Abstract
In the present study, landfill leachate of three landfill sites of Delhi, India, was toxico-chemically analyzed for human risk assessment. Raw leachate samples were collected from the municipal solid waste (MSW) landfills of Delhi lacking liner systems. Samples were characterized with relatively low concentrations of heavy metals while the organic component exceeded the upper permissible limit by up to 158 times. Qualitative analysis showed the presence of numerous xenobiotics belonging to the group of halogenated aliphatic and aromatic compounds, polycyclic aromatic hydrocarbons (PAHs), phthalate esters, and other emerging contaminants. Quantitative analysis of PAHs showed that the benzo(a)pyrene-toxic equivalence quotient (BaP-TEQ) ranged from 41.22 to 285.557 ng L−1. The human risk assessment methodology employed to evaluate the potential adverse effects of PAHs showed that the cancer risk level was lower than the designated acceptable risk of 10−6. However, significant cytotoxic and genotoxic effects of leachates on HepG2 cell line was observed with MTT EC50 value ranging from 11.58 to 20.44 % and statistically significant DNA damage. Thus, although the leachates contained low concentrations of PAHs with proven carcinogenic potential, but the mixture of contaminants present in leachates are toxic enough to cause synergistic or additive cytotoxicity and genotoxicity and affect human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Landfill is the most widely employed method for the disposal of municipal solid waste (MSW). Specifically, it has been reported that almost 95 % of total MSW is being disposed off in landfills worldwide (El-Fadel et al. 1997). However, this process of disposal results in the generation of a complex liquid effluent, commonly known as leachate, due to excess rainwater percolating through the waste layers. In the absence of leachate collection systems in the unengineered landfill sites, leachate is a potential source of ground water pollution, rendering the ground water unusable for domestic and other purposes (Bakare et al. 2000). Therefore, leachate is recognized as an important environmental problem, and its risk assessment and management is thereby considered essential.
The composition of leachate varies considerably among landfills depending on various factors such as hydrogeology, amount of rainfall, age of the landfill, as well as waste composition and degradation stage of waste (Kjeldsen et al. 2002). For general purpose, pollutant load of leachate can be divided into four major groups, such as dissolved organic matter, inorganic salts, metals, and xenobiotic organic compounds (Christensen et al. 1994). Previous studies have reported presence of hazardous organic compounds like aromatics, chlorinated aliphatics, phenols, phthalates, and pesticides in leachate (Baun et al. 2004; Schwarzbauer et al. 2002). Different heavy metals including lead, chromium, copper, and iron have also been reported by different studies as reviewed by Baun and Christensen (2004). Apart from these major groups of contaminants, a huge number of other chemicals may also present in leachate in trace amounts (Kalcikova et al. 2011). Thus, a cocktail of chemicals present in leachate may act in a synergistic and additive manner causing toxic effects to biological organisms (Baderna et al. 2011; Baun et al. 2004).
A limitation of using chemical analyses alone is that the compounds present in low concentrations below the detection limit of the instrument remain unidentified, and hence their potential biological effects are underestimated. Therefore, ecotoxicological and toxicological risk assessment methodologies are gaining importance as knowledge of the chemical composition along with the toxic potential of the leachate is necessary not only to assess the risk but also to make projections on its long-term impact and possible adverse effects on human and ecosystem health (Tsarpali and Dailianis 2012). However, very few studies have combined both chemical and toxicological characterization of leachate, required for proper risk assessment, lesser so in the Indian scenario where majority of the landfills are unengineered and stringent management practices for pollution control are lacking (Narayana 2009; Vij 2012).
A lot of studies have focused on leachate toxicity on different organisms, such as marine invertebrates (Tsarpali and Dailianis 2012), fish species (Deguchi et al. 2007), plant species (Li et al. 2008; Sang et al. 2010), and mammals (Chandra et al. 2006; Sang and Li 2005). In this context, in vitro bioassays using mammalian cell lines can be a suitable option, as they are rapid, simple, and sensitive as well as cost-effective (Talorete et al. 2008). Human hepatocarcinoma cell lines HepG2 are model cell lines for toxicological evaluation due to the expression of xenobiotic metabolizing enzymes cytochrome P450 (CYP) 1A1 (Chaloupka et al. 1994).
Given the lack of studies evaluating the risk posed by leachate from unlined landfill sites of India, the present investigation analyzed the leachate generated in MSW landfill sites of Delhi by both chemical analyses and in vitro toxicity assays using HepG2 cell line. Bioassays assessing cytotoxicity and genotoxicity were used along with chemical analyses in order to assess the impact of leachates on environment. Also, a risk assessment study on health impact was carried out to estimate the potential carcinogenic health risks due to polycyclic aromatic hydrocarbons (PAHs) present in groundwater contaminated with leachates.
