Abstract
Southern Tuscany (Italy) is characterized by extensive arsenic (As) anomalies, with concentrations of up to 2000 mg kg soil−1. Samples from the location of Scarlino, containing about 200 mg kg−1 of As, were used to study the influence of the inoculation of an arbuscular mycorrhizal (AM) fungus (Rhizophagus irregularis, previously known as Glomus intraradices) and of phosphorus (P) application, separately and in combination, on As speciation in the rhizosphere of Zea mays on plant growth and As accumulation. Also, P distribution in plant parts was investigated. Each treatment produced a moderate rise of As(III) in the rhizosphere, increased As(III) and lowered As(V) concentration in shoots. P treatment, alone or in combination with AM, augmented the plant biomass. The treatments did not affect total As concentration in the shoots (with all the values <1 mg kg−1 dry weight), while in the roots it was lowered by P treatment alone. Such decrease was probably a consequence of the competition between P and As(V) for the same transport systems, interestingly nullified by the combination with AM treatment. P concentration was higher with AM only in both shoots and roots. Therefore, the obtained results can be extremely encouraging for maize cultivation on a marginal land, like the one studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since before the first millennium BC, southern Tuscany (Italy) was a region of concerted mining and metallurgical development: the Colline Metallifere, the Tolfa region and Monte Amiata were characterized by a large availability of a variety of metal ores (Harrison et al. 2010). During the Etruscan times (10th–1st century BC), the region showed highly sophisticated production of bronze and, increasingly, iron artefacts of all types. This activity would tend to put significant amounts of undesirable elements, especially arsenic, into the environment.
Also, due to natural deposits (alluvia from the Colline Metallifere) dating back to the Pleistocene and Holocene epochs, nowadays Tuscany exhibits extensive arsenic (As) anomalies in several areas of the region so that arsenic concentrations in soil may range from 5 to 2000 mg kg soil−1, both in topsoil and in deeper soils (Mascaro et al. 2001).
The dangerousness of this element for human and animal populations has been demonstrated by several studies (Valberg et al. 1997; Hughes 2002; Mandal and Suzuki 2002; Markley and Herbert 2009). Any risk assessment for soil contamination by trace elements needs an estimation of the bioavailability (concentration of exposition) to living organisms, which mainly depends on the soil chemistry and on the chemical behaviour of the element itself (Adriano 2001): pH, redox potential, organic matter content, texture, clay mineralogy, microbial activity and especially the presence of Fe- and Al-oxide/hydroxides (Ultra et al. 2007) and the competition between As(V) and phosphate for sorption sites strongly affect As availability (Jackson and Miller 2000). The main form (about 90 %) of arsenic in aerobic soil is pentavalent arsenate [As(V)O4 −3], which is adsorbed more strongly but with a faster sorption/desorption rate than phosphate (Lambkin and Alloway 2003). Trivalent arsenite [As(III)O3 −3] is the more labile but also the more toxic species in soil (Chatain et al. 2005). In terrestrial plants, as in soils, As occurs mostly in inorganic forms (Chen et al. 2004). In plants, As(V) acts as a phosphate analogue and is transported across the plasma membrane via a phosphate co-transport system (Meharg and Hartley-Whitaker 2002). Arsenate reductase (AR), detected in different terrestrial plants, plays an important role in reduction and detoxification of As(V) to As(III) and in its accumulation in plants (Yu et al. 2009). Concentrations of As commonly found in plants vary from 0.009 to 1.5 mg kg−1 dry weight (Kabata-Pendias and Pendias 2001).
Meharg et al. (1994), Abedin et al. (2002) and Esteban et al. (2003), in very-short-duration uptake studies, demonstrated that phosphate can effectively reduce plant uptake of As(V), depending on the different plant resistance characteristics to As and on the amounts of soluble P and As in the rhizosphere. In long-term studies with different plant species, on the contrary, Ultra et al. (2007), Pigna et al. (2009) and Puckett et al. (2012) observed increasing or decreasing uptake and translocation of As with P addition, depending on the experiment setup and mainly on the plants chosen.
As reported by Yu et al. (2009), arbuscular mycorrhizal (AM) fungi, which are ubiquitous in the rhizosphere, form symbiotic association with roots of the majority of plant species and are able to enhance the tolerance of host plants to soil contamination, generally decreasing metal concentration in the plant tissues, also in the case of arsenic (Smith and Read 1997; Gonzalez-Chavez et al. 2002; Bai et al. 2008). Nonetheless, the effect of AM on As depends on many factors and may vary between studies. For example, Chen et al. (2006) reported no effect of different arbuscular mycorrhizal fungi (Glomus mosseae, Glomus caledonium and Glomus intraradices) on As concentrations in tissues of Pteris vittata L. grown in a glasshouse experiment on soil polluted by both As and uranium. Xia et al. (2007), on the other hand, examined the influence of G. mosseae inoculation and P addition on maize after cultivation in a glasshouse on As-contaminated soil, and they found that AM decreased shoot As concentrations when no P was added and increased shoot and root As concentrations with P addition.
