Abstract
This study investigated the inactivation of two antibiotic resistance genes (ARGs)—sul1 and tetG, and the integrase gene of class 1 integrons—intI1 by chlorination, ultraviolet (UV), and ozonation disinfection. Inactivation of sul1, tetG, and intI1 underwent increased doses of three disinfectors, and chlorine disinfection achieved more inactivation of ARGs and intI1 genes (chlorine dose of 160 mg/L with contact time of 120 min for 2.98–3.24 log reductions of ARGs) than UV irradiation (UV dose of 12,477 mJ/cm2 for 2.48–2.74 log reductions of ARGs) and ozonation disinfection (ozonation dose of 177.6 mg/L for 1.68–2.55 log reductions of ARGs). The 16S rDNA was more efficiently removed than ARGs by ozone disinfection. The relative abundance of selected genes (normalized to 16S rDNA) increased during ozonation and with low doses of UV and chlorine disinfection. Inactivation of sul1 and tetG showed strong positive correlations with the inactivation of intI1 genes (for sul1, R 2 = 0.929 with p < 0.01; for tetG, R 2 = 0.885 with p < 0.01). Compared to other technologies (ultraviolet disinfection, ozonation disinfection, Fenton oxidation, and coagulation), chlorination is an alternative method to remove ARGs from wastewater effluents. At a chlorine dose of 40 mg/L with 60 min contact time, the selected genes inactivation efficiency could reach 1.65–2.28 log, and the cost was estimated at 0.041 yuan/m3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics are often used and even abused in human medicine and livestock operations, and their need for treatment of infectious diseases and growth promotion is understood and accepted (Luo et al. 2010). Among these antibiotics, tetracycline and sulfonamide are the most commonly used (Munir et al. 2011). Overuse of antibiotics results in the increase and spread of antibiotic resistance genes (ARGs) and bacteria (ARB) in the environment (Rizzo et al. 2013b), which reduce the pharmaceutical defenses against infection (Zhang et al. 2009a). In recent years, various ARGs have been found and at least 38 tetracycline resistance genes (tet R) have been identified. Particularly, tetG encodes an efflux pump and has been found in integrons or plasmids (Auerbach et al. 2007). Sul1 is the most detected sulfonamide resistant gene in the environment (Munir et al. 2011). Due to the ability to capture and spread gene cassettes containing ARGs (Zhang et al. 2009b), integrons are always investigated, especially class 1 integrons, which contain the intI1 gene (Chen and Zhang 2013).
Wastewater treatment plants (WWTPs) represent important reservoirs containing various ARGs (Zhang et al. 2009a). Wastewater biological treatment steps may serve as a suitable process for the increase and spread of ARGs and ARB because a high density of active bacteria promotes horizontal gene transfer (Chen and Zhang 2013; Chen et al. 2009). Effluents from WWTPs are recognized as sources of ARGs and ARB release into the environment (LaPara et al. 2011), and ARGs detected in effluents from WWTPs are at levels far above those in typical aquatic environments (McKinney and Pruden 2012). Therefore, it is important to reduce ARGs and ARB in the effluents from WWTPs to reduce the spread of ARGs in the environment.
Disinfection is often considered to destroy most pathogens in WWTP effluents before they are discharged into receiving waters. Chlorination, ultraviolet (UV), and ozonation disinfection are commonly selected in wastewater treatment processes, and chlorination is the most common treatment applied. These disinfection processes generally effectively decrease ARB (Huang et al. 2011; Rizzo et al. 2013a). However, intact remnants of DNA may survive and confer ARGs to downstream bacteria by transformation and/or transduction; in fact, even ARB can be fully inactivated (Dodd 2012). So, an urgent need exists to evaluate the effects of disinfection on destruction of ARGs. Some studies have reported that chlorination and UV processes could not achieve significant reduction of ARGs in WWTPs operations (Auerbach et al. 2007; Munir et al. 2011; Gao et al. 2012). However, several laboratory reports from research dedicated to disinfection results of wastewater have observed the notable inactivation of laboratory cultured ARGs or ARB by UV light or chlorine disinfection (McKinney and Pruden 2012; Huang et al. 2013). The differences between the actual WWTP’s operation reports and the laboratory research may be due to the variations of the predominant ARGs, ARB, and disinfection dosages; however, the disinfection doses in the WWTPs are always not given. It is important to compare the destruction of ARGs in real wastewater effluents under specific disinfection operations to find the potential pathway to decrease the spread of ARGs into natural water.