Material and methods
Chemicals
All chemicals and cell culture-related reagents were procured from Sigma-Aldrich (St. Louis, MO, USA) except 17-PAH standard solution purchased from AccuStandard, Inc. (New Haven, USA). All solvents were purchased from Merck (Darmstadt, Germany) and were of HPLC grade.
Sampling sites, leachate collection, and preliminary analysis
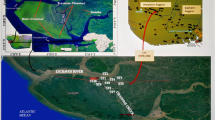
The national capital produces more than 9000 tonnes of MSW daily (Zafar and Alappat 2004). Presently, there are four functioning landfill sites in Delhi—Ghazipur, Okhla, Bhalswa, and Narela of which the first three are unengineered sites and already oversaturated with waste. Absence of base liners in the unengineered landfills results in continuous groundwater contamination. Close proximity of these landfills to river Yamuna also results in polluting the river. Furthermore, no environmental impact assessment has been carried out prior to selection of these sites. Sampling of leachate was therefore carried out at the three unengineered landfill sites—Ghazipur (28° 37ʹ 25.11ʹʹ N, 77° 19ʹ 36.1ʹʹ E), Okhla (28° 30ʹ 48ʹʹ N, 77° 17ʹ 4ʹʹ E), and Bhalswa (28° 44ʹ 26ʹʹ N, 77° 9ʹ 26ʹʹ E) shown in Fig. 1 to estimate their pollution potential. Sampling from the selected sites was carried out during summer season of May 2012. Samples were collected from three sampling points within each landfill in glass bottles cleaned by pre-soaking in 1 M HNO3 for 24 h followed by thorough rinsing with deionized water. For heavy metal analysis, samples were preserved by the addition of concentrated HNO3 (1 mL L−1). Samples collected from the three sampling points of a landfill site were later combined to obtain a homogeneous sample and denoted as OL, BL, and GL for Okhla leachate, Bhalswa leachate, and Ghazipur leachate, respectively. Parameters such as electrical conductivity (EC), pH, and total dissolved solids (TDS) were measured using Cyberscan PC 510 m, COD by open reflux method, and color using platinum-cobalt method (APHA 2005) without delay, and samples were stored at 4 °C until complete analysis.
Extraction of organic contaminants and GC-MS analysis
Classical liquid phase separation with a separating funnel was used for the extraction of organic compounds from leachate samples. Briefly, 100 mL of 1:1 v/v dichloromethane (DCM), and acetone was added to 250 mL of leachate. Extraction procedure was repeated thrice. The organic fraction was collected, concentrated using a vacuum rotator evaporator, and finally dissolved in 1 mL of DCM as the crude organic extract for gas chromatography mass spectrography (GC-MS) analysis. The analysis was done using a Shimadzu GC-MS-QP 2010 Plus equipped with a capillary column Rtx-5 (dimensions: 0.25 μm film thickness, 0.25 mm internal diameter, 30 m in length). Injection volume was 1.0 μL, and the pulsed splitless time was set at 1 min. Detection was carried out in scan mode for qualitative screening as well as in selective ion monitoring (SIM) mode for quantification of PAHs. The GC oven temperature was programmed as follows: 1 min at 70 °C, first ramp 10 °C min−1 to 230 °C, 2 min at 230 °C; second ramp 10 °C min−1 to 250 °C, 2 min at 250 °C; third ramp 10 °C min−1 to 275 °C, 2 min at 275 °C; and fourth ramp 10 °C min−1 to 310 °C, 10 min at 310 °C. Identification of compounds in scan mode was based on the comparison of their mass spectra with those of reference compounds from NIST-05 and Wiley-8 mass spectral library. PAHs were detected using SIM mode based on unique identifier ions chosen for each target compound.
Heavy metal analysis using ICP-AES
For heavy metal analysis, samples were digested according to Ogundiran and Afolabi (2008). Briefly, 10 mL of HNO3 (69 %) was added to 50 mL of sample taken in a digestion tube. The mixture was then evaporated to around 10 mL, cooled to room temperature, filtered through 0.45-μ syringe filter, and finally diluted to 50 mL with double distilled water. Samples were digested and analyzed using Jobin Yvon ICP-OES (Ultima 2) in triplicate.
Toxicological assays
Cell culture and treatments
The toxicity of leachate samples was evaluated by MTT and comet assays. HepG2 cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10 % foetal bovine serum, 1 % antibiotic antimycotic solution in 5 % CO2 at 37 °C. The test samples were filter sterilized using 0.22-μm syringe filter before performing the assays. In MTT assay, 50 μM benzo(a)pyrene (BaP) (positive control), 0.5 % v/v Milli-Q (negative control), and test samples were added to the cell culture in different dilutions to work out the dose-response relationship. In comet assay, 4 % v/v of test samples was used.
