Abstract
Amending polycyclic aromatic hydrocarbon (PAH)-contaminated soils with biochar may be cheaper and environmentally friendly than other forms of organic materials. This has led to numerous studies on the use of biochar to either bind or stimulate the microbial degradation of organic compounds in soils. However, very little or no attention have been paid to the fact that biochars can give simultaneous impact on PAH fate processes, such as volatilization, sorption and biodegradation. In this review, we raised and considered the following questions: How does biochar affect microbes and microbial activities in the soil? What are the effects of adding biochar on sorption of PAHs? What are the effects of adding biochar on degradation of PAHs? What are the factors that we can manipulate in the laboratory to enhance the capability of biochars to degrade PAHs? A triphasic concept of how biochar can give simultaneous impact on PAH fate processes in soils was proposed, which involves rapid PAH sorption into biochar, subsequent desorption and modification of soil physicochemical properties by biochar, which in turn stimulates microbial degradation of the desorbed PAHs. It is anticipated that biochar can give simultaneous impact on PAH fate processes in soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous contaminants in environmental matrices across the globe (Johnsen et al. 2005; Chen et al. 2004). They are divided into low molecular weight (LMW) and high molecular weight (HMW). The LMW PAHs (Fig. 1) are toxic (Sims and Overcash 1983) and are mostly found in crude oil (Meckenstock et al. 2004; Raza et al. 2013). The HMW PAHs are products of non-exhaustive combustion of materials, e.g. bush fires, fossil fuels, eruptions from subsurface rocks (volcanic rocks), intake of roasted food, water and air (Diggs et al. 2011; Chen et al. 2004). The HMW PAHs (Fig. 2) may lead to cancer in humans (Fu et al. 2012; Fagernäs et al. 2012). Furthermore, PAHs are less reactive but can be activated by physicochemical reactions to become toxic, resulting in DNA damage, mutations and tumours (Harvey 1991; Fu 1990; Fu et al. 2012). Wilson and Jones (1993) had also reported that PAHs are teratogenic, i.e. they can hinder the development of foetus, causing deformities in newly born children. Other toxic effects of PAHs on humans are well documented (Vazquez-Duhalt 1989; Fu et al. 2012; Diggs et al. 2011; Sinha et al. 2005).
The sources and distribution of PAHs in environmental media are critically important in understanding their mobility and persistence in soils. PAHs usually get to surface water, soils and sediments by dry and wet deposition, runoff from roads, industrial effluents, as well as dissolution from creosote-treated woods (Chen et al. 2004). In the air, the HMW PAHs usually partition to aerial particulate matter, due to their low vapour pressure, while the LMW PAHs partition to both atmospheric moisture and particulate matter, due to their high vapour pressure (Jones and De Voogt 1999; Baker and Eisenreich 1990; McVeety and Hites 1988). PAHs persist in soils and sediments by partitioning to their organic matter fraction (Chen et al. 2004). Subsequently, the soil is the major uptake medium of PAH by plants and animals (Phillips 1999; Diggs et al. 2011). The same authors reported that benzo[a]pyrene can be found in concentrations of up to 2–500 ng/day in human diets, and therefore having a possibility for bioaccumulation. Diggs et al. (2011) reported a good correlation between meat consumption and cancer of the oesophagus and colon. However, this is compound specific as the authors also reported lack of correlation between cancer of the colon and benzo[a]pyrene residing in meat. Nevertheless, studies have shown that there is a relationship between PAH intake from meat and colon cancer (Diggs et al. 2011). This reiterates the fact that the risks posed by PAHs are compound specific (Canadian Council of Ministers of the Environment (CCME) 2008; Edwards et al. 1997)
The above-mentioned risks posed by PAHs are due to the water-soluble concentrations, and the use of biochar reduces the transport of PAH in soils (Oleszczuk et al. 2012). Although the amendment with biochar took a long time to bind PAHs in the studies mentioned above, its advantages over other forms of amendment are well documented (Sparrevik et al. 2011; Oleszczuk et al. 2012; Barrow 2012; Tsai et al. 2006). Biochar stands out as the only material that may give simultaneous impact on the three major fate processes (volatilization, sorption and biodegradation). However, volatilization was not discussed in this review because PAHs are mainly semi- or no volatiles. Additionally, volatile petroleum hydrocarbons are only toxic in a enclosed space, but most oil spills occur in open space. Some review papers have documented a large set of materials that can be used as potential feedstock for biochar production (Gupta et al. 2009; Ali et al. 2012).
Studies have come up with different definitions for biochar, and these definitions are similar in approach, i.e. biochar is considered only as a sorbent (Cornelissen et al. 2005; Beesley et al. 2011). A more encompassing definition of biochar was that by Lehannes and Joseph (2009), which takes into account biochar potentials to give simultaneous impact on sorption and biodegradation. The authors defined biochar as a biological material produced in the absence of oxygen, at temperatures below 700 °C, to generate more permeable, less dense and carbon-rich product. As a result of their surface areas, aromatic and aliphatic structures, which are a function of production temperature, can bind PAHs tenfold greater than organic matter (Cornelissen et al. 2005). Also, due to their recalcitrance, pore structures and nutrient properties, they can influence microbial degradation of PAHs in soils. This definition implies that a biochar nutrient property (nitrogen) starts to diminish at temperatures above 700 °C (Bagreev et al. 2001; Chan and Xu 2009).
Although studies (Bushnaf et al. 2011; Meynet et al. 2014; Qin et al. 2013; Chen et al. 2012a) have used biochars produced at temperatures above 700 °C and applied it either as a sorbent for VPH remediation or in combination as biostimulant or as a carrier of microbes to augment biodegradation by bacteria. Conversely, these studies cannot adequately explain the scientific mechanisms that may underpin the use of biochar as a soil amendment to stimulate biodegradation by bacteria. This is because N which is also required for microbial growth and metabolism starts to diminish at temperatures above 700 °C, and again, relying on one production temperature amendment alone will neither result in proof of mechanism nor the formulation of adequate hypotheses to draw overarching conclusions. Similarly, Beesley et al. (2011) reported that sorption into biochars alone may not be adequate to contain soil contaminants. Consequently, the authors suggested a combination of biochar and other amendments to improve remediation performance, but the present review has shown that biochars can give simultaneous impact on sorption and biodegradation. The conceptual model showing how biochar may give simultaneous impact on PAH fate processes is represented in Fig. 3. The possible mechanisms represented in Fig. 3 through which biochar can give simultaneous impact on sorption and stimulation of microbial degradation of PAHs in soils are discussed following a review of literatures relating to biochar as an amendment for soil remediation, questions that needed to be addressed before biochar remediation can be considered a possibility were raised and attempts were made to answer these questions by either using data from literature or from initial experimental results of this study. Conclusions were made, and future research areas have been highlighted.
Triphasic concept of PAH degradation in the presence of biochar; (i) Spilt PAH is sorbed by biochar thereby reducing transport and volatilization. (ii) Biochar modifies soil properties; pH, nutrient, moisture, microbial population, oxygen and sorbed PAH desorbs over time. (iii) Microbial degradation of desorbed PAH
How does biochar affect microbes and microbial activities in the soil?
Studies (Saito and Marumoto 2002; Warnock et al. 2007; Ennis et al. 2012) have reported that biochar can affect microbes and microbial activities through the following biochar physicochemical properties: (i) shelter; (ii) effects of water holding capacity, oxygen and nutrients; (iii) pH; and (iv) carbon and energy. These biochar properties are discussed below:
-
(i)
Shelter
The presence of pores, high surface area, ability to bind and retain PAHs as well as nutrients (N and P), makes biochar a good shelter for microbial growth (bacteria and fungi) and reproduction (Thies and Rillig 2009). Increases in biochar pore spaces is a function of production temperature and nature of feed stock (Thies and Rillig 2009; Ennis et al. 2012; Downie et al. 2009), and the accessibility of microbes to these pore spaces will in turn depend on the pore size. In particular, these pore spaces provide protection for the microbes against predators (Saito and Marumoto 2002; Warnock et al. 2007). Samonin and Elikova (2004) reported that for ideal attachment, the biochar pore sizes should be greater than the microbial cell size by a factor of 2 or 5, e.g. Bacillus mucilaginosus and Actinobacter sp. can enter a pore size of 2–4 μm.
Studies have also contrasted granular biochar (GBC open pores) versus powdered biochar (PBC; collapsed pores) to investigate the effect of pore size on microbial habitation of biochars. Ezawa et al. (2002) found that microbes such as arbuscular mycorrhyza fungi were more abundant in soils amended with PBC than those of GBC. Their observation was also attributed to biochar porosity and high rate of biochar application. However, it is not clear if this response was also a result of the acidic condition of soil (pH 5) which favours growth of fungi.
The ability of biochar to serve as a shelter for microbes is also exhibited by the appearance of microbial attachment to its surface (Thies and Rillig 2009), and therefore decreasing the rate of microbial leaching in soil (Pietikäinen et al. 2000). Hence, this would result in greater bacterial biomass, while the fungal biomass remains unchanged as a result of decreased fungal mobility due to their hyphal arrangement (Lehmann et al. 2011). This will also favour microbes that rely on their extracellular enzymes to degrade PAHs in soils into compounds that can be absorbed by their cells and consumed during metabolic activity (Paul 2006). Consequently, microbes prefer to remain closer to surfaces where they release extracellular enzymes into their surrounding (Thies and Rillig 2009). For example, in PBC, where the enzyme active spot is exposed, increased microbial activity will likely take place. Conversely, in GBC where the enzyme active spot is shielded, it may lead to reduction in activity (Thies and Rillig 2009). Hence, enzyme activity will partly depend on the intensity with which the microbe is retained on the biochar surface (Thies and Rillig 2009).
More recently (Jindo et al. 2012), the effect of biochar porosity, which serves as a microhabitat for soil microbes has been highlighted. The mechanism of microbial attachment to biochar surfaces have been reported to be due to hydrophobic or electrostatic attraction (Lehmann et al. 2011). Although biochars have a low isoelectric point (i.e. they are electrically neutral) (pH <4) (Cheng et al. 2008; Zimmerman 2010), electrostatic attraction can be facilitated by the presence of minerals or bio-oils on biochar surfaces (George and Davies 1988).
Ennis et al. (2012) also claim that biochar surfaces can adsorb nutrients and cations in soil solution, thus leading to an increase in the concentration of available nutrients for microbial metabolism (Wardle et al. 1998). Yamato et al. (2006) found greater P uptake following, biochar amendment, which is connected to increased soil pH. Other authors (Ortega-Calvo and Saiz-Jimenez 1998) found that adsorption of microbes adjacent to substrate increases bioavailability, which will in turn attract microbes closer to the surfaces.
Biochars can also improve soil structure, which in turn leads to greater microbial populations. For example, biochar addition to soils reduces soil compactness and aids fluid movement, which indirectly affects diverse population of soil microbes (Lehmann et al. 2011). However, the fact that biochar surface area and pore volume may be altered by obstructions from sorbed organic matter (Pignatello et al. 2006) and nutrients (Joseph et al. 2010) or soil particles shows that this assertion may not always be the case. Additionally, this may alter the sorption properties of soils (Liang et al. 2006a) amended with biochar as well as carbon substrate for energy (Kasozi et al. 2010) and pore spaces available to microbes (Lehmann et al. 2011).
-
(ii)
Effects of water holding capacity, oxygen and nutrients
It is well established that the pore spaces in biochars increases its water holding capacity, hence greater potential to shelter microbes (Thies and Rillig 2009) and increased air/water retention (Downie et al. 2009). These increases are greater with increasing production temperature owing to increased surface area (Ennis et al. 2012). When the water holding capacity of two biochars, (i) humus and (ii) wood, were compared with those of activated carbon and pumice, respectively, it was found that the two biochars had greater water holding capacity compared with the latter materials by a factor of 2 (activated carbon) and 3 (pumice) (Pietikäinen et al. 2000).