This paper compared the reduction of sul1, tetG, intI1, and 16S rDNA (which served to reveal the abundance of background bacteria) in the secondary effluents of WWTP by three disinfection processes (chlorination, UV irradiation, and ozonation), under laboratory conditions. The purpose of this study was: (1) to investigate the most ARG inactivation possible by disinfection with a wide range of dosages; (2) to assess whether disinfection technology is a promising way to reduce ARGs from WWTP effluent.
Materials and methods
Wastewater samples
Wastewater samples were obtained from the effluent of a municipal WWTP in Nanjing, China. After being collected in sterile containers, samples were stored in ice and transported to the laboratory for immediate processing. The average daily flow rate is 50,000 m3. The chemical oxygen demand (COD), total nitrogen (TN) and ammonia nitrogen (NH4 +–N) in samples were 13–29, 17–19, and 9–12 mg/L, respectively. The suspended solid (SS) of effluent was 9–9.5 mg/L, and the bicarbonate alkalinity (CaCO3) was 165–185 mg/L. Original gene copies of wastewater effluent were 105.28 to 106.34 copies/mL for sul1, 105.22 to 106.18 copies/mL for tetG, 105.72 to 106.52 copies/mL for intI1, and 107.64 to 109.57 copies/mL for 16S rDNA.
Lab testing
For lab testing, water samples were firstly exposed by three disinfection methods (chlorination, UV, and ozonation). A wide range of disinfectant dosages was used to test the most ARG inactivation possible. After disinfection, 200-mL wastewater samples were taken and concentrated by passing through the 0.22 μm mixed cellulose ester filter membranes (Xinya, Shanghai, China) to concentrate microbial biomass for gene copies quantification (Munir et al. 2011; Pruden et al. 2006). After filtration, the filters were immersed in 50 % ethanol for DNA protection and stored at −20 °C prior to DNA extraction and subsequent quantification. All disinfection treatments of wastewater were conducted in duplicates for statistical analysis.
Disinfection procedures
Chlorination experiments were carried out using sodium hypochlorite (NaClO, available chlorine ≥ 8 %, XiLong, China) as free chlorine. Five hundred milliliter aliquots of samples were distributed to 500-mL sterile beakers. Sodium hypochlorite was spiked into the sample to establish desired doses of chlorine of 5, 20, 40, 80, 120, and 160 mg/L, respectively, and concentrations of chlorine were analyzed by the DPD method (Environmental Protection Agency of China 2002). After chlorine was added, the samples were stirred immediately to mix and allowed a contact time of 120 min. The sodium thiosulfate solution (Na2S2O3, 1.5 %, (w/v)) was added into the samples to terminate chlorination. To investigate the effect of contact time of chlorination on the inactivation of genes, a series of contact periods were set at 5, 15, 30, 60, 120, and 180 min for chlorine dosages of 40 and 80 mg/L, respectively.
In the UV experiments, a cylinder made of Plexiglas was used as the reactor. The reactor was 31 cm high with radius 4.5 cm. A quartz sleeve with a low-pressure Hg vapor 254-nm lamp inside (Model TUV 16WT5 4P-SE, Philips) was placed in the center of the reactor. The light intensity outside the sleeve was 9.85 mW/cm2 measured by an ultraviolet radiation meter equipped with a UV254 detector (Beijing Normal University). The effective dose of the light was 4.16 mW/cm2 measured by the Point Source Summation Method (Yang 2009). A 1.8-L sample was injected into the reactor and mixed gently by an electric stir bar at 300 rpm, after which the sample was withdrawn having obtained the desired UV irradiation (62∼12,477 mJ/cm2).