Orlowska et al. (2012), after having inoculated Plantago lanceolata L. grown on As-rich soil with AM, found higher root and shoot biomass and lower As concentration in roots than the non-inoculated plants, with significant differences in As concentration and accumulation between AM isolates. Yu et al. (2009), moreover, observed that AM inoculation increased P concentration in both roots and shoots. Maize is able to create particularly good environmental conditions for soil microorganisms and microfauna (Cattani et al. 2006). It is also the main crop cultivated in the sampling area to be used as livestock animal feed or for human consumption. Therefore, it was chosen for this study, which was designed to assess the amount of As(III) in the rhizosphere, the accumulation of As(III) and As(V) in the plant and their possible changes in relation to mycorrhizal inoculation and phosphorus application in a naturally contaminated soil. The obtained results represent a step in understanding the plant biology, with the ultimate purpose of understanding the best agronomic practices.
Materials and methods
Soil characterization and growth container
The soil (topsoil to a depth of approximately 0.3 m) used was collected from an agricultural field at Scarlino (Southern Tuscany, 42°55′45″ N, 10°48′31″ E), in a locality where As concentration is very high and much greater than the mean concentration of the earth crust (1.5 mg kg−1). In the work of Cattani et al. (2009), soil samples from the same location were used in observing the soil texture (typically clay loam), characterized by low permeability, which gave rise to significant stagnation of water, affecting plant growth and root development. For this reason, the soil was not directly used as a cultivation substrate, but it was mixed with coarse river sand (<2 mm) in the ratio 2:1 soil to sand. The analyzed properties of the modified soil were pH (1:2.5 soil to water) 8.22, organic matter 15.4 g kg−1, total CaCO3 35.1 g kg−1, total P 412 mg kg−1, extractable P (Olsen) 4.74 mg kg−1 and total As 194 mg kg−1. After having been previously sieved to 2 mm, homogenized, pasteurized and left to stabilize as reported by Orlowska et al. (2012), the modified soil was used to fill the rhizobox system created by Wenzel et al. (2001), adjusted to a bulk density of 1.3 g cm−3. This system is provided with an upper soil-plant compartment (416 cm3) and a lower compartment (409.5 cm3) where the root only compartment is physically separated from the rhizosphere-soil compartment with a nylon membrane inhibiting root penetration. After termination of the experiments, the soil compartment can be divided in layers parallel to the root plane. All construction materials are selected to minimize interaction with any element or organic compound.
Plants and AM fungus
Seeds of maize (Zea mays L.) were found in the market, washed and sterilized. The granular propagules of the endomychorrhizal fungus Rhizophagus irregularis DAOM197198 (Italpollina, Rivoli Veronese, Italy) used comprised a mixture of spores, mycelium, sandy soil and root fragments, containing 100 spore/cm3. According to Mathimaran et al. (2005), this fungus has been found to colonize rapidly a variety of cultivated plants, including maize, and also to be predominant in soils with high clay and As content, similar to that under investigation. Moreover, R. irregularis is the only fungus able to control nutrient uptake by individual hyphae, depending on differing phosphorus levels in the surrounding soil (Cavagnaro et al. 2005).
Experimental conditions
Plant cultivation was conducted from June to August 2013 in a phytotron, with a 14-h photoperiod, relative humidity constantly at 80 % and 28 °C/20 °C day/night temperatures for both germination and growth. During the light period, the light gradually increased to the maximum and decreased to the minimum to mimic sunrise and sunset and minimize the potential disturbance of plant processes by changing the light suddenly. The experiment comprised the following trials: (1) AM−/P− (not inoculated and not P-fertilized), (2) AM+/P− (inoculated and not P-fertilized), (3) AM−/P+ (not inoculated and P-fertilized) and (4) AM+/P+ (inoculated and P-fertilized), and each one was repeated in three replicates. The bulk density of the modified soil after the rhizobox filling was 1.5 g cm−3. Eight maize seeds were placed directly in the upper compartment of the rhizobox to test the growth efficiency in the different examined conditions. Where it was planned, a propagule was sown together with a seed, and phosphorus was added—as KH2PO4—at the rate of 25 mg kg−1, which corresponds to 100 kg ha−1. The P− treatments did not receive any supplemental potassium. After a period of about 2 weeks, the upper compartments were fixed on the lower compartment. The irrigation water was provided by the irrigation wicks, and root elongation was checked daily through the transparent acrylic window of the lower compartment. Plant harvest and soil sampling were realized after 4 weeks after rhizobox assembling, when P+ trials exhibited the maximum root elongation allowed by the compartment length and P− trials began to show growth inhibition and stress symptoms. Harvested plants were divided into roots and aerial parts, counted, weighed, air-dried and immediately analyzed.