Similarly, Anderson et al. (2011) found that biochar amendment (in 3 months) significantly increased soil moisture by 3.5 % compared with the non-amended soil that significantly decreased soil moisture content by >5 %. Apart from water holding capacity, biochar amendment also affects the oxygen status in the soil. Antal and Grønli (2003) reported that gases (e.g. CO2 and O2) can dissolve into the pore water, fill up air spaces or form bonds with the biochar surfaces. Depending on the magnitude of the air to water-saturated pore spaces, concentration of CO2 to O2 and the degree of sorption, any of aerobic or anaerobic state will prevail in the pore spaces of biochar (Thies and Rillig 2009). This suggests that biochar may support both aerobic and anaerobic hydrocarbon degradation in soils.
Further evidence for increased water holding capacity with biochar additions has also been investigated with two biochars: (i) Zea mays and (ii) Quercus sp., manufactured at 350 and 600 °C, respectively, and then subjected to different soil moisture regimes, e.g. saturated, non-saturated and mixture of saturated/non-saturated. It was found that biochar degradation increased under the non-saturated and saturated/non-saturated regimes compared with the completely saturated regime (Nguyen and Lehmann 2009). This was attributed to the presence of oxygen for microbial degradation in the latter regimes (Morris et al. 2004), with the saturated/non-saturated supporting the highest degradation (Wu and Brookes 2005), owing to loosening of soil particles (Denef et al. 2001) and nutrients (Thanh Nguyen and Marschner 2005). Since oxygen and water are rate-limiting parameters to hydrocarbon degradation, any amendment that will ensure moderate levels of these two parameters or greater O2 concentration (Thies and Rillig 2009), may greatly enhance biodegradation. The effects of environmental restricting parameters following amendment of soils with biochar are worth investigating.
Another example of biochar chemical property is that of nutrient retention, which is a function of the surface chemistry of the biochar, which in turn affects microbial activity. For example, newly produced biochars have net positive charges on the surface or anion exchange capacity (AEC) which vanishes with time (Cheng et al. 2008), due to the binding of phosphates (Beaton et al. 1960) and nitrates in soils. Similarly, nutrient retention is also enhanced by cation exchange capacity (CEC) (Chan and Xu 2009). While contrasting the ability of high production temperature (HPT) and low production temperature (LPT) biochars to bind ammonia, studies found that the LPT biochars were able to efficiently bind ammonia than the HPT biochars (Day et al. 2004; Asada et al. 2002); the greater binding to the LPT biochars was suggested to be a result of the presence of acidic functional groups (carboxyl), which is a product of decomposition of cellulose and lignin at LPTs (Asada et al. 2002). These nutrients are then taken up by microbes attached to the biochar surfaces during metabolic activity. However, as the nutrients are utilised by the microbes, this does not lead to their replenishment, since the AEC vanishes as earlier reported.
This has led to other improvements. Recently, Chen et al. (2011) used a new kind of biochar, having magnetic properties, for greater nutrient retention. They used chemical co-precipitation of Fe3+ and Fe2+ on orange peel feedstock, after which they pyrolysed the resulting mixture at LPTs 250 and 400 °C and HPT 700 °C, respectively, to generate both magnetite (Fe3O4) and biochar. This sorbed PAH (naphthalene) in addition to retaining nutrients more efficiently than pure biochars. In particular, when sorption of PAH and phosphate was compared simultaneously, the LPT 250 and 450 °C magnetic biochars, showed greater capacity to bind PAH (naphthalene) and phosphate simultaneously than their non-magnetic counterparts. Interestingly, this study provides basic evidence showing that PAH and phosphate can be sorbed simultaneously by biochar and with further microbial attachment, this study can key into the triphasic concept proposed in this review.
-
(iii)
Effect of pH
Another physicochemical parameter that influences microbial diversity and activity in the presence of biochar, is pH (Wardle 1998). Studies (Blagodatskaya and Kuzyakov 2008; Wardle et al. 1998) have found that the diversity and abundance of bacteria in soils were greater at the neutral pH ranges (6.6–7.3) compared with acidic pH ranges (<6.5). For example (Farrell et al. 2013), found that the biochar-amended soil, increased in pH from 4.82 to 8.96. This effect is dependent on the pH of biochars which differs according to the feed stock, production temperature (Thies and Rillig 2009) and the extent of oxidation (Cheng et al. 2006), which generates organic acids. Farrell et al. (2013) reported that a significantly higher pH was observed in eucalyptus shoot biochar containing 1.47 mg g−1 carbonate in comparison with that of wheat shoot biochar with 0.9 mg g−1 carbonate; this demonstrated a direct correlation between pH and carbonate concentration.
The pH of biochar is commonly in the range from <4 to >12 (Lehmann 2007; Chan and Xu 2009). Furthermore, while fungi can survive under acidic pH, bacteria mainly survive at neutral or alkaline pH (Rousk et al. 2010). Therefore, the incorporation of biochar in soils may lead to variations in soil microbial population, by altering the bacteria to fungi ratio, in addition to the prevalence of different genera in the community (Thies and Rillig 2009). Biochar pH may also lead to changes in soil functions by influencing both enzyme activities and total microbial activity (Thies and Rillig 2009). When acid soils were amended with biochar, a positive correlation was observed between pH and microbial biomass (Steiner et al. 2004; Van Zwieten et al. 2010; Rousk et al. 2010). The authors concluded that microbial reproduction was favoured following biochar additions to the acidic soil. Several studies have also confirmed that biochar has a continuous influence on soil pH (Farrell et al. 2013; Hass et al. 2012; Ippolito et al. 2012; Anderson et al. 2011).
-
(iv)
Biochar as a source of electrons (carbon) and energy
It well known that biochar is resilient to microbial degradation (Thies and Rillig 2009). Evidence for this was reported by Liang et al. (2006a) whose observation found that surface oxidation of biochar particles took place after a long period of time. Hilscher and Knicker (2011) observed an increase in the number of O-functional groups, with decrease in the aryl C- and N-heterocyclic groups in humus-amended soil. This implies that biochar does not serve as a significant substrate during microbial metabolism. Instead, residues such as bio-oils and other molecules that sorb into the biochar particles are likely the only substrates present to aid microbial growth and metabolism (Thies and Rillig 2009; Ennis et al. 2012). Additionally, the nature and amount of biochar applied to the soil can also affect soil microbial population (Thies and Rillig 2009; Ennis et al. 2012).
There are evidences that PAHs are generated in biochar with slow carbonization (Fagernäs et al. 2012). Conversely, studies have also reported that slow carbonization reduces the risk of yielding PAHs (Barrow 2012). Flash and LPT carbonization, results in residual bio-oils and concentrated materials (Steiner et al. 2008) that may serve as substrates for microbes at low concentrations. At higher concentrations (Steiner et al. 2008; Deenik et al. 2010), they became toxic (PAHs) to certain microbes (Thies and Rillig 2009; Painter 1998). Consequently, only the microbes that possess enzymes needed to degrade these compounds will settle on the biochar surfaces (Thies and Rillig 2009). The authors also note that microbes that settle on newly made biochar with these compounds on the surfaces differ from those that settle on the biochar surfaces overtime, when these compounds must have been metabolised. Nonetheless, Ogawa (1994) and Zackrisson et al. (1996) have found that PAH can be degraded by some microbes, though the degradation will not have any effect on the microbial community structure (Thies and Rillig 2009). Smith et al. (1992) suggested that a combination of both nutrients and compounds adsorbed by biochar can change the microbial community structure. In conclusion, biochar is known to contain some bio-oils some of which may include PAHs and other hydrocarbon fractions, to serve as substrate for microbial metabolism.
Experimental evidences for the role of biochar in providing substrate to soil microorganisms were reported by Durenkamp et al. (2010), Smith et al. (2010), Das et al. (2008), Steiner et al. (2008), Hamer et al. (2004), Wardle et al. (2008), Zimmerman et al. (2011) and Cheng et al. (2006). They observed rapid release of CO2 and greater degradation of soil C when fresh biochars were applied to soils. Even though, this later reverted to control levels, within some days. The authors concluded that the bio-oil from the carbonization were the probable substrates. Evidences to show that bio-oils serve as short-term substrates were reported by Steinbeiss et al. (2009), whose study observed decreased levels to lack of differences in CO2 evolution in soils amended with glucose and yeast biochars after 3 months of incubation. This implies that the bio-oils have a short effective half life. Conversely, when the effect of biochar degradation on other soil organic carbon components was investigated, Wardle et al. (2008) attributed the greater degradation of soil C in biochar-amended soils to higher microbial biomass rather than the bio-oil.
Other evidences to support the effect of bio-oils are discussed: When two contrasting biochar materials were compared with activated carbon and pumice, even when the activated carbon was found to have sorbed greater quantity of liquefied organic carbon, than the two biochars and pumice, respectively (Pietikäinen et al. 2000), the authors found that microbial respiration was highest in the two biochar materials with the microbial communities different to those of the two non-biochar materials. Furthermore, other investigators using different approaches, sequencing (O’Neill et al. 2009) and genetic finger printing (Grossman et al. 2010) found variations in soil microbial community make up in biochar-rich soils even after long periods, compared with nearby soils of similar mineralogy. It, therefore, follows that the presence of biochar has a prolonged effect on soil microbial community even after the bio-oils have been metabolised (Ennis et al. 2012). Wardle et al. (1998) attributed this to higher substrate concentration and availability over longer periods. As a result, soil microbes that settle following biochar amendment differ based on biochar feedstock (electron donor source), quantity, frequency of amendment and residence time (age), all influence biochar effects over longer periods (Joseph et al. 2010). Unfortunately, when bio-oils obtained from biochar was used to prepare agar media, it was found to be toxic to Bacillus and Bordetella pertussis (Pollock 1947), thus confirming the presence of toxic compounds in biochars.
The effects of biochar on soil microbial activity such as nitrogen fixation, has also been investigated, e.g. studies on the effect of biochar to N-fixation showed similar findings where microbes (bacteria and archaea) associated with biochar-amended soils having the gene coding for the nitrogenase enzyme, required for N2 fixation (Ogawa 1994; Rondon et al. 2007). When the effect of biochar on transformation of soil nutrients as affected by microbes was investigated, several investigators (DeLuca et al. 2006; Gundale and DeLuca 2006; MacKenzie and DeLuca 2006; Ball et al. 2010) found that nitrification was greater in forest soils amended with biochar, and this was evident by biochar retention of phenolics that usually hinders nitrification (DeLuca et al. 2006) together with higher abundance of NH3-oxidising bacteria (Ball et al. 2010).
In contrast to the studies above, when arable and grassland soils were amended with biochar, neither N transformation or decrease was observed (DeLuca et al. 2006; Rondon et al. 2007). More recent investigators attributed this to N sorption during transformation of a degradable fraction of biochar having high C/N ratio. In addition, the higher the fraction of degradable biochar concentration, the higher the N sorption, reducing N availability (Deenik et al. 2010). These effects can also be explained by the large population of microbes following biochar amendments (Lehmann et al. 2011). Steinbeiss et al. (2009) investigated the effect of 13C-labelled glucose (N-free) and yeast (5 % N) biochars, respectively, they found that the biochar produced from yeast supported fungal growth in the soil, while that produced from glucose supported gram-negative bacteria. This implies that the presence of N favours fungal activities in soils. Other investigators (Zavalloni et al. 2011) found that biochar did not have any effect on soil organic N. The presence of biochar in soils has also been reported to support the activities of Bradyrhizobiaceae and Hyphomicrobiaceae, both of which are important in nitrogen cycling (Anderson et al. 2011). The influence of biochar in N cycling has also been corroborated by other authors (e.g. Chan et al. 2008a, b; Major et al. 2009) who found that biochar additions support phosphate solubilising bacteria. The addition of biochar also modifies C movement by increasing the presence of bacterial population that has the ability to utilise PAHs (Anderson et al. 2011).