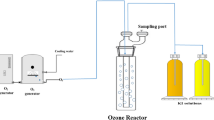
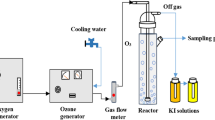
During the ozonation experiments, a 6-L wastewater sample was poured into the ozone contact reaction tower (190 cm high and with a diameter of 10 cm, ZhongTong, China) with sampling done at intervals by adding potassium iodide (KI solution, 2 % (w/v)) to terminate reaction. The ozone concentration was 2.66 mg/L with a flow of 8.3 L/min. Gaseous ozone was produced from air by corona discharge in an ozonator (Model CF-G-2-50G, ZhongTong, China). Ozone was bubbled through porous titanium at the bottom of the reactor, and unreacted ozone, which had escaped from the top of the reactor, was absorbed by 2 % KI solution. The inlet- and off-gaseous ozone concentration was measured by an ozone probe (Model DR70C-03, Wo ST, Shenzhen, China), while aqueous ozone was measured following the DPD method (Jiang et al. 2011). The consumed ozone dose in this paper (27∼178 mg/L) is calculated by the equation:
Ozone consumption by unit of wastewater = (Ozone concentration × contact time − Exit gas dose calculated by integrating the obtained ozone concentration curve during the contact time)/water volume − Residual ozone in the water.
DNA extraction
DNA was extracted using the FastDNATM Spin Kit for soil (MP Biomedicals, Santa Ana, CA) following the protocol of the manufacturer. Single DNA extraction was performed for each wastewater sample. The concentration and quality of the extracted DNA were measured by spectrophotometry (Biodropsis BD2000, Oriental, Beijing, China). The extracted DNA samples were stored at −20 °C until quantification analysis.
Quantitative PCR
Quantitative polymerase chain reaction (qPCR) was applied to quantify sul1, tetG, intI1, and 16S rDNA. Quantification of objective genes was conducted using an Applied Biosystems 7500 qPCR detection system (Life Technologies, USA). The qPCR mixtures consisted of 10 μL 2 × power SYBR Green PCR Master Mix (Life Technologies, USA), 0.16 μL each primer (20 μM), 2 μL template DNA (DNA extracts diluted near to 2 ng/μL), and 7.68 μL ddH2O to a total volume of 20 μL. The temperature protocol consisted of 10 min at 95 °C, 40 cycles of 15 s at 95 °C and 1 min at annealing temperature, followed by a melt curve stage to verify specificity. The information on the qPCR primers is shown in Table 1. qPCR efficiency of target genes ranged from 90 to 100 % with R 2 values of more than 0.99 for all standard curves.
All qPCR runs included a standard curve using at least 5 points. The qPCR standards were prepared by diluting tenfold the plasmids got from positive clones. The PCR purified products were cloned using pMD® 18-T Vector (TaKaRa, Japan).
Transformed clones were screened by PCR to validate cloning of target genes. Plasmids carrying the target genes were extracted and purified according to the MiniBest Plasmid Purification Kit Ver. 3.0 (TaKaRa, Japan). The concentrations of extracted plasmid DNA were measured by spectrophotometry (Biodropsis BD2000, Oriental Co., Ltd, Beijing, China). Absolute quantification was done in this research.
Statistical analysis
The degree of log removal of specific genes was quantified as follows.
-
Inactivation of specific gene j = log (C j0 /C j i )
Here, j indicates specific genes, which include sul1, tetG, intI1, and 16S rDNA, respectively;
- C j0 :
-
Indicates the gene copy number of the specific gene j in the original wastewater samples (copies per milliliter)
- C j i :
-
Indicates the gene copy number of the specific gene j survived to disinfection process at a dosage of i (copies per milliliter)
One-way ANVOA was conducted to compare the inactivation efficiency of genes responding to different disinfectant doses with SPSS Version 17.0. The difference was considered statistically significant at a p value less than 0.05. The relationships among inactivation of different genes were analyzed using OriginPro 8.0.