After the plant harvest, the slicing device developed by Fitz et al. (2003) was used to divide the rhizosphere-soil compartment into different slices: 0–2 mm (rhizosphere, strictly speaking), 2–10 mm (‘extended rhizosphere’) and >10 mm (bulk soil). This distinction was realized because the membrane, characterized by a mesh size of 30 μm, can be penetrated by fungal hyphae and root hairs (with consequent rhizosphere widening), but not by roots (Luster et al. 2009), and the root-induced changes in the soil may be observed up to a distance of more than 7 mm from the root, depending on the soil texture and structure, the plant species, the monitored property and the presence of fungal hyphae (Norvell and Cary 1992). The mixed growth substrate allowed for easier management of the slicing device during sampling. The obtained soil samples were immediately submitted for analysis.
Mycorrhizal colonization was detected according to the modified method of Philips and Hayman (1970).
P and As measurements
Total arsenic
Ground soil samples (approximately 0.3 g dried weight) were analysed by ICP-MS (Agilent, 7500 ce) following nitric acid-hydrochloric acid digestion (Digiprep, Jr Model, SCP Science) and under vacuum filtration with a 0.45-μm Teflon filter membrane. ICP-MS operated in helium gas mode to remove any possible interference of 40Ar35Cl on m/z. The plant sample was analysed with the same procedure, following nitric acid-hydrogen peroxide-assisted digestion. Spike additions, certified reference materials at different arsenic concentrations (BCR 141 calcareous loam soil, BCR 143 sewage sludge amended soil for soil and BCR 402 white clover and BCR 482 lichen for plants) and analytical blanks were included for quality assurance. The recoveries ranged between 93 and 106 % with repeatability better than 8 %.
Total phosphorus
After digestion, soil and plant samples were analysed for P concentration by ICP-OES (Perkin Elmer, Optima™ 2100 DV) after having verified two wavelengths: 213.617 and 178.221 nm.
Arsenic species
Immediately after slicing, drying, grinding and sieving at 2 mm, 0.5 g of the soil samples was added with 10 mL of 1 M orthophosphoric acid and placed in PTFE vials at 60 W for 10 min. When the solution reached room temperature, it was transferred to a volumetric flask, diluted to 50 mL with mQ water and filtered through a 0.45-μm Millipore membrane as reported by Rahman et al. (2009). Spike additions of 1:1 As(III)/As(V) solutions were realized to verify any possible interconversion between the inorganic As forms during the extraction. Then, 0.1 g of the ground plant samples was added with 10 mL of water/methanol (1:1 v/v) mixture containing 1 % nitric acid in 15-mL polyethylene vials, which were shaken for 16 h at room temperature and centrifuged at 4000 rpm for 20 min for arsenic compound extraction. The supernatant was collected, and the residue was washed ultrasonically assisted with 10 water/methanol twice. The obtained combined supernatants were evaporated at 37 °C to dryness by Rotavapor, and the dry residue was redissolved in 20 mL of water and centrifuged. The final supernatant was filtered through a 0.22-μm nylon membrane. Efficiency of extraction was checked by analysing NIST 1568a-certified rice flour as standard reference material. The recovery was 92 % of As(III) and 94 % of As(V) (103 % of total As). Paik et al. (2010) used a very similar procedure to perform extraction and quantification of arsenic species in polished rice, obtaining very good validation results.
Analysis of arsenic species in soil, root and shoot plant extracts was carried out by HPLC (Agilent, LC 1100 series) interfaced with the described ICP–MS, according to the method described by Van den Broeck et al. (1998). Arsenic species were separated using an anion-exchange As speciation column (Agilent G3154-65001), fitted with a guard column (Agilent G3154-65002). The mobile phase was a solution of 3 mM NaH2PO4 and 0.2 mM Na2-EDTA (pH 6.0), which was pumped through the column isocratically at 1 mL min−1. The outlet of the separation column was connected to a MicroMist nebulizer and a Peltier-cooled spray chamber of the ICP–MS. Signals at m/z 75 (As) and 35 (Cl) were collected with a dwell time of 500 and 10 ms, respectively. Any possible polyatomic interference of 40Ar35Cl on m/z 75 was removed as for total arsenic. The calibration standards used for As speciation were previously controlled for their total As concentrations. Analysis of the samples at the beginning of the run was repeated at the end, and no changes in As speciation were observed during this period.
Statistics
Analysis of variance (ANOVA) and post hoc analysis with Tukey HSD test were performed using SAS (1996).