Cell viability assay
The number of viable cells was determined by measuring the conversion of the tetrazolium salt MTT to formazan, according to Nwagbara et al. (2007). Briefly, cells were seeded at 5 × 104 cells mL−1 in 96-well plates and treated with 0.5 % v/v Milli-Q, 50 μM BaP, and different doses of test samples (5, 10, 15, and 20 % v/v) after the cells reached 90 % confluency. After 24 h of treatment, medium was removed and replaced by fresh medium containing MTT at a final concentration of 0.5 mg mL−1 and further incubated for 2 h. Then, solubilisation solution (DMSO) was added into each well and incubated at room temperature for 1 h for proper solubilisation. Absorbance was read at 570 nm and background absorbance at 650 nm was later on subtracted. Sigmoid dose-response curves for different test samples along with their EC50 values were derived from the global curve fitting analysis with four-parameter logistic curve equation (Das et al. 2012).
Alkaline single-cell gel electrophoresis (comet assay)
The genotoxicity of the test samples was evaluated using comet assay as described by Ghosh et al. (2014a). HepG2 cells were seeded in six-well plates at a density of 5 × 105 cells mL−1. After attachment, cells were treated with test samples (OL, BL, GL) and positive (50 μM BaP) and negative (0.5 % MQ) control for 24 h. At the end of the exposure, cells were harvested, mixed with 1 % low-melting agarose, and added to slides pre-coated with 1 % agarose. Cells were then denatured with lysis buffer (2.5 M NaCl, 0.1 M Na2EDTA, 10 mM Tris-HCl, pH 10) for overnight, unwinded with alkaline electrophoresis solution (10 M NaOH, 0.2 M EDTA, pH 13) for 20 min, and subjected to electrophoresis at 25 V for 15 min. At the end of electrophoresis, cells were neutralized with 70 % ethanol for 15 min and stained with ethidium bromide (2 μg mL−1, 100 μL per slide). The comets were visualized with Axio Carl Zeiss fluorescent microscope (Carl Zeiss Micro-imaging, Germany, ×40 magnification), equipped with epifluorescence and Axiocam Camera system coupled with Axio Vision software at excitation and emission setting of 518/605 nm. In all cases, cell viability measured with the use of MTT assay was not below 80 %, which is considered appropriate for conducting comet analysis (Tice et al. 2000). The percentage of DNA in tail, tail moment (TM), and olive tail moment (OTM) of 40 randomly selected cells were analyzed from each slide by using CometScore Freeware Software (www.tritekcorp. com) according to the criteria established by Ritter and Knebel (2009), in order to exclude abnormal comets from comet counting and scoring. Comets were classified according to Miyamae et al. (1998) into five classes on the basis of DNA in the tail; class I, less than 1 % DNA in tail (intact nucleus); class II, 1–20 % DNA in tail; class III, 20–50 % DNA in tail; class IV, 50–75 % DNA in tail; and class V, more than 75 % DNA in tail. Background levels of DNA damage in control cells showed low variability, thus ranging within similar levels with those measured in HepG2 cells and other cellular types as previously reported (Tran et al. 2007; Toufexi et al. 2013).
Human risk assessment
In order to estimate possible adverse effects on humans, an accidental leachate spilling was hypothesized resulting in 1:100 dilution of leachate percolating into groundwater (Fig. 2). Similar hypothesis with the same dilution factor has been previously proposed by Baderna et al. (2011). As the leachate mixes with the groundwater, all the compounds present in the leachate will also be subjected to dilution (Christensen et al. 2001). For each carcinogenic PAH quantified, chronic daily intake (CDI (mg kg−1 day−1)) and cancer risk (CR) were calculated according to Baderna et al. (2011) and Palmiotto et al. (2014) using the following formulas:
where, Cwater = pollutant’s concentration in water (mg L−1); WI = water intake = 2 L day−1; ED = exposure duration = 30 years; EF = exposure frequency = 365 days year−1; BW = body weight = 70 kg (adult); AT = exposure average time: 70 years (lifetime).
where, SF (slope factor, kg day mg−1) represents the chemical’s carcinogenic potency for a unit dose. PAHs equivalent to BaP have a SF value of 7.30 (Palmiotto et al. 2014; USEPA 2011). Values of CR <10−6 were deemed negligible for human risk assessment (Health Canada 2004).
Statistical analysis
All experimental data were expressed as means ± standard deviation of three replicates. All statistical analyses including global curve fitting were performed with sigma plot 11 statistical package (Systat Software, San Jose, CA, USA). Statistical comparisons of the results between the control and treated cells were made using analysis of variance (ANOVA) followed by multiple comparisons (Dunnett’s method). A value of P < 0.05 was used to determine significance in statistical analyses.