Results and discussion
Inactivation of ARGs by chlorination disinfection
Figure 1 shows the effect of chlorine doses (contact time of 120 min) on the elimination of ARGs. Generally, ARGs decreased dramatically when adding chlorine from 5 to 40 mg/L. However, the curve leveled off as the dose of chlorine further increased from 80 to 160 mg/L (p > 0.05), which might have been due to the presence of ARB (Macauley et al. 2006). The maximum inactivation of ARGs by chlorine disinfection at 160 mg/L was 3.16 log for sul1 genes and 3.24 log for tetG genes. In view of the gene response to chlorination treatment, tetG, sul1 and intI1 genes were more effectively removed than 16S rDNA gene.
The inactivation of genes was monitored as chlorination disinfection proceeded at original doses of 40 and 80 mg/L, respectively. As shown in Fig. 2, at the chlorine dose of 40 mg/L, the effective inactivation of ARGs was achieved from 0 to 60 min (p < 0.05). After 60 min, the inactivation efficiencies were not significant (p > 0.05). At the chlorine dose of 80 mg/L, however, ARGs reduced dramatically from 0 to 120 min (p < 0.05); hence, extending chlorination contact time could improve inactivation efficiencies. Changes of sul1, tetG, intI1, and 16S rDNA revealed similar patterns when increasing chlorination dose or contact time. In this study, 120 min was enough for inactivation of ARGs at the investigated chlorine doses.
The inactivation of genes involving different chlorination operation modes was also observed. CT value (the initial concentration product of free chlorine multiplied by contact time) is always used for calculating disinfectant dosages for chlorination. At a CT value of 2400 mg Cl2 min/L, 40 mg/L chlorine with 60 min contact time, and 80 mg/L chlorine with 30-min contact time could achieve significantly more inactivation of ARGs and intI1genes (1.65–2.28 log reduction of genes for 40 mg/L and 1.56–1.87 log reduction of genes for 80 mg/L) than 20 mg/L chlorine with 120 min contact time (0.75–1.18 log reductions of genes). It indicated that at the same CT value, increasing the chlorine dose improved the inactivation efficiency when compared to the smaller doses. Based on view of economy, the chlorine dose of 40 mg/L and contact time of 60 min would be enough to achieve high inactivation of ARGs.
Inactivation of ARGs mostly depends on the damage to DNA structure (extracellular ones or intracellular ones). In reporting the effect of disinfection on DNA structure, this study’s laboratory protocol mostly focused on extracellular biomolecules. For extracellular biomolecules, CT values over 180 mg Cl2 min/L have been reported to exhibit measurable fragmentation of isolated pETBlue plasmid DNA (Suquet et al. 2010). Van Aken and Lin (2011) also reported that 2 log 16S rDNA reduction could be achieved at a dosage of 1,980 mg Cl2 min/L toward DNA extracted from E.coil. However, in this study, even at 2400 mg Cl2 min/L, only 0.92–1.87 log inactivation of 16S rDNA was achieved. It could be anticipated that higher CT values or chlorine doses were needed to inactive intracellular ARGs than extracellular ones. Gao et al. (2012) and Shi et al. (2013) indicated neither significant inactivation nor enrichment of sul1 or tet R in the water environment by chlorination, which may be due to the low chlorine doses or CT values, which, unfortunately, were not given.
It has been generally accepted that the amount of chlorination needed to create inactive ARGs in wastewater varies considerably depending on the quality of the effluent which can vary in content (with different amounts of COD, NH3–N, and organic matter); it has also been established that a higher dose of chlorine is required for low-quality wastewater (Anastasi et al. 2013). During chlorination, the quantity of NH3–N present in the wastewater plays a critical role in the removal of ARGs. Higher NH3–N concentration leads to lower ARG removal, which may attribute to its rapid competition for free chlorine to form combined chlorine, such as monochloramine and dichloramine (Wang et al. 2007).