Results and discussion
Arsenic species in rhizosphere
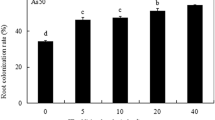
The mycorrhizal colonization was satisfying (more than 60 % of mycorrhizal frequency estimated on the basis of aniline blue staining) for all the AM+ trials. No AM colonization was detected in the non-inoculated plants. The concentrations of As(III) in the soil layers are shown in Fig. 1. The As(III) concentration was very low, less than 1 % of total As (about 200 mg kg−1); thus, total As was mainly represented by As(V) in the studied soil. In the control (AM−/P−) rhizoboxes, no statistically significant differences were found in As(III) concentrations among the layers. A significant difference (p < 0.05) in As(III) concentration between the 0–2-mm layer and the other two layers was found for all treatments with respect to the control. Differences among treatments for the same layer were not significant. The effect of P on arsenic speciation in soil was less perceptible than that of AM. In any case, the effect observed for such nutritive element could be either direct, on As chemistry in soil, or indirect, through its interaction with development of both the roots and the rhizosphere microorganisms in the polluted soil. Regarding As speciation in the soil, Carbonell-Barachina et al. (1998) highlighted that the decreases of pH and redox potential induced in the rhizosphere by Spartina spp. may produce reduction of As(V) to As(III). Ultra et al. (2007) demonstrated that As(III) and As(V) showed gradients toward the root surface of Helianthus annuus L. and that the increase was more noticeable for As(III) in the P+ trials, and for As(V) in the AM+ trials, with results conflicting with those ones about our plants. To this end, Gonzalez-Chavez et al. (2002) reminded that the endomycorrhizal fungus Hymenoscyphus ericae, responsible for the colonization of arsenic-contaminated mine spoils by its host Calluna vulgaris, achieves the resistance to As(V) of its symbiont by reducing cellular As(V) to As(III) and then effluxing the As(III), which is also the mechanism used to achieve As(V) resistance in bacteria and yeasts.
Concentrations of As(III) in the soil layers collected at the end of the rhizobox experiments from the different trials. Black bars are for 0–2 mm, grey bars are for 2–10 mm, white bars are for bulk soil, AM − /P − not inoculated and not P-fertilized, AM+/P − inoculated and not P-fertilized, AM − /P+ not inoculated and P-fertilized and AM+/P+ inoculated and P-fertilized. Values are mean of three replicates. Significant differences (Tukey HSD test, at least p < 0.05) appear with different letters, uppercase for inter-treatment and lowercase for intra-treatment comparisons
Most probably, the effect of P and AM treatments could be species and soil specific, peculiarly and simultaneously affecting not only the root activity but also the rhizosphere microbial activity, thus resulting in specific effects on As(V) reduction. In this context, and relatively to the microbiological aspect, Fitz and Wenzel (2002) already showed that the number of microorganisms in the rhizosphere is typically one order of magnitude greater than in the bulk soil, and the reduction of As(V) to As(III) may be also a widely distributed process of soil microbes.
Rahman et al. (2009), after having demonstrated the effectiveness of the extractant chosen (orthophosphoric acid) for both organic and inorganic arsenic extraction by soil, analysed 20 different soil samples from an As-contaminated area of Bangladesh and found that DMA was barely detectable as the only organic species in just two samples. It is normally expected in aerobic conditions, like in our case: as reminded by Fitz and Wenzel (2002), methylated arsenicals are generally produced under anaerobic conditions, and in very few cases trimethylarsine oxide (TMAO) and arsenobetaine (AB) have been detected as minor compounds in soil extracts. Regarding the organic forms of As, DMA and MMA were not detectable in any of our trials.
Plant growth and arsenic and phosphorus concentrations
For the P+ trials, germination rate and root and shoot dry biomass were significantly higher than for the P− trials (Table 1). Such effect might be ascribed to improved phosphorus nutrition, even if at the same time P-mediated alleviation of soil As toxicity could not be excluded. Gulz et al. (2005) reported similar results after arsenic application on two different soils, cultivated with maize (Z. mays), English ryegrass (Lolium perenne), rape (Brassica napus) and sunflower (H. annuus). Our findings, on the other hand, partially conflict with the results of Ultra et al. (2007), who observed that the As toxicity symptoms of sunflower (H. annuus L.), cultured in rhizoboxes, were most clearly alleviated in the AM+/P− treatment, whereas plant growth was promoted most strongly in the AM+/P+ treatment.