Results and discussion
Physicochemical characterization
Results of the physicochemical analysis of the leachate samples from unlined landfills of Delhi are presented in Table 1. All of them are characterized by dark color, unpleasant odor, alkaline pH, high conductivity, and relatively high concentrations of organic matter. Unfortunately, no standard maximum allowable discharge limit for landfill leachate is there in India, so the limits set by developed countries such as Germany are used as a guideline in the present study (Kurniawan et al. 2006). Leachate samples were found to exceed the German permissible limits of leachate discharge for iron and chromium. High concentrations of cadmium and copper were also found in the OL and GL, respectively. Compared to previous studies on landfill leachates from Croatia by Gajski et al. (2012) and Garaj-Vrhovac et al. (2013), the concentrations of iron and chromium were much higher. But the concentration of other heavy metals particularly lead was much less compared to leachate from other landfill sites of Greece (Tsarpali et al. 2012) and India (Singh and Mittal 2009). However, metals are commonly present as organic complexes, with free metal ions constituting less than 10 % of the metal concentration and thus pose serious implications on chemical-based risk assessment (Baun and Christensen 2004).
Compared to the low concentrations of most of the heavy metals detected, the organic load of the leachate represented by COD was extremely high, exceeding the maximum allowable values of discharge by 145, 134, and 158 times for OL, BL, and GL, respectively. Organic micropollutants detected in the leachate using scan mode of GC-MS (Table 2) included compounds belonging to aliphatics, terpenoids, alcohols, benzenes, ketones, pharmaceuticals, phthalates, as well as halogenated compounds. A large number of other organic contaminants can also be expected to be present in the leachates at concentrations below the detection limit of the analytical methods used. But their presence in low concentrations does not eliminate the threat posed to human and aquatic health. Analysis of the leachate organic extracts in SIM mode of GC-MS showed the presence of many PAHs (Table 3) which remained undetected in scan mode with the total PAHs being the highest in GL. Percentage of naphthalene, pyrene, and anthracene were highest in OL, BL, and GL, respectively (Fig. 3). The concentration level and the type of PAHs in the three sampling sites differ due to differences of pollution sources and other environmental factors. Previous studies on chemical characterization of leachate from Italy (Baderna et al. 2011), Oklahoma (Andrews et al. 2011), Poland (Matejczyk et al. 2011), Qingdao (Gong et al. 2014), and Thailand (Boonyaroj et al. 2012) have shown the prevalence of compounds such as bisphenol A and phthalates commonly used as plasticizers, PAHs associated with combustion of organic matter, and ibuprofen used as a pharmaceutical drug which were also detected in the present study. Bearing in mind the percolation of leachate into ground and surface water, the persistence of these compounds, and assimilation by aquatic organisms, these compounds can pass through food chain and bioaccumulate (Toufexi et al. 2013).
Leachate-induced cytotoxic effects on HepG2 cell line
Cytotoxicity of the leachate samples was evaluated by measuring the cell viability of HepG2 cells using MTT assay. The positive control and test samples were added in different dilutions to work out the dose-response relationships. Cell viability was expressed as percentage of the corresponding control (0.5 % v/v Milli-Q). MTT assay derived EC50 values (Table 4), and the dose-response curves (Fig. 4) show the level of cytotoxicity of the test samples. After 24 h of treatment, lowest EC50 value was observed in Ghazipur leachate (11.581 %) and highest in Bhalswa leachate (20.4472 %).
Overall results of MTT assay clearly suggest that the leachate from the landfill sites contained significant load of cytotoxic compounds. Leachate-induced cytotoxicity may be due to generation of oxidative stress-related free radicals causing DNA damage and blocking cell cycle progression and mitosis (Baderna et al. 2011). Disturbance in cellular proliferation can be attributed to the presence of compounds such as PAHs (Kang et al. 2010), phthalate esters (Erkekoglu et al. 2010), dioxins (Aly and Khafagy 2011), and bisphenol A (Baderna et al. 2011) found in the leachates. Heavy metals like Cr (Naik et al. 2014) and Cd (Koizumi et al. 1996) have also been reported to have cytotoxic effects. Garaj-Vrhovac et al. (2013) showed significant cytotoxicity of leachate from the Piškornica (Croatia) sanitary landfill in human peripheral blood lymphocytes using differential staining with acridine orange (AO) and ethidium bromide (EtBr). Previous studies have assessed the cytotoxicity of leachates using human breast cancer MCF-7 cells (Talorete et al. 2008), liver cancer HepG2 cells (Ghosh et al. 2014a), and mussel hemocytes (Toufexi et al. 2013), and shown that the mixture of contaminants are highly cytotoxic at high concentrations.