The effects of biochar amendment on total and functional soil microbial community have been discussed elsewhere. Khodadad et al. (2011) used quantitative polymerase chain reaction (qPCR), nested PCR-automated ribosomal intergenic spacer analysis (ARISA) and culture-dependent approach to investigate the effects of two contrasting biochar feedstock on soils associated with and without bushfires. They found that the soils amended with HPT biochar had increases in viable cell counts as well as the quantity of 16S rRNA gene. Whereas the soils not impacted by bushfire and amended with LPT biochar showed increases in CO2 evolution but with a decrease in bacterial diversity. The 16S rRNA sequence analysis showed an increase in population of Actinobacteria and Gemmatimonatedes, prompting the authors to suggest that biochar amendments usually lead to an enrichment of particular taxa; Actinobacteria is known to support organic matter decomposition and humus formation (Ventura et al. 2007).
An increased population of Verrucomicrobia has also been observed to respond to biochar amendments in soils (Grossman et al. 2010). Other microbial taxa that were associated with biochar applications have also been reviewed by Lehmann et al. (2011). However, this review is not focused on taxa associated with biochar, since not all of them are associated with PAH degradation. In addition to the molecular techniques mentioned above, other strategies that are yet to be applied to biochar-amended soils are metagenomic analysis in which PCR biases could be bypassed to recover inaccessible communities (Delmont et al. 2011). Microarrays are capable of bypassing PCR/amplification distortions; they are quantitative, high thorough put and permit analysis of community composition, structure and functions concurrently (Zhou et al. 2010). It is noteworthy that DNA extraction from biochar-amended soils is a critically important step towards the application of any molecular technique. This is because DNA released into biochar from microbial cells can also be sorbed by the biochar (Lehmann et al. 2011). Further research is needed to develop techniques that can lead to total liberation of DNA sorbed into biochars so as to avoid underestimation of microbial population and activity in biochar-amended soils relative to their non-amended counterparts.
One principal explanation for the increased presence of microbes with biochar amendment was reported by Kolb et al. (2009). They used fresh biochars made from livestock manure and found increased microbial activity in biochar-amended soils. The authors attributed this to the high N and P content in the livestock manure biochar, as well as a degradable fraction of carbon in the biochar as evidenced by reduced substrate-induced respiration when greater quantity of biochar was added. This implies that biochar C from manure source is easily degradable and that microbes prefer manure biochar C to the substrate. These findings show that livestock manure biochar can aid co-metabolism of HMW PAHs. Further evidences to support greater N and P contents as well as degradable C in livestock manure biochar were reported by Cantrell et al. (2012). They found greater N ranging from 1.51 HPT 700 °C to 4.45 % LPT 350 °C and P contents; 10 g kg−1 LPT 350 °C to 59 g kg−1 HPT 700 °C, respectively. C was reported in the range of 49.28 % (LPT 350 °C to 56.67 % HPT 700 °C. Similarly, Cimò et al. (2014) in their study reported livestock manure biochar C ranging from 25 to 32%, while % N ranged from 1.08 to 2.3. Although the livestock manure in the studies discussed above had high N and P contents, they are still referred to as class 2 biochar because of their low C content (International Biochar Initiative 2012). This suggests that livestock manure biochar may not be a good candidate for sorption since they are not thoroughly carbonised but may have greater impact on microbial degradation due to the high N and P contents. In contrast, our locally generated biochar from coconut shell (Cocos nucifera) feedstock falls under class 1 biochar (Table 1), having greater C but lower N and P contents compared with the livestock biochars. This implies that it may be a better candidate to give simultaneous impact on sorption and biodegradation. It can therefore be concluded that for biochar to be able to impact on both fate processes simultaneously, class 1 biochars will be better candidates as they have greater capacity to sorb spilt oil than class 2 biochars and unlike the C in class 2 biochar that is degradable, biochar C from class 1 biochar has greater recalcitrance and hence the spilt oil will be the major substrate to support microbial activity.
In contrast to the studies reviewed above, negative effects in soils amended with biochar have also been reported. Several studies found reduced degradation of soil organic carbon in biochar-amended soils (Murage et al. 2007; Kuzyakov et al. 2009; Spokas and Reicosky 2009; Kimetu and Lehmann 2010) or absence of degradation of soil organic carbon, respectively (Haefele et al. 2009; Steiner et al. 2009). Evidences to support the above observations were further reported by Zavalloni et al. (2011) whose work showed that biochar amendment did not increase soil microbial populations as well as that of Jin (2010) who observed reduced soil microbial activity with biochar. Lehmann et al. (2011) attributed the observations above to the presence of recalcitrant C in the biochar or the sorption of the easily degradable organic carbon by the biochar.
Other investigators have also reported negative effects with biochar amendments: Prolonged incubations and field investigations have revealed that biochar decreases degradation of soil C (Kuzyakov et al. 2009; Kimetu and Lehmann 2010; Zimmerman et al. 2011). However, this may not always be the case as Lehmann et al. (2011) suggested that lower CO2 evolution observed at the condition where microbial biomass is high, may be due to the precipitation of CO2 in the form of carbonates on the surfaces of biochars with alkaline pH, thereby reducing the concentration of measured CO2 evolved. Evidences of lower microbial biomass in biochar-amended soils have also been reported. Several investigators (Jin 2010; Bailey et al. 2011) found decreased enzyme (glucosidase and cellobiosidase) activities following amendment of soils with biochar. Again, Lehmann et al. (2011) attributed the above observations to the possibility of both the degradable carbon and microorganisms lying adjacent to each other on biochar surfaces, which may in turn result in greater utilisation of the C and curtailing the demand for enzyme production. The authors concluded that the above processes may be the likely reasons for reduced CO2 evolution as well as enzyme activity in biochar-amended soils. There are also evidences showing that biochar additions could give positive and negative responses simultaneously. Jin (2010) reported that biochar applications give rise to greater abundance of certain microbes, e.g. Zygomycota a fungi known for the degradation of easily degradable C compounds was found in Alfisols of temperate climates amended with corn biochar, while presence of other fungi such as Basidiomycota associated with lignin degradation and Ascomycota decreased, respectively.
On the other hand, reduced presence of microbes that degrade HMW compounds may result in the decreased degradation of aromatic structures of biochar, making the biochar more stable (Lehmann et al. 2011). For example, two PBC derived from glucose and yeast showed different behaviours when added to soil (Steinbeiss et al. 2009). The authors found that the glucose biochar led to a decrease in soil microbial biomass. Whereas the yeast biochar did not show any change in the phospholipid fatty acid (PLFA) composition of the soil except for support of fungal growth observed with the yeast biochar. When terminal restriction fragment length polymorphism (TRFLP) was used to investigate changes in soil microbial community, it was observed that soils amended with biochar significantly differed from the controls by having >5 % increases in bacteria families of Bradyrhizobiaceae, (∼8 %), Hyphomicrobiaceae (∼14 %), Streptosporangineae (∼6 %) and Thermomonosporaceae (∼8 %), whereas the presence of biochar decreased the abundance of Streptomycetaceae (approx. −11 %) and Micromonosporaceae (approx. −7 %) (Anderson et al. 2011). In conclusion, biochar additions can result in negative or positive effects on soil microbes with biochars of alkaline pH favouring positive effects.
What are the effects of adding biochar on sorption of PAHs?
Three mechanisms have been suggested for the sorption of PAH to biochars. According to Sander and Pignatello (2005) and Cornelissen et al. 2005), the strong sorption of PAH to biochar is by π–π interactions between the benzene rings of the PAH and those of the biochars; secondly, sorption into the nanopores of biochars (Cornelissen et al. 2005); and thirdly, rapid adsorption at lower aqueous PAH concentrations due to greater energy of adsorption and absorption at higher aqueous concentrations due to the attainment of maximum adsorption (Allen-King et al. 2002). Against this backdrop, sorption into biochar is unlikely to be feedstock dependent. Several investigators (Schmidt and Noack 2000; Titirici et al. 2007) also showed that irrespective of feedstock, biochar have the same chemical structures, heterocyclic (O-containing) pyran and furan rings of carbohydrates or phenol-like structures, which are the major components of lignin. They form benzene and other PAHs due to the conversion of the aliphatic to aromatics as the biochar is generated (phenolic, carbonyl and hydroxyl functional groups) (Titirici et al. 2007)
Consequently, sorption of PAHs by biochar are mainly a function of the production temperatures and PAH chemical structures. Aromatic hydrocarbons are slightly polar and can be covalently bonded to polar surfaces of biochar (Cornelissen et al. 2005). As a result, sorption of PAHs into biochar is related to its aromaticity (Chen et al. 2008a; Chen et al. 2009b); the intensity of aromaticity varies with production temperature (Wang et al. 2010). HPT results in higher aromaticity in biochars (Brewer et al. 2009) and lower CEC, respectively, owing to higher surface area obtained at HPT (600 °C) and loss of volatiles, which harbours most of the negative charges and CEC in the form of organic acids (Lehmann et al. 2009b). As a result, increases in sorption of polar organic compounds, e.g. catechol into biochars are observed mostly at 400 to 650 °C, owing to higher nanopores (Kasozi et al. 2010). In addition, catechol is toxic to microorganisms (Chen et al. 2009) and has been found to sorb tenaciously to HPT biochars made from corn stover (Kasozi et al. 2010). While this may reduce its risks in soils, Hockaday et al. (2007) reported that aromatic fraction of black carbon were established in surface and ground water, as dissolved organic carbon (DOC), constituting 7–9 % of the total organic carbon (TOC) in the surface waters (Mannino and Harvey 2004) and 8–17 % of TOC in river water (Masiello and Druffel 2001). Mannino and Harvey (2004) also noted that the black carbon bearing PAH was either from particulate matter deposition or an attachment to surface sediment. These differ from biochar incorporated as an amendment into the soil that may not be subjected to aerodynamic transport, after absorbing the spilt PAHs. Similarly, the PAH from black carbon found in ground water by the same authors may also be due to the fact that the subsurface contains less microbes and organic matter that may help to degrade and bind the PAH from particulate matter. Although the International Biochar Initiative (2012) listed PAHs as one of the toxicants that need to be tested in biochars, PAH yield in biochar depends on production conditions, whereby fast pyrolysis may yield more PAHs compared with slow pyrolysis (Thies and Rillig 2009). As a result, the effect of production conditions and temperatures on biochar PAHs is worth investigating. For example, studies by Chen and Yuan (2011) found that concentrations of both chemical extractable and subsequently released biochar PAHs in water were negligible. In conclusion, biochars should always be incorporated into the soils as an amendment and not deposited on the surface to avoid transport. Likewise, biochars incorporated into the top soil should be mixed together with the subsoil for greater microbial activity and binding by organic matter to avoid its transport.
Huang and Chen (2010), investigated aqueous sorption of PAH (naphthalene); using straw ash biochars under contrasting ash composition. The authors found that the sorption isotherm followed Freundlich behaviour (non-linear) for all biochar types. Similarly, Zhu et al. (2005) investigated aqueous sorption of non-polar hydrocarbons, such as cyclohexane, 1,4-xylene, 1,2,3,5-tetramethylbenzene and 1,3,5-triethylbenzene, using five contrasting wood biochars. The authors found that higher molecular weight aromatic compounds were sterically blocked from a segment of the biochar pore space and platinum catalyst promoted hydrogenation, thus removing O-functional groups. This greatly enhanced sorption of the non-polar aromatic compounds by decreasing competition for adsorption by water molecules. This highlights the importance of pore size when considering biochar for sorption purposes.
Evidence for the effect of biochar production temperature on the retention of non-polar compounds was reported by Chun et al. (2004); the retention in water of benzene (non-polar) by two wheat biochars produced in the range of 300–700 °C. The authors found that sorption into the HPT 700 °C biochar was by retention on the carbonised surfaces, while those of the LPT biochar were by both surface adsorption and absorption into the organic matter components of the LPT biochars. This suggests a dual-mode sorption concept at LPT and also a slower sorption rate compared with HPT.