Figure 2 reports the concentrations of arsenic (total As, As(III) and As(V)) and P detected in maize after the different treatments. For what concerns total As, in all the treatments its shoot concentration was below the toxicity limits estimated by Kabata-Pendias and Pendias (2001). This result could be the effect of a very low bioavailability of the soil arsenic combined with a very strong retention of arsenic in the roots, the latter suggested by the huge difference in its root and shoot concentrations. Recent findings (Zhao et al. 2009) show that rice (Oryza sativa) is particularly efficient in As uptake from paddy soil, leading to accumulation in grain at concentrations hazardous for health. The same authors remind that As is generally only partially translocated from roots to shoots in plants, except in hyperaccumulators: for example, in wild-type Arabidopsis thaliana, only 2.6 % of As taken up by roots was found in shoots. The explanation is that arsenate is reduced to arsenite rapidly in roots, probably followed by complexation with thiols and possibly sequestration in the root vacuoles. The arsenic uptake and accumulation characteristics of maize, together with Chinese cabbage and tomato, were investigated by Yan et al. (2013) by using culture solutions with different arsenic concentrations. The transfer coefficient of maize exhibited a rising tendency with increasing arsenic concentrations, whereas in Chinese cabbage and tomato it increased and then declined.
Concentration of As and P in shoots and roots at the end of the rhizobox experiments. a Total As concentration. Black bars are for roots and white bars are for shoots. b As(III) and As(V) concentration. Light grey bars are for As(III) and dark grey bars are for As(V) in roots and shoots. c P concentration. Black bars are for roots and white bars are for shoots. AM−/P− not inoculated and not P-fertilized, AM+/P− inoculated and not P-fertilized, AM−/ P + not inoculated and P-fertilized and AM+/P+ inoculated and P-fertilized. Mean of three replicates. Values with different letters are significantly different at Tukey HSD test (p = 0.05)
The low shoot arsenic levels observed by us (<1 mg kg−1 dry weight) were extremely encouraging for cultivating maize on a marginal land like the one studied here, even if further analyses are needed to assess the effective arsenic concentration in the maize edible parts. Many studies on arsenic around the world (Bhattacharya et al. 2010) have found that arsenic concentration in plants from arsenic-contaminated agricultural lands varied from less than 0.01 to about 5.0 mg kg−1, as in our study. The same authors reported that, in arsenic-affected areas of Murshidabad, West Bengal, India, the accumulation of arsenic in various food composites (potato skin, leaves of vegetables, rice, wheat, cumin, turmeric powder and cereals) ranged between <0.0004 and 0.693 mg kg−1. Rice grain can reach arsenic accumulation much above the WHO-recommended permissible level of 1.0 mg kg−1. Studies on the agricultural plants of China and Nepal have found that arsenic accumulated in the range 0.003–0.116 and <0.01–0.55 mg kg−1 dry weight, respectively. Similarly, a study of different plant parts in soils with varying arsenic levels found that the amount of arsenic in root crops, such as potatoes and onions, was correlated with the amount of arsenic in the soils in which they were grown (Dahal, et al. 2008) but did not exceed the FDA standard. Huang et al. (2006) demonstrated that radishes and onions accumulated arsenic when grown in soils with approximately 1 to 25 mg kg−1 of arsenic, although not in concentrations of concern. Finally, another study by Gaw et al. (2008) found that radishes and lettuce grown in soils that had formerly been treated with arsenical pesticides also accumulated arsenic, although not in concentrations that exceeded the FDA standard.
In all our treatments, arsenic concentration did not change in shoots, whereas in roots it significantly decreased (P < 0.05) with P application, in the absence of AM.
To evaluate if the differences in element concentrations could be related to any difference in the uptake, the apparent element uptake was calculated as in Arnetoli et al. (2008) and expressed as the total amount of element per plant per g root d.w. (Table 2). Arsenic uptake was significantly lower only in AM−/P+ treatment in respect to the other ones, and this effect, together with the data on element concentrations, intriguingly suggests a probable apparent nullification of the P effect in the presence of AM, either on plant As concentration or on apparent uptake. These results are partially in conflict with those of Ultra et al. (2007), who cultured sunflower (H. annuus L.) in rhizoboxes for 6 weeks and found that P application increased water-soluble As in the rhizosphere and also that shoot As concentration was slightly reduced by AM inoculation but enhanced by P application. In a long-term (2 months) study like the one presented in this work, Pigna et al. (2009), on the other hand, observed that P fertilization minimizes the translocation of As to the shoots and grain of wheat (Triticum durum L.), grown in uncontaminated soil irrigated with solutions containing As at three different concentrations. Also, the studies of Puckett et al. (2012) on arsenate uptake by an As-tolerant willow (Salix viminalis × Salix miyabeana) and an As-sensitive willow (Salix eriocephala) in hydroponic systems indicate that the presence of phosphate generally decreased plant uptake and translocation of arsenate.