Leachate-induced genotoxic effects on HepG2 cell line
The potential genotoxicity of the leachates was investigated by the alkaline single cell gel electrophoresis using HepG2 cells. The outcome of the assay is shown in Fig. 5 and Olive tail moment in Table 4. The assay indicated that genotoxicity followed the order OL > GL > BL. In case of cells treated with OL, 37.5 and 62.5 % comets fell under classes IV and V, respectively. Whereas, only 15 and 10 % comets fell under classes IV and V, respectively, in case of treatment with BL. According to the results of one-way ANOVA, both the parameters TM and OTM showed statistically significant difference (Dunnett’s method P < 0.05) with respect to the negative control (0.5 % Milli-Q) confirming the genotoxic nature of the leachates from all the landfills.
Genotoxicity of leachate samples from three landfill sites of Delhi. a The tail moment and the olive tail moment plotted against different samples. Tail moments of 40 randomly selected comets are presented as quantile box plots. The edges of the box represent the 25th and the 75th percentiles; a solid line in the box represents the median value while dash represents mean value. Error bars indicate 90th and 10th percentiles and the black circles indicate outlying points beyond 5th and 95th percentiles. Olive tail moments of the same 40 comets are shown as the mean ± standard deviation. b Representative images of different classes of comets seen under fluorescent microscope after ethidium bromide staining
Though highest cytotoxicity was observed in GL, highest genotoxicity was found in OL as the cytotoxic compounds necessarily do not affect the genome but may result in a variety of cell fates like loss of membrane integrity or lead to apoptosis. High genotoxicity of OL may be attributed to the additive effects of chemicals present even in trace amounts. Since relatively low concentrations of most of the heavy metals were found, genotoxic effect may be connected to high concentrations of organic biorefractory compounds. Genotoxicity can be induced by a diverse group of chemicals present in landfill leachate like benzene and its derivatives, PAHs and phthalate esters (Ghosh et al. 2014b; Yuan et al. 2011). The present study is also in agreement with a series of similar studies from other countries that evaluated direct impact of leachate on genome using plant (Sang et al. 2006, 2010) and animal (Li et al. 2004, 2006) species and indirectly through water contamination (Amahdar et al. 2009; Baun et al. 1999). The genetic toxicity of leachates has been shown using different bioassays, such as the Ames Salmonella/microsome mutagenicity bioassay (Omura et al. 1992), the umu-test using Salmonella typhimurium (Kwasniewska et al. 2012), the Bacillus subtilis DNA repair bioassay, and the diploid Aspergillus nidulans chromosome damage bioassay (Schrab et al. 1993). Tewari et al. (2005) reported leachate-induced genotoxicity in mouse bone marrow cells using chromosomal aberrations (CA), micronucleus test (MT), and comet assay. The DNA damage induced by landfill leachate implicate that humans consuming leachate-contaminated water are at increased risk of developing adverse health effects. Consequently, it has become important to monitor the potential toxicity of landfill leachate.
Human risk assessment due to PAHs present in leachates
The risk posed due to exposure to drinking water contaminated with leachates was estimated considering a hypothetical situation of 1:100 dilution of leachate in drinking water. The exposure dose (CDI) and cancer risk (CR) due to PAHs are shown in Table 3. Concentration of carcinogenic PAHs in contaminated drinking water as indicated by BaP TEQ’s was found to range from 41.22 × 10−8 to 285.557 × 10−8 mg L−1. Cancer risk varied between 3.6843 × 10−8 and 25.5253 × 10−8. The toxicological ranking based on CR for the leachates is GL > BL > OL. The cancer risk due to PAHs measured as CR was 100 times lower than the risk threshold of 10−6 (Health Canada Health 2004). Thus, the cancer risk due to PAHs did not exceed the risk threshold under hypothesized conditions.
One of the factors affecting the quality of groundwater at a particular location in an aquifer is chemical composition of the infiltrated water recharging the aquifer. Though no significant alert resulted from the present investigation due to contamination of groundwater from leachates containing PAHs, but the carcinogenic effects due to other organic as well as inorganic contaminants cannot be neglected as indicated by the cytotoxicity and genotoxicity assay results of the study. The previous study by Baderna et al. (2011) has shown high cancer risk in drinking water due to PCBs, PCDDs, and arsenic. Jurado et al. (2012) also reported the presence of emerging organic contaminants in ground water of Spain at concentrations above the European groundwater quality standards. The mixture of contaminants present in trace amounts may induce cytogenic abnormalities and DNA damage implicating that humans consuming leachate-contaminated water are at a risk of adverse health effects. A deeper knowledge of the risks associated with the landfills is required as the residents living in the vicinity tend to be seriously affected by the toxic emissions and health-related issues ranging from immunotoxicity to reproductive disorders, developmental effects, and cancer induction in different organs (Palmiotto et al. 2014).
