zsolt U (2012) GLUT10 is required for the development of the cardiovascular system and the notochord and connects mitochondrial function to TGF-β signaling. Hum Mol Genet 21(6):1248–1259
Yaji AJ, Auta J (2007) Sub-lethal effect of monocrotophos on growth and food utilization of the African Cat Fish Clarias gariepenus (Teugels). J Fish Int 2(2):127–129
Yamauchi M, Kim EY, Iwata H, Tanabe S (2005) Molecular characterization of the aryl hydrocarbon receptors (AHR1 and AHR2) from red seabream (Pagrus major). Comp Biochem Physiol C 141:177–187
Yang D, Lauridsen H, Buels K, Chi LH, La Du J, Bruun DA, Olson JR, Tanguay RL, Lein PJ (2011) Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol Sci 121(1):146–159
Yen J, Donerly S, Levin ED, Linney EA (2011) Differential acetylcholinesterase inhibition of chlorpyrifos, diazinon and parathion in larval zebrafish. Neurotoxicol Teratol 33(6):735–741
Yong T, Xiayang X, Steven W, Meera S, David JK, Jeff SM, John KC (2011) Loss of Zebrafish lgi1b leads to hydrocephalus and sensitization to pentylenetetrazol induced seizure-like behavior. PLoS ONE 6(9):e24596, 1-10
Zhang X, Gao L, Yang K, Tian H, Wang W, Ru S (2013a) Monocrotophos pesticide modulates the expression of sexual differentiation genes and causes phenotypic feminization in zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol 157(1):33–40
Zhang X, Tian H, Wang W, Ru S (2013b) Exposure to monocrotophos pesticide causes disruption of the hypothalamic–pituitary–thyroid axis in adult male goldfish (Carassius auratus). Gen Comp Endocrinol 193:158–166
Acknowledgments
The authors are thankful to the Director, IICT for providing the facilities and for the constant encouragement throughout the study. Rajesh Pamanji is also thankful to the Council of Scientific and Industrial Research (CSIR), Govt. of India, New Delhi, and Leelavathi is thankful to the Indian Council of Medical Research for the grant of research fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Henner Hollert
Rights and permissions
About this article
Cite this article
Pamanji, R., Bethu, M.S., Yashwanth, B. et al. Developmental toxic effects of monocrotophos, an organophosphorous pesticide, on zebrafish (Danio rerio) embryos. Environ Sci Pollut Res 22, 7744–7753 (2015). https://doi.org/10.1007/s11356-015-4120-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4120-8