Inactivation of ARGs by UV irradiation
For UV disinfection of selected genes, results are shown in Fig. 3. Generally, the genes decreased greatly as the UV irradiation doses increased from 0 to 12,477 mJ/cm2. However, for intI1 and 16S rDNA, the reductions tended to be flat as the UV irradiation doses increased from 1,248 to 3,743 mJ/cm2. Considering the high-energy consumption, UV disinfection does not seem to be economically feasible. Furthermore, the most effective dosages, which result in considerable inactivation of ARGs can range from 10 to 100 times higher than the common UV dosages applied for wastewater disinfection, which is often less than 100 mJ/cm2 (Macauley et al. 2006). tetG seemed to be slightly resistant to UV irradiation than other genes. The removals of sul1 and intI1 were at similar levels.
UV light can penetrate the UV–transparent structures in the cell and primarily be absorbed by the nucleobases comprising DNA and RNA (Dodd 2012). Various wavelengths toward different organisms can lead to different degrees of DNA damage (Suquet et al. 2010). For ARGs, it was reported at dosages of 200–400 mJ/cm2, ARGs in extracellular and existing within ARB could result in a 3 to 4 log reduction (McKinney and Pruden 2012), which was more effective than the results observed in this investigation. The higher removal in McKinney and Pruden’s study may be interpreted based on their use of pure strains as objectives. In this study, the microbial communities were more complex due to the use of actual wastewater. Besides, the amplicon lengths of selected genes were shorter than those investigated in McKinney’s study, which can limit the potential capture of DNA damage. Auerbach et al. (2007) reported that a UV dosage of 30,100 mJ/cm2 showed no reduction in the detectable tetG and tetQ in wastewater effluent. Hence, Auerbach’s finding is not consistent with the results shown in this study, which shows that a dose of 12,477 mJ/cm2 can achieve 2.48 log reductions on tetG. The difference may be attributed to different water quality (such as turbidity, COD, UV absorbance) and different starting quantities of genes. Du et al. (2014) analyzed the correlation between the reduction of ARGs and reduction of water parameters (COD, NH4 +–N, TP, and TN) and 16S rDNA at a municipal WWTP in China and found that COD exhibited significant correlation with tetW (R = 0.636, p < 0.05), intI1 (R = 0.829, p < 0.01), and sul1 (R = 0.832, p < 0.01), respectively, which may possibly be attributed to COD changing the microbial community composition. Effects of water quality parameters on removal of ARGs from WWTPs need to be explored.
Inactivation of ARGs by ozonation
Figure 4 shows the effect of ozone disinfection on the reduction of ARGs. At a dose of 27 mg/L, ozonation results showed a slight removal of ARGs with only 0.60 log inactivation on 16S rDNA. This result can be attributed to the consumption of ozone by organic matter in wastewater (Macauley et al. 2006). For ozone consumption by organic constituents such as humic substances, carbohydrates, and fatty acids, major pathways involve oxidation followed by fragmentation (Zeng et al. 2014). Inactivation of selected genes noticeably increased when the ozone concentration was increased from 27 to 61 mg/L (p < 0.05). At a higher dosage (above 97.7 mg/L), the decrease of inspected genes tended to be flat (p > 0.05) as per our examination of the results involving tetG. The 16S rDNA was more efficiently removed than ARGs by ozone disinfection.
On the whole, the inactivation of genes by ozone disinfection from easy to hard was: 16S rDNA > tetG > sul1 > intI1. 16S rDNA was removed most efficiently, which can be attributed to its high original copies, which existed in most background bacteria. However, for chlorination and UV disinfection, 16S rDNA was not the ARG most efficiently removed, suggesting that chlorine and UV were more selective on the bacteria harboring ARGs.
When ozone exposure increases, the protein leakage and membrane permeabilization also increases (Cho et al. 2010). It can be inferred that the ozone reacts first with the cell envelope before it touches ARGs, and the intact ARGs will probably still exist when the ARB are totally inactivated (Dodd 2012). It suggests that more ozone is needed to achieve a desirable ARG deactivation, which can then lead to a total ARB removal.
In this study, chlorination showed the most removal of selected genes, while ozonation showed the least. But for the inactivation of 16S rDNA, both ozonation and chlorination disinfection were more effective than UV irradiation. It was pointed out that ozone can react too rapidly with the cell envelope leading to an inability of the dose to penetrate into the cytoplasm and achieve ARG damage, while chlorine typically reacts moderately with cell envelope and apparently penetrates into the cell to deactivate the ARGs effectively (Dodd 2012). This ability to penetrate the cell envelope is one of the main reasons for chlorine exhibiting the best removal of ARGs in wastewater in this study.