Conclusions
The present study evaluated the landfill leachate by chemical analysis and toxicological bioassays, for assessment of human risk resulting from percolation of leachate into the ground water. The results indicated that the landfill leachate may act as a cytotoxic and genotoxic agent in mammalian cells. Due to absence of base liners in the landfills of Delhi, the mixture of contaminants could result in water contamination. Exposure to leachate-contaminated water may thus pose a potential risk to organisms. An integrated approach of using chemical characterization, bioassays, and human risk assessment can be used efficiently to assess the effect of leachate pollution on the environment.
References
Aly HA, Khafagy RM (2011) 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced cytotoxicity accompanied by oxidative stress in rat Sertoli cells: possible role of mitochondrial fractions of Sertoli cells. Toxicol Appl Pharmacol 252:273–280
Amahdar L, Anouar A, Ababou B, Verschaeve L, Hilali A (2009) In vitro genotoxicity of Settat town landfill leachate, Morocco. Arh Hig Rada Toksikol 60:179–184
Andrews WJ, Masoner JR, Cozzarelli IM (2011) Emerging contaminants at a closed and an operating landfill in Oklahoma. Ground Water Monit R 32:120–130
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, USA
ATSDR (2009) Public Health Assessments & Health Consultations. Available from: http://www.atsdr.cdc.gov/hac/pha/pha.asp?docid=59&pg=2. Accessed June 2014
Baderna D, Maggioni S, Boriani E, Gemma S, Molteni M, Lombardo A, Colombo A, Bordonali S, Rotella G, Lodi M, Benfenati E (2011) A combined approach to investigate the toxicity of an industrial landfill’s leachate: chemical analyses, risk assessment and in vitro assays. Environ Res 111:603–613
Bakare AA, Mosuro AA, Osibanjo O (2000) Effect of simulated leachate on chromosomes and mitosis in roots of Allium cepa. J Environ Biol 21:263–271
Baun A, Kløft L, Bjerg PL, Nyholm N (1999) Toxicity testing of organic chemicals in groundwater polluted with landfill leachate. Environ Toxicol Chem 18:2046–2053
Baun A, Ledin A, Reitzel LA, Bjerg PL, Christensen TH (2004) Xenobiotic organic compounds in leachates from ten Danish MSW landfills—chemical analysis and toxicity tests. Water Res 38:3845–3858
Baun DL, Christensen TH (2004) Speciation of heavy metals in landfill leachate: a review. Waste Manage Res 22:3–23
Boonyaroj V, Chiemchaisri C, Chiemchaisri W, Theepharaksapan S, Yamamoto K (2012) Toxic organic micro-pollutants removal mechanisms in long-term operated membrane bioreactor treating municipal solid waste leachate. Bioresource Technol 113:174–180
Chaloupka K, Santostefano M, Goldfarb IS, Liu G, Myers MJ, Tsyrolv IB, Gelboin HV, Krishnan V, Safe S (1994) Aryl hydrocarbon (Ah) receptor independent induction of CYP1A2 gene expression by acenaphthylene and related compounds in B6C3F1 mice. Carcinogenesis 15:2835–2840
Chandra S, Chauhan LK, Murthy RC, Gupta SK (2006) In vivo genotoxic effects of industrial waste leachates in mice following oral exposure. Environ Mol Mutagen 47:325–333
Christensen TH, Kjeldsen P, Albrechtsen HJ, Heron G, Nielsen PH, Bjerg PL, Holm PE (1994) Attenuation of landfill leachate pollutants in aquifers. Crit Rev Environ Sci Technol 24:119–202
Christensen TH, Kjeldsen P, Bjerg PL, Jensen DL, Christensen JB, Baun A, Albrechtsen HJ, Heron G (2001) Biochemistry of landfill leachate plumes. Appl Geochem 16:659–718
Das MT, Budhraja V, Mishra M, Thakur IS (2012) Toxicological evaluation of paper mill sewage sediment treated by indigenous dibenzofuran-degrading Pseudomonas sp. Bioresource Technol 110:71–78
Deguchi Y, Toyoizumi T, Masuda S, Yasuhara A, Mohri S, Yamada M, Inoue Y, Kinae N (2007) Evaluation of mutagenic activities of leachates in landfill sites by micronucleus test and comet assay using goldfish. Mutat Res 627:178–185
El-Fadel M, Findikakis AN, Leckie JO (1997) Environmental impacts of solid waste landfilling. J Environ Manag 50:1–25
Erkekoglu P, Rachidi W, Yuzugullu OG, Giray B, Favier A, Ozturk M, Hincal F (2010) Evaluation of cytotoxicity and oxidative DNA damaging effects of di(2-ethylhexyl)-phthalate (DEHP) and mono(2-ethylhexyl)-phthalate (MEHP) on MA-10 Leydig cells and protection by selenium. Toxicol Appl Pharmacol 248:52–62