Further evidence for the dual-mode sorption concept (Xing and Pignatello 1997) was investigated by Chen and Chen (2009), showing sorption in aqueous solution of non-polar (naphthalene) by biochars produced from orange peels generated at nine different temperatures, ranging from 150 to 700 °C, as well as carbonised organic matter phase with the non-carbonised phases, respectively. The authors found that for LPT 150 °C biochar, the sorption isotherms of the PAH exhibited almost linear behaviour, suggesting that absorption had a greater effect owing to the presence of an amorphous aliphatic fraction (Chen et al. 2008b). Whereas sorption isotherms of the HPTs exhibited a Freundlich behaviour (non-linear) as a result of increased adsorption arising from greater aromaticity of the HPT biochars (Chen et al. 2008b).
A complex scenario was presented when sorption of naphthalene was investigated (Chen et al. 2012b) using biochars made from pine wood at 150–700 °C; naphthalene showed a fast sorption rate to the LPT 150 °C biochar, due to greater oxygen content and lower surface area, which consequently resulted in the absorption of naphthalene into the non-carbonised organic matter of the LPT biochar. In contrast, naphthalene diffusion into LPT 250/350 °C biochars occurred at a slower rate owing to the incomplete removal of their polar-group constituents, resulting into a higher density of the absorption medium. Whereas, sorption of naphthalene to HPT 700 °C biochars with low oxygen contents and high surface area exhibited faster rates due to close saturation of absorption sites and fast adsorption into the carbonised phase of the biochar. The results showed that the density of the absorption phase directly affects the diffusivity of naphthalene.
It is worth noting that the studies mentioned above were based on sorption into pure biochars. Several studies investigating on the ability of biochars to improve the binding of PAHs in soils are presented below. Zhang et al. (2010) contrasted biochars made from Pinus radiata at 350 and 700 °C, for their sorption properties using different soils. They found that the amendment with HPT 700 °C biochar exhibits greater sorption capacity in soils than those of LPT 350 °C biochar. When desorption of the sorbed phenantherene was studied, sorption hysteresis was observed in both treatments. Following 28 days of equilibration, the capacity of the soils treated with biochar to sorb Phenantherene decreased significantly. This implies that desorption of sorbed contaminants from biochars is a slow process.
More recent study by Zhang et al. (2013) found that sorption of pyrene into a biochar-amended soil was significantly lower than the predicted values, following summation of the values obtained for single soil and biochar sorption, implying that sorption of pyrene into soil and biochar were influenced by each other. The authors attributed the low sorption capacity of biochar in soil to the presence of resident organic matter (ROM), thereby leading to loss of pyrene sorption onto biochar by 18.7–40.3 %. Additionally, biochar interacted with other soil components thereby altering its sorption capacity. Similarly, kaolinite clay showed decreased pyrene sorption with biochar amendment, though the authors highlighted that the mechanism for this effect is not yet clear. Hale et al. (2011) also found decreased sorption capacity for d10 pyrene, in soil amended with biochar.
In contrast to the studies above, Chen and Yuan (2011) using pine needle biochars produced at (100, 300, 400 and 700 °C), found that at HPT, PAHs (naphthalene, phenantherene and pyrene) were totally sorbed to the biochar with little adsorption to the soil. Although the authors reported loss of sorption sites by HPT biochar, but this was also attributed to ROM in soils which may struggle for or block biochar adsorption sites. Evidences for ROM blocking or struggling for sorption sites were reported by Kwon and Pignatello (2005). Decrease in the total surface area of biochar was observed, leading to a decrease in benzene sorption by the biochar. They used vegetable oil (triglycerides extracts) to mimic the probable response of soil lipids or humic fractions. They were able to show that total surface area as measured by N2 (77 K) reduced significantly following addition of lipid equivalent of 40 % biochar weight, however, sorption of benzene at 20 °C, were rarely affected. The authors suggested that ROM inhabit pore openings, which together with the sorbate are accessible to internal pore sites.
Further evidence also exists to prove that blocking of sorption sites at 20 °C is not significant. When the sorption properties of PAHs were contrasted, Nguyen et al. (2007) observed that the isotherms were all non-linear. They found that lower concentration of PAH were extracted from both the wood char and its residue component. Sorption of PAHs was found to be higher in the biochar residue, which they attributed to the resident organic chemicals in the biochar residue and the condensed states of the PAHs. They also found that maximum sorption increased as the diameter of PAH increased: phenantherene < naphthalene.
Other studies have also contrasted the effect of sorbate concentration on ROM. Nguyen and Ball (2006) used different soots: two diesel exhaust soots, such as ordinary hexane soot and ozone-generated hexane soot. The authors found that the diesel exhaust soot with a greater concentration of ROM exhibited lower sorption of 14C-labelled phenantherene at lower concentrations but exhibited greater sorption at high phenantherene concentrations; sorption isotherms were all non-linear. Whereas, the ordinary hexane soot showed greater sorption than the ozone-generated hexane soot, owing to differences in their physicochemical properties. The authors concluded that adsorption to the surface of biochar occurs at lower phenantherene concentrations, while both adsorption and absorption were responsible at higher concentration of sorbate. This further highlights the influence of contaminant concentrations when modelling the sorption of organic compounds.
Studies have also contrasted the possibility of competing for the sorption sites by both ROM and water. Endo et al. (2009) investigated the effects of aqueous sorption of soot, with and without the ROM (methanol extracted) and surface water coverage on sorption behaviour of soot, using naphthalene. The study demonstrated that removal of ROM by methanol led to an increased non-linearity of the isotherms, compared with the treatment with ROM. On the other hand, the surface water coverage hindered sorption into the soot particles.
The bulk of studies discussed above have compared organic compound sorption in the presence or absence of ROM, but studies have also showed that apart from extractable ROM, soot is a heterogeneous material made up of other chemical and physical domains; comprising ash, extractable ROM, amorphous carbon and stable aromatic condensed carbon (Chen and Huang 2011). However, just like ROM, some of these domains may decrease the sorption capacity of soot. It is, therefore, of interest to know the effects of the removal of these components on improving the sorption of PAH. To this end, a four-step sequential extraction procedure with various chemicals was used by Chen and Huang (2011) to extract ash, ROM and amorphous carbon from soot to enhance its sorption capacity in comparison with a non-extracted soot. The authors found that the sorption isotherm for the non-extracted soot was linear, and therefore exhibits both adsorption and partitioning, while for the extracted soot, the sorption isotherms were non-linear, indicating adsorption. The authors showed that the removal of the ROM in the extracted soot enhanced adsorption. When other soot components such as the external amorphous carbon and the internal amorphous core of the soot were completely removed, this gave rise to greater surface area and an aromatic structure, which in turn enhanced further π–π attraction between the three-ringed PAH (phenantherene) and the chemical extracted soot. The study suggests that rapid sorption of PAH into biochar can be achieved by removing all the unwanted soot domains thereby leaving the stable condensed aromatic structure as the principal sorption site.
Bornemann et al. (2007) contrasted the sorption of benzene and toluene under single and bi-sorbate systems on grass and wood biochars prepared at 250, 450 and 850 °C, respectively. They found that the sorption by the HPT 850 °C biochars increased significantly as well as the non-linearity of the isotherms, implying pore saturation mechanisms. While for LPT biochars, sorption of toluene was greater than that of benzene. However, sorption of benzene increased in the bi-sorbate system. They attributed these observations to the greater hydrophobicity of toluene, diverse capacity for expansion of the biochar materials and pore distortion by the two sorbates. Due to its lower surface area and high cation content, the grass biochar had a significantly lower sorption capacity compared with the wood biochar.
Further to the two studies discussed above, PAHs are known to be a complex mixture of individual fractions (Chen et al. 2004). In addition, PAH contamination is ubiquitous in most soils around the globe as reported earlier. As a result, PAHs may exhibit bisolute and different thermodynamic behaviours in different soils such as when comparing temperate and tropical soils or laboratory versus field soils (Chen et al. 2012c). Chen et al. (2012c) investigated the bisolute sorption and the behaviour of organic compounds under different sorbate solution temperature regimes to mimic field temperatures, using various biochars produced at HPT 700 °C and LPT 300 °C. The authors found that the adsorption of 1-naphthol, a polar organic compound in the HPT biochar was non-linear and was diminished by a co-solute phenol. The authors attributed this to competition for the carbonised adsorption sites mainly found in HPTs by the two competing solutes. Conversely, both adsorption and partitioning were the dominant mechanisms in the LPT biochar, which was further enhanced by a co-solute phenol, with the sorbed concentration increasing as the concentration of the phenol increases. The authors attributed this to cohabitation of the carbonised and non-carbonised organic matter in the LPT biochars. Regarding the thermodynamic behaviour, the LPT biochar sorbed more PAH (naphthalene) as the solution temperature increased. In contrast, the sorption of PAH (naphthalene) to HPT biochar had little or no effect to the increases in solution temperature. The authors attributed this to gradual exposure of adsorptive sites in the LPT biochar. The study suggests that the adsorption capacity of the LPT biochars can be further enhanced by increased solute temperature and that greater sorption at HPT may not always be the case.
Other researchers (Oleszczuk et al. 2012) have also investigated the sorption behaviour of PAHs into biochar; they found that biochar sorbed 0–57 % of PAHs. Further biochar sorption increased significantly overtime between 7 and 30/60 days agitation period. All the studies discussed above show that sorption by LPTs is by both absorption and adsorption, which are usually linear while sorption by HPTs is by adsorption onto the carbonised surfaces of biochar, which is non-linear.
What is the effect of adding biochar on degradation of PAHs?
The effect of adding biochar on degradation of PAHs is best understood by discussing how biochar affects the degradation of its aliphatic and aromatic components. Lehmann et al. (2009a) proposed a biphasic degradation concept for biochar: (i) a biodegradable and (ii) recalcitrant concentrations of carbon. Their chemistry involves a degradable aliphatic fraction that is present at lower concentrations in HPTs and a non-readily degradable/abiotically degradable aromatic fraction, which forms surface oxygen-containing functional groups such as carboxylic acid. Similar mechanisms (biphasic model) have also been proposed by (Cheng et al. 2006; Liang et al. 2006b) for biochar degradation: (i) structural alterations in the form of surface oxidation and (ii) degradation of C by microorganisms.
Experimental evidence for the biphasic model of biochar degradation has also been investigated in soils by Farrell et al. (2013), describing 13CO2 evolution from biochar-amended soils as biphasic, which followed a double first-order exponential decay model. The authors found that after 74 days of incubation, 99.7 % of the biochar C was not degraded. Also, due to the presence of carbonate C in the biochars, discrimination between abiotic and biotic losses was not possible. However, the authors speculated that the initial phase of CO2 evolution involving carbonate-C may be abiotically mediated. This was further supported by a mean residence time of 1.91 days for the initial stages of carbonate-C evolution compared with 50.4 days for the incubation period. Further freshly produced biochar contains an aliphatic fraction that may undergo more rapid degradation compared with the aromatic fraction (Cheng et al. 2006). These studies suggest that the degradation of aromatic and aliphatic components of biochar is not significant probably due to their presence in low concentrations.
Experiments on biodegradation of organic compounds (OC) in the presence of biochars (Ortega-Calvo and Saiz-Jimenez 1998) suggested that adsorption of both the OC and microbes to biochar surfaces may give rise to a greater concentration of OCs close to the colonising bacterial cells and therefore may increase the rate of biodegradation of these compounds. Additionally, (Wessels 1999) reported that saprophytic fungi can penetrate the interior surfaces of biochar with the aid of their hyphae; in addition, their enhanced enzymatic ability suggests that they can degrade biochars. Laborda et al. (1999) have shown that two species of saprophytic fungi; Trichoderma and Penicillium can degrade coal, by producing enzymes such as Mn-peroxidase and phenoloxidase, and laccase can also degrade biochar (Hockaday 2006). Although Warnock et al. (2007) showed that biochars are mainly colonised by arbuscular mycorrhizal fungi rather than saprophytic fungi, Ennis et al. (2012) suggest that the effectiveness of biochar to stimulate the degradation of OCs depends on the activities of saprophytic fungi, which relies on the action of extracellular enzyme and hyphal proliferation/penetration to degrade OCs. Other investigators have also suggested that the presence of substrates such as hydrocarbons, as well as biochar feed stock, can favour the predominance of saprophytic fungi (Thies and Rillig 2009).