Relative abundance of ARGs in three disinfections
The relative abundance of ARGs (defined as the gene copy numbers of ARGs and intI1 normalized to that of 16S rDNA) is also often used to evaluate the change of ARGs during different water treatments (Shang et al. 2007). As shown in Fig. 5, gene quantities are also normalized to 16S rDNA as an indicator of the proportion of bacteria carrying ARGs (Ma et al. 2011). Decreases of the relative abundance of ARGs and intI1 genes were observed in chlorination at doses from 20 to 160 mg/L, except for sul1, at 40 mg/L. After UV irradiation, the decreases were also observed in many situations, while for ozonation, the relative abundance of ARGs and intI1 genes increased. In this study, the reduction of genes were achieved in three disinfection processes, but the relative abundance of ARGs increased in many cases, which might be due to the fact that 16S rDNA genes were also affected by disinfection. A similar result has also been reported in other UV disinfection processes (Shang et al. 2007), where the relative abundance of intI1 and sul1 genes increased although ARGs concentrations decreased after UV irradiation. The increases of relative abundance of ARGs might be because more decreases of 16S rDNA than ARGs were achieved in some experiments.
Relative abundance of ARGs in three disinfections with different doses. A1–A6 represented chlorination by dosage of 5, 20, 40, 80, 120, and 160 mg/L, respectively; B1–B5 represented UV irradiation by dosage of 249.54, 1,247.7, 3,743.1, 8,733.9, 12,477 mJ/m2, respectively; C1–C5 represented ozonation by dosage of 27, 61, 97.7, 136, 178 mg/L, respectively
Studies showing the inactivation of ARGs have been partially attributed to the inactivation of 16S rDNA at the disinfection stages at the same time, and the relative quantities cannot give a direct assay of effects of the disinfectants on ARGs (Gao et al. 2012; Moura et al. 2012; Shi et al. 2013).
In terms of relationships among inactivation of ARGs, intI1 and 16S rDNA genes in the three disinfection processes, it was found that correlations between log inactivation of ARGs and intI1 genes (sul1, R 2 = 0.929 with p < 0.01 and tetG, R 2 = 0.885 with p < 0.01) were stronger than the correlations between log removals of ARGs and 16S rDNA genes (sul1, R 2 = 0.634 with p < 0.01; tetG, R 2 = 0.692 with p < 0.01). This implies more ARGs might be contained in integrons or ARGs contained in integrons are more easily inactivated. Generally, class 1 integrons include an intI1 gene and a conserved segment, which contains sul1 encoding resistance to sulfonamide , so, sul1 and intI1 might show similar responses to various disinfectants (Chen and Zhang 2013).
Comparison of different technologies on removal of ARGs from WWTPs effluents
Among the three disinfection methods studied herein and shown in Figs. 1, 2, 3, and 4, chlorination is better than the others in view of the inactivation efficiency and cost, while ozonation is unadvisable. Ozonation will not be considered for further assessment. Based on the average daily flow rate of a selected WWTP of 50,000 m3, we estimated the economic costs of different technologies (disinfection, advanced oxidation and coagulation) on inactivation of ARGs. As shown in Table 2, under relatively suitable operation conditions, Fenton oxidation was an effective technology to remove ARGs, but was unlikely to be economically viable due to its high chemical requirements. Compared to other technologies, chlorination is an alternative method to remove ARGs. With a chlorine dose of 40 mg/L with 60-min contact time, the genes inactivation efficiency could reach 1.65–2.28 log and the cost was estimated at 0.041 yuan/m3 wastewater. Considering the inactivation of ARGs and costs, more combined methods such as UV/Chlorination need further investigation.