Gajski G, Orescanin V, Garaj-Vrhovac V (2012) Chemical composition and genotoxicity assessment of sanitary landfill leachate from Rovinj, Croatia. Ecotox Environ Safe 78:253–259
Garaj-Vrhovac V, Orescanin V, Gajski G, Geric M, Ruk D, Kollar R, Radic Brkanac S, Cvjetko P (2013) Toxicological characterization of the landfill leachate prior/after chemical and electrochemical treatment: a study on human and plant cells. Chemosphere 93:939–945
Ghosh P, Das MT, Thakur IS (2014a) Mammalian cell line-based bioassays for toxicological evaluation of landfill leachate treated by Pseudomonas sp. ISTDF1. Environ Sci Pollut Res 21:8084–8094
Ghosh P, Swati TIS (2014b) Enhanced removal of COD and color from landfill leachate in a sequential bioreactor. Bioresource Technol 170:10–19
Gong Y, Tian H, Wang L, Yu S, Ru S (2014) An integrated approach combining chemical analysis and an in vivo bioassay to assess the estrogenic potency of a municipal solid waste landfill leachate in Qingdao. PLoS ONE 9:e95597. doi:10.1371/journal.pone.0095597
Health Canada (2004) Federal contaminated site risk assessment in Canada. Part 1: Guidance on human health preliminaryquantitative risk assessment (PQRA). Environmental Health assessment services, Safe Environments Programme
Jurado A, Vàzquez-Suñé E, Carrera J, de Alda ML, Pujades E, Barceló D (2012) Emerging organic contaminants in groundwater in Spain: a review of sources, recent occurrence and fate in a European context. Sci Total Environ 440:82–94
Kalcikova G, Vavrova M, Zagorc-Koncan Z, Gotvajn AZ (2011) Seasonal variations in municipal landfill leachate quality. MEQ: Int J 22:612–619
Kang Y, Cheung KC, Wong MH (2010) Polycyclic aromatic hydrocarbons (PAHs) in different indoor dusts and their potential cytotoxicity based on two human cell lines. Environ Int 36:542–547
Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, Christensen T (2002) Present and long-term composition of MSW landfill leachate: a review. Crit Rev Environ Sci Technol 32:297–336
Koizumi T, Shirakura H, Kumagai H, Tatsumoto H, Suzuki KT (1996) Mechanism of cadmium-induced cytotoxicity in rat hepatocytes: cadmium-induced active oxygen-related permeability changes of the plasma membrane. Toxicology 114:125–134
Kurniawan TA, Lo WH, Chan GYS (2006) Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. J Hazard Mater B129:80–100
Kwasniewska J, NaŁęcz-Jawecki G, Skrzypczak A, PŁaza GA, Matejczyk M (2012) An assessment of the genotoxic effects of landfill leachates using bacterial and plant tests. Ecotox Environ Safe 75:55–62
Li G, Sang N, Wang Q (2006) Oxidative damage induced in brains and livers of mice by landfill leachate. Ecotox Environ Safe 65:134–139
Li G, Sang N, Zhao YC (2004) Micronucei induced by municipal landfill leachate in mouse bone marrow cells in vivo. Environ Res 95:77–80
Li G, Yun Y, Li H, Sang N (2008) Effect of landfill leachate on cell cycle, micronucleus, and sister chromatid exchange in Triticum aestivum. J Hazard Mater 155:10–16
Matejczyk M, Płaza GA, Jawecki GN, Ulfig K, Markowska-Szczupak A (2011) Estimation of the environmental risk posed by landfills using chemical, microbiological and ecotoxicological testing of leachates. Chemosphere 82:1017–1023
Miyamae Y, Yamamoto M, Sasaki Yu F, Kobayashi H, Igarashi-Soga M, Shimoi K, Hayashi M (1998) Evaluation of a tissue homogenization technique that isolates nuclei for the in vivo single cell gel electrophoresis comet assay: a collaborative study by five laboratories. Mutat Res Gen Tox En 418:131–140
Naik UC, Das MT, Sauran S, Thakur IS (2014) Assessment of in vitro cyto/genotoxicity of sequentially treated electroplating effluent on the human hepatocarcinoma HuH-7 cell line. Mutat Res : Genet Toxicol Environ Mutagen 762:9–16
Narayana T (2009) Municipal solid waste management in India: from waste disposal to recovery of resources? Waste Manag 29:1163–1166
Nwagbara O, Darling-Reed SF, Tucker A, Harris C, Abazinge M, Thomas RD, Gragg RD (2007) Induction of cell death, DNA strand breaks, and cell cycle arrest in DU145 human prostate carcinoma cell line by benzo[a]pyrene and benzo[a]pyrene-7,8-diol-9,10-epoxide. Int J Environ Res Publ Health 4:10–14
Ogundiran OO, Afolabi TA (2008) Assessment of the physicochemical parameters and heavy metals toxicity of leachates from municipal solid waste open dumpsite. Int J Environ Sci Tech 5:243–250