Majority of the studies mentioned above were carried out in the presence of biochars alone, i.e. the organic compounds (substrates) were generated during biochar production. Experiments investigating bulk organic compound degradation in biochar-amended soils are also discussed. The studies by Bushnaf et al. (2011) using 2 % biochar amendment to soils, found that even with increased sorption of BTEX (benzene, toluene, ethylbenzene and xylene) compounds, CO2 evolution were similar in the biochar-amended and biochar-non-amended soils. The authors attributed this to the greater degradation of the linear, cyclic and branched alkanes in the soils that received biochar additions. The authors concluded that the TPH (total petroleum hydrocarbon) degradation rate was not only a function of substrate availability but the retention of the BTEX compounds in the soils amended with biochar also resulted in the biodegradation of other petroleum components. The study also suggest that bulk concentrations of the BTEX compounds are not deleterious to microbes, hence the lack of differences in CO2 evolution in both treatments. A more recent example of VPH degradation in the presence of biochar-amended soils by Meynet et al. (2014) suggests that microbial presence increases upon addition of hydrocarbon substrate. However, these studies are not conclusive enough to be extrapolated with PAHs, in addition to the fact that the biochars used were above 700 °C.
It is worthy to examine the effect of production temperature on biochar/OCs degradation. When comparing the effect of production temperatures 350 and 600 °C (Nguyen and Lehmann 2009) on the degradation of two biochars under different moisture regimes, the authors found that microbial degradation and oxidation was lower with the HPT biochar than those of LPT in the corn residue biochar. In contrast, this was not evident for the oak biochar (Ennis et al. 2012). This could be attributable to the presence of residual bio-oils in both the LPT and HPT biochars from the oak, as oak is known to be hard compared with the maize Stover. However, in addition to the bio-oils, some feedstock dissolve into soil solution and are also degradable (Lehmann et al. 2009b), besides stimulating growth and activity of soil microbes (Steiner et al. 2008). This highlights the importance of feedstock when using biochar as an amendment. This also highlights the potentials biochars may have to degrade PAHs, e.g. introducing PAHs into the corn residue biochar above, especially at HPT, will lead to greater degradation of the PAHs as they will serve as the only substrate available for microbial metabolism. This also suggests that the organic compounds in biochar may contribute to the total organic compound pool especially for woody feedstock. Source apportionment and discrimination between these two pools in bioremediation studies is worth investigating.
Another example of the effect of production temperature on biochar degradation was reflected by abiotic losses (Cohen-Ofri et al. 2007; Zimmerman 2010; Cheng et al. 2006; Moreno-Castilla et al. 2000; Kawamoto et al. 2005) which suggested that abiotic losses predominate with biochar. Baldock and Smernik (2002) observed that 20, 13 and 2 % of the C in red pine wood biochar produced at 150 and 350 °C, was degraded after 120 days as measured by C weight loss technique. Other report on abiotic losses by Hamer et al. (2004) found that 0.8, 0.7 and 0.3 % of C from biochar produced from maize and rye grass at 350 and 800 °C, respectively, were degraded within 2 months as measured by CO2 evolution. Zavalloni et al. (2011) found that 2.8 % of biochar C was degraded within 84 days when using biochars produced at 800 °C as an amendment. Zimmerman (2010) found that C losses from sterilised incubations were lower or similar to the non-sterile incubations. On the other hand, CO2 evolution from the PBC was higher than that from the GBC, with both having similar surface areas, which Zimmerman (2010) attributed to surfaces of the PBC which are easily accessible than those of the GBC. This implies that amendment with PBC may contribute to greater degradation of OCs compared with those with GBC. To date no study has been carried out to compare these effects using PAHs as a substrate.
Further to the above-mentioned studies little variations with regards to the sterile and non-sterile soils can be attributed to occlusion of extracellular enzymes or steric inhibition from a larger portion of the non-sterile biochar surfaces, whereas for the PBC and GBC, limited diffusion of water and oxygen into the inner pores of the GBC may be responsible for its reduced CO2 evolution compared with the PBC. Zimmerman (2010) predicted that aliphatic C are closer to the biochar external surfaces while the balance of C left are constituents of condensed aromatic rings or bio-oils inside the inner pores of biochars. The author attributes the lower degradation of GBC compared with PBC biochars to protection conferred by the GBC. It has been reported that the ratio of non-sterile to sterile microbial C losses is a function of feedstock and incubation temperatures (Zimmerman 2010; Cheng et al. 2006), with biodegradation having greater influence at temperatures <22 °C (Zimmermann et al. 2012). Abiotic release of CO2 may proceed in two ways as proposed by Cheng et al. (2006); (i) it may be due to chemisorbed oxygen attached to unsaturated ring structures, to form carboxylic acid thereby leading to CO2 evolution and (ii) evolution of carbonate-C upon biochar additions to acid soils (Yuan et al. 2011; Farrell et al. 2013).
The authors mentioned above also observed that LPT biochar degraded faster than HPT biochar, implying the effect of bio-oil in LPT biochars as well as potential for greater degradation in HPT in the presence of hydrocarbon substrate. Furthermore, 50 % of the carbon was lost during the first 90–120 days of a 1 year incubation period, following which C losses decreased and stabilised and finally became similar for both sterile and non-sterile incubations. In addition, Cheng et al. (2006) found changes in surface chemistry of biochar functional groups following a 120-day incubation and concluded that abiotic losses were more important than biotic losses. The authors also found that the ratio of sterile to non-sterile CO2 evolution increased during the study period. Since there were no differences between losses from sterile and non-sterile incubations, therefore, the losses attributed to non-sterile incubations may not be entirely due to biodegradation. For example, Farrell et al. (2013) found that CO2 evolution from their study was partly affected by microbes. Suggesting that abiotic degradation also had some effects.
When contrasting the effect of biochar additions on soil organic matter using biochars produced at 350 and 700 °C, respectively, Luo et al. (2011) found that LPT biochar stimulated the degrdadation of soil organic carbon (SOC) over a short period, whereas HPT 700 °C had a much lower effect on SOC degradation. Farrell et al. (2013) concluded that soil bacteria suitable for degradation of aromatic-C (Kramer and Gleixner 2008; Santos et al. 2012) can as well degrade biochar-C. All the studies discussed above suggest that biochar degradation follow a biphasic model.
Relating the biphasic model to bulk PAH degradation is not possible because of its potential toxicity to microbes. Additionally, the biphasic model suggests degradation of the aliphatic component than the PAHs. In contrast, the current study proposes that the aliphatics will go into solution and hence their degradation is negligible. This is due to the fact that the enzymes that can degrade the aliphatics may not survive in the presence of bulk PAH(Thies and Rillig 2009). PAHs desorb overtime since they are either semi- or non-volatiles. Alternatively, a triphasic model (Fig. 3) for degradation of PAHs in the presence of biochar is proposed in the present review.
The probable mechanisms (Fig. 3) by which biochar can simultaneously impact on sorption and biodegradation of PAHs in soils are reviewed. After TPH must have separated into fractions based on fate and transport (Edwards et al. 1997) and as can be seen in Fig. 3, (i) the spilt PAH is sorbed by biochar. It is well known that sorption of PAHs is the main mechanism affecting their fate, transport and biodegradation in soils and has been extensively reviewed by Pignatello and Xing (1995). (ii) The biochar modifies soil pH, nutrients (N and P), moisture and oxygen: as already discussed in the previous sections (this modification could be positive or negative depending on the production temperature. (iii) Desorption and biodegradation of the desorbed PAH; desorption kinetics of PAHs sorbed to biochar in soils has been suggested to be slow due to strong affinity of the PAH to biochars (Jonker and Koelmans 2002; van Noort et al. 2003). Evidences to support this hypothesis include (1) aromatic hydrocarbons sorbed to biochars are found to desorb over long periods (Jonker et al. 2005) and (2) desorption kinetics for aromatic hydrocarbons sorbed to biochar is non-linear (Cornelissen et al. 2000).
Studies (White et al. 1997; Luthy et al. 1997; Kelsey et al. 1997; Tang et al. 1999; Alexander 2000) had suggested that sorption to biochars reduces desorption and biodegradation rates. The authors hypothesised that sorption to biochar results in slow desorption of the sorbed hydrocarbons and that biodegradation takes place after longer periods following a slow desorption. Evidences to support this include (1) the slowly desorbing fraction, i.e. the biodegradable concentration, was equal to the non-biodegradable concentration in the presence of microbes with time (Cornelissen et al. 1998). However, it is worth noting that the authors did not take the losses by volatilization into consideration in their study; (2) a correlation was found between concentrations extracted using supercritical fluid and biodegraded concentrations (Hawthorne and Grabanski 2000; Hawthorne et al. 2001); and (3) those concentrations extracted by water (Hawthorne et al. 2001; Hawthorne et al. 2002). The authors, therefore, suggested that the concentration that could not be extracted by water was the concentration sorbed to biochar and can be biodegraded but after a longer period, (4) total absence of biodegradation of naphthalene in soils amended with HPT biochars (Guerin and Boyd 1997).
Despite the above evidences, Cornelissen et al. (2005) reported that it is not adequate to suggest that hydrocarbons sorbed to biochars and the desorbing concentrations are not biodegradable in the short-term or over longer periods. They suggested that after the readily available PAH concentrations absorbed to the organic matter component are biodegraded, lower pore-water concentrations due to the presence of biochar may decrease beyond the levels needed by microorganisms for biodegradation, i.e. biodegradation may stop after the concentrations sorbed to organic matter has been degraded. Cornelissen et al. (2005) also proposed that this theory not holding as PAHs can be co-metabolised. Again even in the presence of organic matter alone, biodegradation can still be limited by the absence of water and other soil factors (Cornelissen et al. 2005). Hence, hydrocarbons sorbed to biochar are degradable but probably after longer periods (Cornelissen et al. 2005).
In contrast, other studies suggest that gradual desorption alone rather than both gradual desorption and biodegradation accounts for slow degradation rates (Haritash and Kaushik 2009). The authors reported that gradual desorption is caused by slow diffusion of the hydrocarbons through the pore water and the soil organic matter. This is in line with the conclusions in this review that biochar retains water hence biodegradation is not expected to cease since biochar increases the WHC of the soil. Moreover, desorption can also be hastened by increasing soil temperature, which in turn increases the diffusion rates, resulting in dissolution of PAHs in water (Haritash and Kaushik 2009). Likewise, use of chemicals like acetone–water mixture (4:1) can desorb over 95 % of total PAHs in 60 min (Noordkamp et al. 1997). This is also in line with studies that reported that PAHs can desorb readily as a chemical extractant found rapidly desorbing PAH concentration ∑PAH (Gomez-Eyles et al. 2011) with biochars. After the desorption process, the desorbed PAHs are finally utilised by the microbes thus resulting in long-term controlled bioremediation.
Having elucidated on the triphasic concept, we now show an example of a study where PAH degradation was stimulated by biochar. Experiments involving the simultaneous use of bioaugmentation and biostimulation in the presence of biochar have also been reported (Chen et al. 2012a); plant residues and biochars inoculated with two PAH-degrading bacteria as carriers to test the bioremediation of 15 PAHs were investigated. Chen et al. (2012a) showed that the degradation of PAH in soils was a function of molecular weight and carrier treatment. PAH biodegradation was greater for the four- and five-ring PAHs compared with those of three and six rings; only the biochar amendment at 400 °C production temperature carrier biodegraded all PAHs in the soil after 3 months.