To improve the removal rate of ARGs, the disinfectant dosages were much higher than those set as standard in common usage. For example, under chlorination, the maximum removal of selected genes was achieved at the chlorine dose of 160 mg/L with a contact time as long as 120 min. Usually, a free chlorine dose is set at 5–10 mg/L with a contact time of 30 min for wastewater effluent disinfection. However, the reduction of ARGs was 0.15–0.20 log (unpublished data), which is much lower than that achieved by the high dosage. Current practices can only ensure that ARGs are partially eliminated, which means that residual amounts can reach receiving water based on the present disinfection doses used in WWTPs. Further removal of ARGs from WWTPs should be extensively investigated along with their potential risk to ecosystems (Baquero et al. 2008).
Conclusions
Inactivation of sul1, tetG, intI1 increased due to increased doses of disinfectors. Chlorination was found to be more effective in the reduction of genes than other methods. Ozonation could significantly decrease 16S rDNA while ARGs were not notably inactivated. Chlorine and UV were more selective when targeting bacteria harboring ARGs. To achieve more ARG removal, the dosage of disinfectant had to be much higher than that defined by common usage. WWTPS can only partially remove ARGs and residual amounts can reach receiving water at present disinfection doses. Other methods including advanced treatment systems and combined methods also need to be investigated to find a more useful and economic way to remove ARGs from WWTP effluents.
References
Anastasi EM, Wohlsen TD, Stratton HM, Katouli M (2013) Survival of Escherichia coli in two sewage treatment plants using UV irradiation and chlorination for disinfection. Water Res 47:6670–6679
Auerbach EA, Seyfried EE, McMahon KD (2007) Tetracycline resistance genes in activated sludge wastewater treatment plants. Water Res 41:1143–1151
Baquero F, Martínez J-L, Cantón R (2008) Antibiotics and antibiotic resistance in water environments. Curr Opin Biotechnol 19:260–265
Chen H, Zhang M (2013) Occurrence and removal of antibiotic resistance genes in municipal wastewater and rural domestic sewage treatment systems in eastern China. Environ Int 55:9–14
Chen RZ, Craik SA, Bolton JR (2009) Comparison of the action spectra and relative DNA absorbance spectra of microorganisms: information important for the determination of germicidal fluence (UV dose) in an ultraviolet disinfection of water. Water Res 43:5087–5096
Cho M, Kim J, Kim JY, Yoon J, Kim JH (2010) Mechanisms of Escherichia coli inactivation by several disinfectants. Water Res 44:3410–3418
Dodd MC (2012) Potential impacts of disinfection processes on elimination and deactivation of antibiotic resistance genes during water and wastewater treatment. J Environ Monit 14:1754–1771
Du J, Ren HQ, Geng JJ, Zhang Y, Ding LL, Xu K (2014) Variation of antibiotic resistance genes in municipal wastewater treatment plant with A2O-MBR system. Environ Sci Pollut Res. doi:10.1007/s11356-014-3552-x
Environmental Protection Agency of China (2002) Water and wastewater monitoring methods, 4th edn. Chinese Environmental Science Publishing House, Beijing
Gao P, Munir M, Xagoraraki I (2012) Correlation of tetracycline and sulfonamide antibiotics with corresponding resistance genes and resistant bacteria in a conventional municipal wastewater treatment plant. Sci Total Environ 421–422:173–183
Huang JJ, Hu HY, Tang F, Li Y, Lu SQ, Lu Y (2011) Inactivation and reactivation of antibiotic-resistant bacteria by chlorination in secondary effluents of a municipal wastewater treatment plant. Water Res 45:2775–2781
Huang JJ, Hu HY, Wu YH, Wei B, Lu Y (2013) Effect of chlorination and ultraviolet disinfection on tetA-mediated tetracycline resistance of Escherichia coli. Chemosphere 90:2247–2253
Jiang L, Tang S, You Q, Wang Y, Peng J (2011) Spectrophotometric determination of ozone in water by the color-fading reaction of sodium indigo disulfonate. Phys Test Chem Anal 47:180–182