Omura M, Inamasu T, Ishinishi N (1992) Mutagenic activity of the leachate of municipal solid waste landfill. Mutat Res 298:125–129
Palmiotto M, Fattore E, Paiano V, Celeste G, Colombo A, Davoli E (2014) Influence of a municipal solid waste landfill in the surrounding environment: toxicological risk and odor nuisance effects. Environ Int 68:16–24
Ritter D, Knebel J (2009) Genotoxicity testing in vitro—development of a higher throughput analysis method based on the comet assay. Toxicol In Vitro 23:1570–1575
Sang N, Han M, Li G, Huang M (2010) Landfill leachate affects metabolic responses of Zea mays L. seedlings. Waste Manag 30:856–862
Sang N, Li G (2005) Chromosomal aberrations induced in mouse bone marrow cells by municipal landfill leachate. Environ Toxicol Phar 20:219–224
Sang N, Li GK, Xin XY (2006) Municipal landfill leachate induces cytogenetic damage in root tips of Hordeum vulgare. Ecotoxicol Environ Safe 63:473–489
Schrab GE, Brown KW, Donnelly KC (1993) Acute and genetic toxicity of municipal landfill leachate. Water Air Soil Pollut 69:99–112
Schwarzbauer J, Heim S, Brinker S, Littke R (2002) Occurrence and alteration of organic contaminants in seepage and leakage water from a waste deposit landfill. Water Res 36:2275–2287
Singh V, Mittal AK (2009) Toxicity Analysis and Public Health Aspects of Municipal Landfill Leachate: A Case Study of Okhla Landfill, Delhi. 8th World Wide Workshop for Young Environmental Scientists, 1-8
Talorete T, Limam A, Mitsuko K, Jenhani ABR, Ghrabi A, Isoda H (2008) Stress response of mammalian cells incubated with landfill leachate. Environ Toxicol Chem 27:1084–1092
Tewari A, Chauhan LKS, Kumar D, Gupta SK (2005) Municipal sludge leachate-induced genotoxicity in mice—a subacute study. Mutat Res 587:9–15
Tice RR, Agurell E, Anderson D, Burlnson B, Hartman A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Toufexi E, Tsarpali V, Efthimiou I, Vidali MS, Vlastos D, Dailianis S (2013) Environmental and human risk assessment of landfill leachate: an integrated approach with the use of cytotoxic and genotoxic stress indices in mussel and human cells. J Hazard Mater 260:593–601
Tran D, Moody AJ, Fisher AS, Foulkes ME, Jha AN (2007) Protective effects of selenium on mercury-induced DNA damage in mussel haemocytes. Aquat Toxicol 84:11–18
Tsarpali V, Dailianis S (2012) Investigation of landfill leachate toxic potency: An integrated approach with the use of stress indices in tissues of mussels. Aquat Toxicol 124–125:58–65
Tsarpali V, Kamilari M, Dailianis S (2012) Seasonal alterations of landfill leachate composition and toxic potency in semi-arid regions. J Hazard Mater 30:163–171
USEPA (2011) Exposure factors handbook. Available at < http://www.epa.gov/ncea/efh/pdfs/efh-complete.pdf>. Accessed May 2014
Vij D (2012) Urbanization and solid waste management in India: present practices and future challenges. Procedia Soc Behav Sci 37:437–447
Yuan Z, Cao Y, Si L, Wang D, Guo C (2011) The effects of nitrobenzene on the genetic toxicity in tobacco seedling leaf cells by comet assay. Mol Cell Toxicol 7:291–298
Zafar M, Alappat BJ (2004) Landfill surface runoff and its effect on water quality on river Yamuna. J Environ Sci Health A Tox Hazard Subst Environ Eng A39:375–384
Acknowledgments
We would like to express our sincere thanks to the Council of Scientific and Industrial Research, Government of India, New Delhi, for providing SRF (Ghosh, P). We also thank Dr. J.K. Tripathi (SES, JNU, New Delhi) for ICP-AES analysis, Dr. Ajai Kumar (Advanced Instrumentation Research Facility—AIRF, JNU, New Delhi), and Mr. Sanjay Sharma (Sigma Test and Research Centre) for GC-MS analysis. We would also like to extend our sincere thanks to Dr. Mihir Tanay Das and Mr. Subhanjan Sengupta for their excellent technical assistance in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Rights and permissions
About this article
Cite this article
Ghosh, P., Gupta, A. & Thakur, I.S. Combined chemical and toxicological evaluation of leachate from municipal solid waste landfill sites of Delhi, India. Environ Sci Pollut Res 22, 9148–9158 (2015). https://doi.org/10.1007/s11356-015-4077-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4077-7