All the studies discussed above show that the presence of moisture is critical for effective stimulation of microbial degradation by biochar. It is well known that biodegradation takes place in the oil–water interphase (Brändli et al. 2008), and this may lead to a reduction in microbial activity in biochar-amended soils compared with the non-amended soils where PAHs could be less rigidly bound and could be rapidly desorbed (Hilber et al. 2009). In laboratory studies using spiked soils amended with biochar, a reduction in the biodegradation of PAH was observed by Rhodes et al. (2008), hence, highlighting the need to maintain soil-pore water in biochar-amended soils. However, comparing non-amended soils with those amended with biochar may not give an accurate account of biodegradation since the microbial activities may not be similar (Meynet et al. 2012; Bento et al. 2005). In other studies, Bento et al. (2005) suggested the use of sterile soils for individual treatments to compare the non-sterile soils in the laboratory to provide greater account for the concentration biodegraded.
It is therefore noteworthy that studies reporting greater degradation of hydrocarbons in non-amended soils compared with biochar-amended soils may not be satisfactory. This is because most of those studies neither made use of sterile soils nor applied biomarker analysis to monitor the concentration degraded. As a result, it will be difficult to suggest that it was only biodegradation that took place in their experiments. Rather, a combination of appropriate strategies during the study period such as identification and optimization of physicochemical conditions to ensure that over 20 % of the microbial populations are active (Verstraete et al. 2007) suggest that biochar has a great potential to impact on the biodegradation of PAHs. Additionally, biochar has an advantage compared with non-amended and organic matter-amended soils as proposed by Vasilyeva et al. (2010) that biochar addition reduces the toxicity of hydrocarbons to microbes in soils, thereby increasing the rate of microbial degradation.
Similar to the suggestions above, when comparing laboratory versus field studies on the impact of biochar amendment Cho et al. (2012), laboratory studies have found an advantage because the production temperature and amendment concentration could be varied and optimised for mass transfer of hydrocarbon contaminants for biodegradation. In field studies, it is difficult to measure or vary production temperature, and again it has been reported (Young and Ball 1994; Luthy et al. 1997; Rügner et al. 1999) that due to the heterogeneous nature of soils, migration into soil particles is slow thereby retarding desorption of petroleum hydrocarbons for biodegradation.
Kinney et al. (2012) found that biochars are also sources of PAHs despite their potential role in enhancing microbial degradation. Wengel et al. (2006) found that wood-degrading fungus, Schizophyllum commune, degraded biochar produced at 400 °C after 84 days study period, resulting in the liberation of dissolved OC high in aromatic compounds. PAHs have been found in aqueous phase of Birch wood biochar, produced at 450 °C as reported by Fagernäs et al. (2012), who found 16 EPA-listed PAHs in concentrations up to 10 mg kg−1. The authors also reported that though the above concentration are within the total concentration of PAH approved by EPA (6–20 mg kg−1), however still, it is greater than the 4 and 12 mg kg−1 approved for both commercial and basic biochars, respectively (Hilber et al. 2012). This also reiterates the suggestions in the previous section of the present review on how to sequester and minimise the effect of biochar PAH.
What are the factors that we can manipulate in the laboratory to enhance the capability of biochars to degrade PAHs?
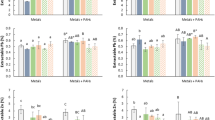
This section discusses some of the factors that can be manipulated to enhance the capability of biochars to stimulate the degradation of PAHs. They include: (i) addition of a co-metabolite (substrate), (ii) inoculation with known PAH degraders, (iii) production temperature, (iv) pH, (v) CEC, (vi) surface area, (vii) aromaticity and (viii) particle size. In this section, comparisons were made between our initial experimental results using locally generated coconut shell biochar; C. nucifera (CSB) and those reviewed elsewhere.
-
(i)
Addition of a co-metabolite as substrate
It is well established that PAHs can be degraded through co-metabolism (Keck et al. 1989; Wilson and Jones 1993) either through the addition of a co-metabolite substrate or those naturally present in the soil such as organic matter. There is evidence to show that biochar degradation was increased several fold in the presence of glucose (Hamer et al. 2004; Kuzyakov et al. 2009), which will in turn result into the degradation of the PAH content in the biochar. Hamer et al. (2004) attributed this to co-metabolism as evidenced by increased growth of microbes and enzyme production concurrently. This has also been corroborated by other investigators (Steiner et al. 2008; Kuzyakov et al. 2009; Steinbeiss et al. 2009; Hamer et al. 2004) as evidenced by the greater degradation and CO2 evolution in the presence of glucose in their studies.
Another example of co-metabolic stimulation of degradation of biochar has been reported (Kuzyakov et al. 2009; Keith et al. 2011; Luo et al. 2011). The authors found that soil organic carbon (SOC) degradation either increases or decreases with biochar. A decrease in the degradation of SOC in the presence of biochar results in a stable SOC (Cross and Sohi 2011; Keith et al. 2011; Blagodatskaya et al. 2011), and these momentary degradation have been attributed to the co-metabolic effects of bio-oils in biochars (Luo et al. 2011).
Evidences for the existence of bio-oils in biochar was investigated by Spokas et al. (2011), who found 140 organic compounds present in 70 biochars of contrasting feedstock, additionally, most of the volatile organic compounds present may hinder or increase microbial activity, and owing to their solubility in water, these volatile organic compounds (VOCs) may go into soil solution to form part of dissolved organic carbon pool in soils amended with biochar (Farrell et al. 2013).
In contrast to the studies above, Zimmerman (2010) reported that a degradable co-metabolite such as glucose is not needed in the degradation of biochar. Other investigators (Wardle et al. 2008) found that humic fraction of the soil does not contribute to biochar degradation and vice versa. Zavalloni et al. (2011), in their investigations, found that 56 % of wheat straw used as an amendment with biochar were degraded, while only 2.8 % of the biochar was degraded. The authors concluded that wheat straw did not contribute to biochar degradation but degradation was effected by the presence of dissolved organic carbon in soil. Wardle et al. (2008) also found that biochar addition led to degradation of carbon from humus in soils and attributed it to the ability of the biochar to support microbial decomposition.
Despite the conflicting evidences in favour of co-metabolism of biochar in the presence of glucose and other substrates, the bulk of evidence suggests that addition of a co-metabolite like glucose or the presence of bio-oils can enhance the degradation of PAHs in the presence of biochar. To date, no study has tested the degradation of bulk PAH using glucose as a co-metabolite in biochar-amended soils.
-
(ii)
Inoculation with known PAH degraders
Microbial species associated with biochar that may be beneficial to the degradation of HMW compounds have been isolated in biochar-amended soils, e.g. Rhodopseudomonas can degrade aromatic structures of lignin (Anderson et al. 2011). As a result, they can be targeted for the degradation of PAHs. Furthermore, Mycobacterium also increased (∼16 %) in abundance due to biochar additions; they are known to degrade PAHs (Anderson et al. 2011). Sphingomonadaceae are known to increase in the presence of biochar; they are known to degrade recalcitrant compounds (Anderson et al. 2011). Actinobacteria have been reported to degrade recalcitrant organic compounds, and due to their branched structure, they can penetrate the inner pores of biochar thereby increasing the exchange of nutrients at the surface (O’Neill et al. 2009).
More recently, Farrell et al. (2013) found a shift in microbial community structure in favour of gram-positive bacteria, which are known to degrade aromatic C in soils (Kramer and Gleixner 2008; Santos et al. 2012). This implies that inoculation with any of these families using biochar as a carrier can greatly enhance PAH degradation. It has also been reported that inoculation with biosurfactant-generating microbes such as Pseudomonas aeruginosa can increase the bioavailability of PAHs (Haritash and Kaushik 2009). It is worth noting that most of the results favour accessible microbes (Anderson et al. 2011).
-
(iii)
Production temperature
It is worth noting that majority of the factors that can be manipulated to enhance biochar ability to give simultaneous impact on sorption and biodegradation of PAHs revolve round the production temperature. Similarly, biochar production temperature is critical to understanding the scientific mechanisms that underpin the use of biochar as an amendment for PAH fate processes in soils. The probable influence of biochar production temperature was highlighted by Thies and Rillig (2009), reporting that brief carbonization and LPT generates residual bio-oils and other residues on biochar surfaces. It has also been reported that these materials may be made up of substrates that can aid microbial growth and metabolism (Ogawa 1994). Further, these bio-oils, which are mostly aliphatic, can be biodegraded in a short time when incorporated into the soil. In contrast, it was found that HPT biochars have greater aromatic structures and were abiotically degraded at a faster rate than biochars produced at LPTs with mainly aliphatic structures (Lehmann et al. 2009a). However, the authors above stated this hypothesis with reference to soil molecular response to biochar application but made no reference to PAH degradation.
Extrapolating the above mechanism to PAH degradation in soils is possible, because the soil amendment with HPT biochar should be expected to stimulate biodegradation and oxidation of PAH-contaminated soils at a faster rate compared with amendment with LPT biochar. This is because with the HPT, it is assumed that the recalcitrant aromatic structures and lack of bio-oils will result in the petrogenic PAHs alone to be the major substrate for the microbes to act on, while with the LPT, the microbes will act on both the petrogenic PAH and degradable aliphatic compounds (bio-oils), thereby decreasing the rate of biodegradation with the LPT amendment. To date, no attempt has been made to investigate the influence that biochar production temperature may have to stimulate the biodegradation of PAHs in soils and studies relying on only one production temperature, whether below or above 700 °C are not completely mechanistic. This also forms part of our future studies.
Having elucidated on the mechanisms that underpin the effect of biochar production temperatures, we now present results from our locally generated coconut shell biochar CSB for comparison with studies in the literature.
Regarding the effect of production temperature on elemental composition (total carbon TC, total nitrogen TN and extractable phosphorus EP)
As can be seen on Table 1, HPT 650 °C (84.01 ± 0.24%) had greater TC content than LPT 450 and 350 °C (76.12 ± 1.10 and 81.50 ± 14%, respectively). Similar TC contents were reported by Mukome et al. (2013), who found TC contents in the range of 15.6–87.3 % with 12 biochars from different feed stocks and production temperatures. Similarly, McBeath and Smernik (2009) reported TC content in the range of 27.6–97.4 % for 12 biochars of different feedstock and production temperatures. However, the studies mentioned above investigated multiple feed stock and some of the production temperatures were not reported. Studies using one feed stock, e.g. Chen et al. (2012b) reported a consistent trend in TC content with production temperatures, were the HPT biochar had the highest TC content which decreased in the order 700 (84.9 %) > 500 (69.7 %) > 350 (65.4 %) > 250 (57.7 %) > 150 °C (49.2 %), respectively. Similarly, Kinney et al. (2012) also reported an increase in carbon content with production temperatures: with corn stover and apple wood biochars showing consistent trends of increase in TC content with increasing production temperatures, respectively. Chen et al. (2008c) reported that biochar is made up of both carbonised and un-carbonised organic carbon. Consequently, sorption of PAHs into biochars is a function of the ratio of the carbonised to un-carbonised surfaces as well as other properties (Chen et al. 2008c; Chen and Chen 2009). This implies that the HPT biochar will have the capacity to bind PAHs tenaciously and at a faster rate compared with the LPT biochars. In the present review, TC content was greater with HPT.
For the TN content, HPT 650 °C (0.26 ± 0.02) had the least TN content, whereas the LPT 450 and 350 °C (0.35 ± 0.01 and 0.33 ± 0.03, respectively) had greater TN contents (Table 1). The low TN with HPT may likely be ascribed to volatilization as production temperature increases. Similar TN content values were reported by Mukome et al. (2013), who found TN value of 0.21. Other investigators (Chen et al. 2012b) reported an increase in TN content with production temperature which peaked at 500 °C but then disappeared completely at 700 °C. Similarly, Kinney et al. (2012) reported lower TN contents with HPT 600–700 °C biochars, respectively. In the present review, the HPT biochar had the lowest TN content. All the studies discussed above show that N decreases as production temperature increases.