As for the plant concentrations of the two arsenic forms (Fig. 2), the different treatments did not affect As(III) concentration in the roots and significantly increased it in the shoots. As(V) concentration was decreased in the case of AM−/P+ treatment in roots and in all the treatments in shoots. Therefore, the decrease in total As observed in roots after the P treatment could be ascribed to the well-known competition of P with As(V), which was able to produce a lowering in its accumulation in the roots. Most probably, the unaffected shoot concentration was due to the very low shoot As level that maize showed. Such concentrations were so low to probably obscure any possible effect of reduced As(V) root accumulation on shoot As concentration itself, at least for the experimental conditions used. Anyway, the decreased root concentration was enough to determine a lower apparent uptake at the level of the whole plant (Table 2).
Intriguingly, data on the concentrations of the different As forms showed that the apparently unaffected shoot total As concentration after the treatments was the result of an increased As(III) concentration and of a lowered As(V) concentration. As the uptake of As(III) was unchanged after the treatments, and that one of As(V) changed only in the AM+/P− case for the abovementioned reason, the changes in the As(III) and As(V) concentration in the shoots might be ascribed to an increased reduction of As(V) in this organ after the treatments. Probably, such higher efficiency of arsenic reduction could be a direct effect of the ameliorated plant growth in the case of P treatment, with or without AM. The fact that also the AM treatment alone produced such result, without any apparent effect on plant growth, remains hard to explain. Furthermore, our data did not depose in favour of any possible effect of mycorrhizal inoculation on the selective uptake and transport of different As species, leading to different accumulation of As species in maize, unaccountably in contrast with what was reported by Yu et al. (2009), who found that G. mosseae generally decreased concentrations of As(III) in maize roots and concentrations of As(III) and As(V) in the shoots. They also measured the arsenate reductase activity that was detected in maize roots and was reduced with mycorrhizal inoculation.
With respect to control and to P trials, phosphorus concentrations were significantly higher in roots and shoots only for AM+/P− trials (Fig. 2). As the P apparent uptake was higher in the AM+/P− treatment (Table 2), the difference in P concentration might rely on the improvement in P nutrition by AM. Interestingly, AM treatment increased the plant P concentration without any effect on plant growth. This lack of a higher plant biomass production in consequence of a higher P concentration was not due to a possible increase also in As concentration in the plant tissue. The higher P concentration resulted only in an increase in As(III) concentration and decrease in As(V) concentration in the shoots, without any apparent effect on plant growth. Our results were only partially in agreement with other reports. For example, Wang et al. (2008) verified that AM-treated maize had higher shoot biomass and root P and As concentration than the untreated.
In the P+ trials, the opposite situation occurred and higher plant growth was not coupled with higher P concentration in plants. Such effect could probably be a consequence of the higher plant biomass production stimulated by the higher P availability itself. For this reason, in the treatment AM+/P+, an increase in P concentration and uptake was not detected as it occurred only in the case of AM presence, the P-induced higher biomass production, obscuring the effect of AM on P accumulation and uptake themselves. In our plants, probably when P was supplemented directly, the plant readily absorbed it, and such increased its growth, thus rendering not detectable the higher amount of P taken up. On the contrary, when it was AM treatment to improve P nutrition, the process could have been slower and P could have had the time to reveal its higher accumulation inside the root and the shoot before having its effect on growth amelioration. This aspect could be very important to bear in mind in the use of AM.
Conclusions
The presence of arsenic in the examined soil was considerable, but not of concern, as speciation suggested that availability of this trace element was low and its concentration in the plant shoots was below the toxicity limits. Treatments with AM and P, separately or in combination, increased only moderately As(III) concentration close to the maize root. In plant shoots, all the treatments were able to increase the concentration of As(III) and decrease the concentration of As(V), but the shoot total concentration of this element did not change. In roots, only the treatment with P was able to decrease As(V) concentration. Moreover, the results of our study showed that phosphorus application at agronomic rate was much more effective than AM to improve the plant biomass production. This fertilization could be successfully used for cultivating maize on marginal lands like the one under investigation, whereas the effect of arbuscular mycorrhiza inoculation seemed to be of secondary relevance, at least for the experimental conditions applied. Further studies would be required to assess if different levels of fungal infection could be more helpful for plant resistance and growth.