LaPara TM, Burch TR, McNamara PJ, Tan DT, Yan M, Eichmiller JJ (2011) Tertiary-treated municipal wastewater is a significant point source of antibiotic resistance genes into Duluth-Superior Harbor. Environ Sci Technol 45:9543–9549
Luo Y, Mao DQ, Rysz M, Zhou DX, Zhang HJ, Xu L, Alvarez PJJ (2010) Trends in antibiotic resistance genes occurrence in the Haihe River, China. Environ Sci Technol 44:7220–7225
Ma Y, Wilson CA, Novak JT, Riffat R, Aynur S, Murthy S, Pruden A (2011) Effect of various sludge digestion conditions on sulfonamide, macrolide, and tetracycline resistance genes and class I integrons. Environ Sci Technol 45:7855–7861
Macauley JJ, Qiang Z, Adams CD, Surampalli R, Mormile MR (2006) Disinfection of swine wastewater using chlorine, ultraviolet light and ozone. Water Res 40:2017–2026
McKinney CW, Pruden A (2012) Ultraviolet disinfection of antibiotic resistant bacteria and their antibiotic resistance genes in water and wastewater. Environ Sci Technol 46:13393–13400
Moura A, Pereira C, Henriques I, Correia A (2012) Novel gene cassettes and integrons in antibiotic-resistant bacteria isolated from urban wastewaters. Res Microbiol 163:92–100
Munir M, Wong K, Xagoraraki I (2011) Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res 45:681–693
Pei R, Kim SC, Carlson KH, Pruden A (2006) Effect of river landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Res 40:2427–2435
Pruden A, Pei R, Storteboom H, Carlson KH (2006) Antibiotic resistance genes as emerging contaminants: studies in northern Colorado. Environ Sci Technol 40:7445–7450
Rizzo L, Fiorentino A, Anselmo A (2013a) Advanced treatment of urban wastewater by UV radiation: effect on antibiotics and antibiotic-resistant E. coli strains. Chemosphere 92:171–176
Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy MC, Michael I, Fatta-Kassinos D (2013b) Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci Total Environ 447:345–360
Shang C, Cheung LM, Liu W (2007) MS2 coliphage inactivation with UV irradiation and free chlorine/monochloramine. Environ Eng Sci 24:1321–1332
Shi P, Jia SY, Zhang XX, Zhang T, Cheng SP, Li AM (2013) Metagenomic insights into chlorination effects on microbial antibiotic resistance in drinking water. Water Res 47:111–120
Suquet C, Warren JJ, Seth N, Hurst JK (2010) Comparative study of HOCl-inflicted damage to bacterial DNA ex vivo and within cells. Arch Biochem Biophys 493:135–142
Van Aken B, Lin LS (2011) Effect of the disinfection agents chlorine, UV irradiation, silver ions, and TiO2 nanoparticles/near-UV on DNA molecules. Water Sci Technol 64:1226–1232
Wang LS, Hu HY, Wang C (2007) Effect of ammonia nitrogen and dissolved organic matter fractions on the genotoxicity of wastewater effluent during chlorine disinfection. Environ Sci Technol 41:160–165
Yang B (2009) A pilot study of UV disinfection technology in reclaimed municipal wastewater. Dissertation, Xian University of Architecture and Technology, Xian, China
Zeng T, Wilson CJ, Mitch WA (2014) Effect of chemical oxidation on the sorption tendency of dissolved organic matter to a model hydrophobic surface. Environ Sci Technol 48:5118–5126
Zhang XX, Zhang T, Fang HH (2009a) Antibiotic resistance genes in water environment. Appl Microbiol Biotechnol 82:397–414
Zhang XX, Zhang T, Zhang M, Fang HHP, Cheng SP (2009b) Characterization and quantification of class 1 integrons and associated gene cassettes in sewage treatment plants. Appl Microbiol Biotechnol 82:1169–1177
Acknowledgments
This work was supported by the National Science Foundation of China (no. 51278241) and the Jiangsu Natural Science Foundation (no. BK2011016).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Zhuang, Y., Ren, H., Geng, J. et al. Inactivation of antibiotic resistance genes in municipal wastewater by chlorination, ultraviolet, and ozonation disinfection. Environ Sci Pollut Res 22, 7037–7044 (2015). https://doi.org/10.1007/s11356-014-3919-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3919-z