The extractable P content was greater in HPT 650 °C (0.14 ± 0.02) than LPT 450 and 350 °C biochars (0.09 ± 0.00 and 0.1 ± 0.00) by a factor of 1.4 and 1.56 than those of LPT 450 and 350 °C, respectively (Table 1). Similar results were obtained by Cantrell et al. (2012), who observed greater P contents with production temperatures, with HPT 700 °C biochar from five different manures of animal origin having higher P contents than those of LPT 350 °C. Furthermore, when using nuclear magnetic resonance (NMR) spectroscopy to investigate the characteristic of chars, McBeath and Smernik (2009) reported that biochars in the production temperature ranges of 250 to 450 °C are less sensitive to chemical changes, whereas at production temperatures of between 450 and 850 °C, chemical changes become apparent. In the present review, this is evident by the TN and extractable P data.
pH
It is well known that HPT results in higher pH values irrespective of feedstock for newly produced biochars (Lehmann et al. 2011). However, with ageing, pH may decrease or increase based on type of feedstock (Lehmann et al. 2011). For example, Nguyen and Lehmann (2009) encountered lower pH, from 4.9 to 4.7, in biochars with low mineral content (oak wood), whereas, an increase in pH, from 6.7 to 8.1 was recorded for higher mineral content (corn stover) biochar following a 12-month incubation period. The mechanism for this effect was highlighted by Cheng et al. (2006), where a decrease in pH is as a result of oxidation of C to form carboxylic acids, while a pH increase is attributed to breaking up of alkaline minerals.
Studies have also reported that total bacteria diversity and specific taxa in HPT 650 °C biochar made from oak and grass increased significantly compared with those of LPT 250 °C (Khodadad et al. 2011). Farrell et al. (2013) have also highlighted the effect of production temperature on the degradation of biochar C, e.g. higher degradation was encountered by Hamer et al. (2004) using LPT 350 °C maize biochar and rye biochars compared with lower degradation encountered with LPT 450 °C eucalypt and wheat shoot biochars respectively used by (Farrell et al. 2013).
In the present review, Table 1 shows that CSB pH increased with production temperature with HPT 650 °C (9.48 ± 0.02) having greater pH than LPT 450 and 350 °C (8.87 ± 0.15 and 8.99 ± 0.03, respectively), thus exhibiting potentials for increased bacterial activity.
Cation exchange capacity
Manyà (2012) highlighted the need for determining the impact of production temperature on CEC, although CEC generally accounts for retention of nutrient cations (K+, Ca2+, Mg2+ and NH4 +) (Manyà 2012). However, in the present review, CEC is considered with respect to stimulation of PAH degradation because these cations when retained can bind nutrient anions in the soil such as phosphates and nitrates, in addition to the NH4 + which can be retained by the soil CEC. All these constitute the nutrients needed for microbial growth and metabolism in soils. Evidences for greater CEC in biochar-amended terra preta soils have also been documented (Manyà 2012). However, with respect to production temperature, previous studies by Lehmann (2007) reported that CEC was lower at LPT but increased at HPT, which the author noted needed further confirmation. One explanation was due to the fact that HPT 600–700 °C biochars are oxidised within short periods, resulting in higher CEC (Cheng et al. 2008; Nguyen et al. 2010). When the effect of nutrient retention was tested with HPT biochar, it was found that retention of nutrients by HPT biochar was negligible, due to their hydrophobicity at these production temperatures (Cornelissen et al. 2005). However, the fact that these are nutrient cations and not all of them are needed for PAH transformation shows that this effect is negligible.
On the other hand, this oxidation resulted into perpetuating the initial pore size of non-polar aromatic surfaces (Smernik 2009), and therefore a greater potential to retain PAH. However, it is not adequate to suggest that the higher CEC stemming from this oxidation will increase PAHs sorption. This is because PAHs are non-polar and will not have any electrostatic attraction to biochar surfaces but the sorption will only be as a result of the increased and stable aromatic strucure and pore spaces. In the present review, results from our locally generated CSB (Table 1) showed that CEC decreased at HPT. The LPT 350 and 450 °C had greater CEC (13.12 ± 1.63 and 18.52 ± 1.4 cmol kg−1), than HPT 650 °C biochar (9.73 ± 2.53 cmol kg−1). This implies that the LPT biochars will have greater capacity to retain nutrient cations, which in turn translates into greater retention of nutrient anions in the form of nitrates and phosphates than HPT biochar. The CEC result from the present review is also in line with that reported by Brewer et al. (2009), who found lower CEC at HPT and concluded that HPT results in lower CEC due to loss of volatiles that harbours CEC in form of organic acids. This also reiterates the conclusions by Lehmann (2007) that biochar CEC needs to be tested widely. On the other hand, Lehmann (2007) reported that newly made biochars usually have lower CEC than age-old biochars. This suggests that HPT biochar will be a better candidate than the LPT biochar in terms of PAH remediation because apart from having a greater sorption capacity owing to its wider pore size as reported, its CEC can change overtime and overtake that of LPT, which reported that CEC increases with time. This is also of advantage as degradation of the desorbed PAH by biochar is a slow process.
-
(iv)
Aromaticity
Although aromaticity cannot be manipulated directly, manipulation of production temperature indirectly affects the extent of aromaticity. Lehmann et al. (2011) reported that the inert nature of biochar carbon makes it unavailable to microbes as an energy source, so also the N and other nutrients in the C structure. However, alterations in soil physicochemical properties and presence of bio-oils in the biochar may modify the soil microbial community structure (Anderson et al. 2011; Farrell et al. 2013). Kimetu and Lehmann (2010) found that amendment with biochar reduced CO2 respiration in soils by 27 %. Zimmerman (2010) found a significant relationship between C degradation in biochars and production temperature and duration but not with feedstock type.
Furthermore, Ennis et al. (2012) reported that production temperature (Brewer et al. 2009) and duration (Yip et al. 2010) increase the magnitude of establishment of aromatic structure in biochar. For example, low level of aromaticity and small-sized aromatic zones give rise to high surface activity than larger aromatic zones; the former qualities described above can give rise to higher cation exchange capacity in soils (Lehmann et al. 2009a). The higher the degree of aromaticity of biochar, the more recalcitrant it will be in soils and hence a greater potential to enhance biodegradation (Ennis et al. 2012).
Evidences for the degree of aromaticity of biochar as a function of production temperature were confirmed by several investigators (Baldock and Smernik 2002; Hilscher and Knicker 2011). The authors found greater loss of aliphatic C and increased aromatic C (and O-aryl C) at HPTs as measured by NMR and Fourier transform infrared (FTIR) spectroscopies. On the other hand, degradation of aliphatic C and generation of carboxyl/carbonyl C with incubations (Hilscher and Knicker 2011; Cheng et al. 2006). Zimmerman (2010) further classified biochar composition into a degradable volatile fraction composed of lower C and higher oxygen (aliphatic) and a non-volatile fraction composed of high C and low oxygen (aromatic). Farrell et al. (2013) have also reported the recalcitrance of biochars as evidenced by greater presences of aryl and O-aryl-C, respectively, compared with alkyl, O-alkyl and carbonyl C, all degradable C respectively in soil organic carbon as measured by NMR spectroscopy.
Apart from instrumental evidences described above, laboratory studies by Zimmerman (2010) found that there was no difference in rate of degradation of biochar alone and those of soils amended with biochar, despite the advantages of soil moisture and oxygen availability and interactions with organic and inorganic soil components. Zavalloni et al. (2011) found no differences in CO2 evolution for soils amended with crop residue and those with biochar, but greater CO2 evolution was found in soils amended with crop residue at the initial stages. This also suggests that biochar degradation is not influenced by other soil C.
Other evidences to support biochar aromaticity/recalcitrance is its C/N ratio, e.g. Chan et al. (2008a) found that microbial activity was reduced following soil amendment with biochar. Zavalloni et al. (2011) attributed this to the chemical composition of the biochar utilised. Chan et al. (2008a) used a low N biochar having a C/N ratio of 200. Hamer et al. (2004) reported that biochar recalcitrance is positively correlated to its C/N ratio, which is a function of the production temperature and biochar chemical structure. Thus, a lower N and hence higher C/N ratio confers increased stability and vice versa. Zavalloni et al. (2011) found biochar with C/N ratio of ≈800. In this review, the C/N ratio is presented in Table 1. It was found that HPT 650 °C (323) had greater C/N ratio as well as lower N when compared with LPT 450 and 350 °C (217 and 246, respectively). It can therefore be concluded that the 650 °C (HPT) biochar had the highest C/N ratio hence a greater recalcitrance than LPT biochars.
In the present review, results from our CSB showed that the extent of aromaticity increases with production temperature. The FTIR spectra of our locally generated coconut shell biochar are shown in Fig. 4 and Table 2 below. The results showed that the HPT 650 °C (1633.67 cm−1) shared similar peaks with LPT 450 and 350 °C biochars (1622.22 and 1622.37 cm−1, respectively), which are typical of condensed aromatic ring structure, skeletal stretch, moderate intensity and attributed to C=C bond. Other peaks also common to both HPT 650 °C (2912.08 cm−1) and LPT 450 and 350 °C biochars (2912.08 and 2912.08 cm−1, respectively), which are typical of aliphatic structure (alkyl peaks), strong intensity, asymmetric stretching (C–H). However, only LPT 450 and 350 °C biochars shared similar peaks (2840.65 and 2851.64 cm−1, respectively), which are typical of aliphatic (alkyl peaks), strong symmetrical stretching, as well as 1384.26 and 1384.09 cm−1, respectively, which are characteristic of aliphatic, strong CH3 symmetrical bending (Steinbeiss et al. 2009). This implies that stability and the degree of aromaticity increases with production temperature as evidenced by the lower alkyl peaks at HPT. Similar results (3000–2800 cm−1) were also reported by Kinney et al. (2012). Although Keiluweit et al. (2010) and Chen et al. (2008a) suggested that alkyl functionalities are destroyed at production temperatures of 400–500 °C, the same author (Chen et al. 2008a) also reported peaks in the region of (1620 cm−1), which are characteristics of condensed aromatic rings as observed in the present review.
-
(v)
Surface area (N2 BET single point)
The result (Table 3) showed that HPT 650 °C (80.40 ± 22.69) had greater surface area than LPT 450 °C (76.91 ± 4.88) and LPT 350 °C (4.88 ± 0.70) biochars, respectively. When using a production temperature of 500 °C, Zhang et al. (2004) found similar surface area values: 92, 48 and 38 m2 g−1 for oak, maize hull and maize stover, respectively, which the authors described as low. The low surface area of biochars was also reiterated by Cornelissen et al. (2005). Higher surface area with HPT has also been encountered in previous studies. Brown et al. (2006) found the highest surface areas at 600–750 °C. Also, studies by Bornemann et al. (2007) found greater surface area at HPT than LPT, which also sorbed hydrocarbons at a faster rate than LPT biochars, although the HPT biochar in their study was produced at 850 °C, which is above the production temperature recommended in the present review.