References
Abedin MJ, Feldmann J, Meharg AA (2002) Uptake kinetics of arsenic species in rice plants. Plant Physiol 128:1120–1128
Adriano DC (2001) Trace elements in terrestrial environment; biogeochemistry, bioavailability and risks of metals. Springer, New York
Arnetoli M, Vooijs R, Ten Bookum W, Galardi F, Gonnelli C, Gabbrielli R, Schat H, Verkleij JAC (2008) Arsenate tolerance in Silene paradoxa does not rely on phytochelatin-dependent sequestration. Environ Poll 152:585–591
Bai J, Lin X, Yin R, Zhang H, Junhua W, Xueming C, Yongming L (2008) The influence of arbuscular mycorrhizal fungi on As and P uptake by maize (Zea mays L.) from As-contaminated soils. Appl Soil Ecol 38:137–145
Bhattacharya P, Samal AC, Majumdar J, Santra SC (2010) Arsenic contamination in rice, wheat, pulses and vegetables: a study in an arsenic affected area of West Bengal. India Water Air Soil Poll 213:3–13
Carbonell-Barachina AA, Arabi MA, De Laune RD, Gembrell RP, Patrick WH Jr (1998) The influence of arsenic chemical forms and concentrations on Spartina patens and Spartina alternifolia growth and tissue arsenic concentration. Plant Soil 198:33–43
Cattani I, Fragoulis G, Boccelli R, Capri E (2006) Copper bioavailability in the Zea mays rhizosphere of two Italian soils. Chemosphere 64:1972–1979
Cattani I, Capri E, Boccelli R, Del Re AAM (2009) Assessment of arsenic availability to roots in contaminated Tuscany soils by a diffusion gradient in thin films (DGT) method and uptake by Pteris vittata and Agrostis capillaris. Eur J Soil Sci 60:539–548
Cavagnaro T, Smith F, Smith S, Jakobsen I (2005) Functional diversity in arbuscular mycorrhizas: exploitation of soil patches with different phosphate enrichment differs among fungal species. Plant Cell Environ 28:642–650
Chatain V, Bayard R, Sanchez F, Moszkowicz P, Gourdon R (2005) Effect of indigenous bacterial activity on arsenic mobilization under anaerobic conditions. Environ Int 31:221–226
Chen R, Smith BW, Winefordner JD, Tu MS, Kertulis G, Ma LQ (2004) Arsenic speciation in Chinese brake fern by ion pair high-performance liquid chromatography–inductively coupled plasma mass spectroscopy. Anal Chim Acta 504:199–207
Chen BD, Zhu YG, Smith FA (2006) Effects of arbuscular mycorrhizal inoculation on uranium and arsenic accumulation by Chinese brake fern (Pteris vittata L.) from an uranium mining-impacted soil. Chemosphere 62:1464–1473
Dahal BM, Fuerhacker M, Mentler A, Karki KB, Shrestha RR, Blum WE (2008) Arsenic contamination of soils and agricultural plants through irrigation water in Nepal. Environ Poll 155:157–163
Esteban E, Carpena RO, Meharg AA (2003) High-affinity phosphate/arsenate transport in white lupin (Lupinus albus) is relatively insensitive to phosphate status. New Phytol 158:165–173
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil-rhizosphere-plant system: fundamentals and potential application to phytoremediation. J Biotech 99:259–278
Fitz WJ, Wenzel WW, Wieshammer G, Istenic B (2003) Microtome sectioning causes artifacts in rhizobox experiment. Plant Soil 256:455–462
Gaw SK, Kim ND, Northcoot GL, Wilkins AL, Robinson G (2008) Uptake of ΣDDT, arsenic, cadmium, copper and lead by lettuce and radish grown in contaminated horticultural soils. J Agric Food Chem 56:6584–6593
Gonzalez-Chavez C, Harris PJ, Dodd J, Meharg AA (2002) Arbuscular mycorrhizal fungi confer enhanced arsenate resistance on Holcus lanatus. New Phytol 155:163–171
Gulz PA, Gupta S, Schulin R (2005) Arsenic accumulation of common plants from contaminated soils. Plant Soil 272:337–347
Harrison AP, Cattani I, Turfa JM (2010) Metallurgy, environmental pollution and the decline of Etruscan civilisation. Environ Sci Pollut Res 17:165–180
Huang RQ, Gao SF, Wang WL, Staunton S, Wang G (2006) Soil arsenic availability and the transfer of soil arsenic to crops in suburban areas in Fujian Province, southeast China. Sci Tot Environ 368:531–541
Hughes MF (2002) Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133:1–16
Jackson BP, Miller WP (2000) Effectiveness of phosphate and hydroxide for desorption of arsenic and selenium species from iron oxides. Soil Sci Soc Am J 64:1616–1622
Kabata-Pendias A, Pendias H (2001) Trace elements in soils, 3rd edn. CRC, Boca Raton, 413 pp
Lambkin DC, Alloway BJ (2003) Arsenate-induced phosphate release from soils and its effect on plant phosphorus. Water Air Soil Poll 144:41–56
Luster J, Göttlein A, Sarret G, Nowack B (2009) Sampling, defining, characterising and modeling the rhizosphere—the soil science toolbox. Plant Soil 321:457–482
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Markley CT, Herbert BE (2009) Arsenic risk assessment: the importance of speciation in different hydrologic systems. Water Air Soil Poll 204:385–398
Mascaro I, Benvenuti M, Corsini F, Costagliola P, Lattanti P, Parrini P, Tanelli G (2001) Mine wastes at the polymetallic deposit of Fenice Capanne (Southern Tuscany, Italy). Mineralogy, geochemistry and environmental impact. Environ Geol 41:417–429
Mathimaran N, Ruh R, Vullioud P, Frossard E, Jansa J (2005) Glomus intraradices dominates arbuscular mycorrhizal communities in a heavy textured agricultural soil. Mycorrhiza 16:61–66
Meharg AA, Naylor J, Macnair MR (1994) Phosphorus nutrition of arsenate-tolerant and nontolerant phenotypes of velvetgrass. J Environ Qual 23:234–238
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154:29–43
Norvell WA, Cary EE (1992) Potential errors caused by roots in analyses of rhizosphere soil. Plant Soil 143:223–231
Orlowska E, Godzik B, Turnau K (2012) Effect of different arbuscular mycorrhizal fungal isolates on growth and arsenic accumulation in Plantago lanceolata L. Environ Poll 168:121–130
Paik MK, Kim MJ, Kim WI, Yoo JH, Park BJ, Im GJ, Park JE, Hong MK (2010) Determination of arsenic in polished rice using a methanol–water digestion method. J Korean Soc Appl Biol Chem 53:634–638
Philips J, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Pigna M, Cozzolino V, Violante A, Meharg AA (2009) Influence of phosphate on the arsenic uptake by wheat (Triticum durum L.) irrigated with arsenic solutions at three different concentrations. Water Air Soil Poll 197:371–380
Puckett EE, Serapiglia MJ, DeLeon AM, Long S, Minocha R, Smart LB (2012) Differential expression of genes encoding phosphate transporters contributes to arsenic tolerance and accumulation in shrub willow (Salix spp.). Environ Exp Bot 75:248–257
Rahman MM, Chen Z, Naidu R (2009) Extraction of arsenic species in soils using microwave-assisted extraction detected by ion chromatography coupled to inductively coupled plasma mass spectrometry. Environ Geochem Health 31:93–102
S.A.S. Institute, SAS/STAT user’s guide. Version 6, vols. 1 and 2, 4th ed., SAS Inst., Cary, NC, 1996
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic, London
Ultra VU, Tanaka S, Sakurai K, Iwasaki K (2007) Effects of arbuscular mycorrhiza and phosphorus application on arsenic toxicity in sunflower (Helianthus annuus L.) and on the transformation of arsenic in the rhizosphere. Plant Soil 290:29–41
Valberg PA, Beck BD, Bowers TS, Keating JL, Bergstrom PD, Boardman PD (1997) Issues in setting health-based cleanup levels for arsenic in soil. Reg Tox Pharm 26:219–229
Van den Broeck K, Vandecasteele C, Geuns JMC (1998) Speciation by liquid chromatography-inductively coupled plasma-mass spectrometry of arsenic in mung bean seedlings used as a bio-indicator for arsenic contamination. Anal Chim Acta 361:101–111
Wang Z-H, Zhang J-L, Christie P, Li X-L (2008) Influence of inoculation with Glomus mosseae or Acaulospora morrowiae on arsenic uptake and translocation by maize. Plant Soil 311:235–244
Wenzel WW, Wieshammer G, Fitz WJ, Pushenreiter M (2001) Novel rhizoboz design to assess rhizosphere characteristics at high spatial resolution. Plant Soil 237:37–45
Xia YS, Chen BD, Christie P, Smith FA, Wang YS, Li XL (2007) Arsenic uptake by arbuscular mycorrhizal maize (Zea mays L.) grown in an arsenic- contaminated soil with added phosphorus. J Environ Sci 19:1245–1251
Yan JZ, Ying WY, XiaoLi R (2013) Arsenic uptake accumulation characteristics in varied crops. J South Agric 44:793–796
Yu Y, Zhang S, Huang H, Luo L, Wien B (2009) Arsenic accumulation and speciation in maize as affected by inoculation with arbuscular mycorrhizal fungus Glomus mosseae. J Agric Food Chem 57:3695–3701
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Acknowledgments
The authors wish to thank Roberto M. De Santis, Vincenza Cozzolino, Massimo Pigna and Antonio Violante (Dipartimento di Scienza del Suolo, della Pianta, dell’Ambiente e delle Produzioni Animali- Università degli Studi di Napoli Federico II, Portici (NA), Italy) for their support in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Rights and permissions
About this article
Cite this article
Cattani, I., Beone, G.M. & Gonnelli, C. Influence of Rhizophagus irregularis inoculation and phosphorus application on growth and arsenic accumulation in maize (Zea mays L.) cultivated on an arsenic-contaminated soil. Environ Sci Pollut Res 22, 6570–6577 (2015). https://doi.org/10.1007/s11356-014-3837-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3837-0