-
(vi)
Particle size
Studies (Liang et al. 2006b; Cheng et al. 2008) have reported that the outer surfaces of PBC particles with collapsed pore structures, amended with soils undergo greater oxidation compared with GBC with interior pore surfaces. This is to be expected because the GBC have exposed external surfaces, while their interior is shielded, but for PBC, there is complete exposure or access to the surfaces. Further evidence for the greater potential of the PBC to undergo oxidation compared with the GBC were reported by Zimmerman (2010), whose study found greater CO2 evolution and accessible surfaces with PBC compared with GBC. Field emission scanning electron microscopy (FESEM) images of the CSB in the present review (Figs. 5 and 6) showed that the GBC at 350, 450 and 650 °C have a lot of porous structures when compared with the PBC 650 °C were the pores have collapsed, resulting in well-exposed surfaces. Although this suggests that the PBC may have a greater potential to degrade PAHs at a faster rate than GBC, the GBC, however, is still important especially at the initial stages to ensure rapid sorption of spilt PAH as described in the triphasic concept proposed in this review. This is because the IUPAC as reported by Rouquerol et al. (1994) has classified pores with respect to their accessibility to an extraneous liquid. Based on this classification, pores are divided mainly into closed pores; which are completely secluded from their neighbours. The authors noted that they are not active towards the flow of liquid and adsorption of gases. Conversely, open pores have an unabated network of openings that can permit free flow of liquids and gases. As can be seen from the FESEM images, in the present review (Fig. 6d), PBC biochar showed more closed pores than the images represented in Figs. 5a, b and 6c (GBC), which showed more open pores. As a result, the GBC biochar have greater potential to sorb bulk PAHs than PBC biochar. In addition to being able to retain more water, oxygen provides protection for microbes against predators which are also crucial during microbial degradation of PAHs. In conclusion, since microbial degradation can be limited by oxygen, the PBC will definitely support degradation at a faster rate. As a result, a combination of both GBC and PBC are recommended for greater PAH removal in the present review so as to support rapid sorption and subsequent biodegradation, which is in line with the triphasic concept and simultaneous impact proposed in this review.
Some of the studies discussed in this review had reported that PBC had the same surface area as that of GBC. However, results from CSB in the present review showed that the multipoint surface areas, including single, BET and Langmuir surface areas were greater in the PBC than GBC (Table 4). The results also showed that the total pore volume, t-plot micropore volume were also greater in the PBC than GBC. However, the density functional theory (DFT) pore size of the PBC and GBC compared favourably with each other (Table 4). The pore area showed mesopores for both PBC and GBC while the total pore areas showed mainly micropores. The BET constant (C) showed that the GBC interacted with the adsorbate with greater energy compared with the PBC, which is in line with our analysis above as well as with IUPAC recommendations.
In addition to the mechanisms reviewed in this paper, other biochar-related studies worth investigating have been further highlighted. Studies on the effects of biochar on other soil biota, e.g. soil fauna with respect to their toxicity have been investigated; earthworms (Van Zwieten et al. 2010; Gomez-Eyles et al. 2011; Major et al. 2010; Eckmeier et al. 2007; Noguera et al. 2010), nematodes (e.g. Matlack 2001) and microarthropods (e.g. Phillips et al. 2000), but there is little or nothing in literature on molluscs, e.g. snail with respect to behaviour in biochar and PAH-impacted soils. They are important in the food chain and unlike other soil fauna, they are directly consumed by humans. Although phytoremediation has been used to remove PAHs from soils as reviewed by Haritash and Kaushik (2009), incorporation of biochar as a nutrient source to these plants may enhance greater removal, and this is worth investigating.
Conclusions and perspectives
This review has shown that biochar have potentials to give simultaneous impact on PAH fate processes contrary to the general notion that biochar is only good as a sorbent. Biochar can reduce volatilization, ensure rapid sorption thereby reducing the risk of uptake of spilt PAHs into crops and livestock as well as leaching into ground water or runoff into surface water resources. After which, the biochar modifies the soil physicochemical properties for greater stimulation of microbes for enhanced biodegradation of the desorbed PAHs. However, biochars differ according to their production temperature and feedstock, which in turn will affect soil response towards their addition (McLaughlin et al. 2009). As a result, the characteristics of each biochar need to be examined before making any recommendations for its utilisation (Manyà 2012). The International Biochar Initiative (2012) has classified biochars into grades with respect to the degree of carbonization. Biochars with carbon content ≥60 % are classified as class 1, while those with <60 % are classified as class 2. In the present review, we have produced and characterised CSB which falls under class 1 biochar due to its carbon content. This fits well into the triphasic model because the model aims at rapid binding of the PAHs which can best be achieved with a class 1 biochar, since they are more carbonised with greater surface area and aromatic structures. Conversely, feed stocks of livestock manure origin are not thoroughly carbonised and are class 2 biochars as confirmed in the present review. Although they may contain greater N and P compared with the CSB (class 1 biochar), their importance, however, may only come up after the sorbed PAHs might have been desorbed for microbial degradation. Moreover, bound PAHs constitute less risk to the soil and humans (Bogan and Sullivan 2003). As a result, the nutrient content of the class 1 biochar even though lower than those of the class 2 biochar may still be adequate for stimulation of complete microbial degradation of the desorbed PAHs.
This review also dwelt exhaustively on the mechanisms through which biochar may give simultaneous impact on PAH fate processes; it highlighted gaps in the literature and compared and contrasted results in the present and previous studies. However, the following areas are critically important towards achieving the reviewed mechanisms. Studies considering the effect of production temperatures will find HPT biochars as better candidates to give simultaneous impact on PAH fate processes compared with LPT biochars, whereas in terms of particle size, a combination of GBC and PBC have been recommended in this review. However, the GBC may be mostly important at the initial stage of rapid sorption of PAHs. In addition, GBC may block enzyme active spots of the microbes, thereby reducing enzyme activities compared with PBC. Likewise, soil particles may block pores of GBC, thereby creating an anaerobic condition. Hence, a combination of both PBC and GBC should be used for optimum performance to give simultaneous impact on PAH fate processes in biochar-amended soils.
Experimental design is also critically important when evaluating the effectiveness of biochar-amended soils over non-amended soils. Studies investigating the impact of biochars on sorption and biodegradation of PAHs in soils should design their experiment in such a way that the concentration biodegraded could be determined using sterile soils to contrast to each amendment and not just comparing non-amended versus biochar-amended soils, as the absence of sterile soils means that those results may not be valid. Alternatively, the effectiveness of biodegradation can also be determined using biomarker analysis. Either way, there is clear evidence that biochar can undergo abiotic degradation and therefore, all the losses may not be entirely due to biodegradation alone. Similarly, losses in the non-amended soils may also not be entirely due to biodegradation. As a result, any of sterile amendment or biomarker analysis will help to distinguish between biodegradation and abiotic losses. It is only then that definite observations and conclusions could be reached on the extent to which biochar can impact on fate processes compared with non-amended soils. Similarly, the results of the current work indicated that CEC was greater with the LPT biochars and hence a greater potential to retain nutrients than the HPT biochar, but differentiation should be made between nutrient retention for plant growth and that of microbial action which requires a C/N/P stoichiometric ratio of 100:10:1 for greater microbial degradation. As a result, higher CEC may not confer any advantage and HPT biochars still remains a better option than the LPTs.
The bulk of evidence suggest that sorption into biochars is not feed stock dependent but depends on production temperature. Whereas the ability of LPT biochar to stimulate the microbial degradation of PAHs may be feed stock dependent as woody feed stock generates more bio-oils which serves as a short-term substrates for microbes, livestock manure biochar generates more labile carbon. The triphasic concept proposed in this review aims at proof of principle that rapid sorption of PAHs into biochars will lead to slow release or desorption of PAHs for microbial degradation, leading to long-term controlled bioremediation, i.e. sorption and biodegradation can take place simultaneously. Evidences for this concept have been elucidated in this review, which is worth investigating and hence the bases of our future work.
References
Alexander M (2000) Aging, bioavailability, and overestimation of risk from environmental pollutants. Environ Sci Technol 34(20):4259–4265. doi:10.1021/es001069+
Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manag 113:170–183
Allen-King RM, Grathwohl P, Ball WP (2002) New modeling paradigms for the sorption of hydrophobic organic chemicals to heterogeneous carbonaceous matter in soils, sediments, and rocks. Adv Water Resour 25(8–12):985–1016. doi:10.1016/s0309-1708(02)00045-3
Anderson CR, Condron LM, Clough TJ, Fiers M, Stewart A, Hill RA, Sherlock RR (2011) Biochar induced soil microbial community change: implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 54(5):309–320
Antal MJ, Grønli M (2003) The art, science, and technology of charcoal production. Ind Eng Chem Res 42(8):1619–1640. doi:10.1021/ie0207919
Asada T, Ishihara S, Yamane T, Toba A, Yamada A, Oikawa K (2002) Science of bamboo charcoal: study on carbonizing temperature of bamboo charcoal and removal capability of harmful gases. J Health Sci 48(6):473–479
Bagreev A, Bandosz TJ, Locke DC (2001) Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge-derived fertilizer. Carbon 39(13):1971–1979
Bailey VL, Fansler SJ, Smith JL, Bolton H Jr (2011) Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol Biochem 43(2):296–301
Baker JE, Eisenreich SJ (1990) Concentrations and fluxes of polycyclic aromatic hydrocarbons and polychlorinated biphenyls across the air–water interface of Lake Superior. Environ Sci Technol 24(3):342–352
Baldock JA, Smernik RJ (2002) Chemical composition and bioavailability of thermally altered Pinus resinosa (Red pine) wood. Org Geochem 33(9):1093–1109
Ball P, MacKenzie M, DeLuca T, Montana W (2010) Wildfire and charcoal enhance nitrification and ammonium-oxidizing bacterial abundance in dry montane forest soils. J Environ Qual 39(4):1243–1253
Barrow C (2012) Biochar: potential for countering land degradation and for improving agriculture. Appl Geogr 34:21–28
Beaton J, Peterson H, Bauer N (1960) Some aspects of phosphate adsorption by charcoal. Soil Sci Soc Am J 24(5):340–346
Beesley L, Moreno-Jimenez E, Gomez-Eyles JL, Harris E, Robinson B, Sizmur T (2011) A review of biochars' potential role in the remediation, revegetation and restoration of contaminated soils. Environ Pollut 159(12):3269–3282
Bento FM, Camargo FAO, Okeke BC, Frankenberger WT (2005) Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresour Technol 96(9):1049–1055. doi:10.1016/j.biortech.2004.09.008
Blagodatskaya Е, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45(2):115–131
Blagodatskaya E, Yuyukina T, Blagodatsky S, Kuzyakov Y (2011) Three-source-partitioning of microbial biomass and of CO2 efflux from soil to evaluate mechanisms of priming effects. Soil Biol Biochem 43(4):778–786
Bogan BW, Sullivan WR (2003) Physicochemical soil parameters affecting sequestration and mycobacterial biodegradation of polycyclic aromatic hydrocarbons in soil. Chemosphere 52(10):1717–1726
Bornemann LC, Kookana RS, Welp G (2007) Differential sorption behaviour of aromatic hydrocarbons on charcoals prepared at different temperatures from grass and wood. Chemosphere 67(5):1033–1042. doi:10.1016/j.chemosphere.2006.10.052
Brändli RC, Hartnik T, Henriksen T, Cornelissen G (2008) Sorption of native polyaromatic hydrocarbons (PAH) to black carbon and amended activated carbon in soil. Chemosphere 73(11):1805–1810. doi:10.1016/j.chemosphere.2008.08.034
Brewer CE, Schmidt-Rohr K, Satrio JA, Brown RC (2009) Characterization of biochar from fast pyrolysis and gasification systems. Environ Prog Sust Energ 28(3):386–396
Brown RA, Kercher AK, Nguyen TH, Nagle DC, Ball WP (2006) Production and characterization of synthetic wood chars for use as surrogates for natural sorbents. Org Geochem 37(3):321–333
Bushnaf KM, Puricelli S, Saponaro S, Werner D (2011) Effect of biochar on the fate of volatile petroleum hydrocarbons in an aerobic sandy soil. J Contam Hydrol 126(3–4):208–215. doi:10.1016/j.jconhyd.2011.08.008
Canadian-Council-of-Ministers-of-the-Environment-(CCME) (2008) Canada-wide standard for petroleum hydrocarbons (PHC) In soil-technical supplement
Cantrell KB, Hunt PG, Uchimiya M, Novak JM, Ro KS (2012) Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428
Chan KY, Xu Z (2009) Biochar: nutrient properties and their enhancement. Biochar for Environmental Management: Science and Technology Earthscan, London, pp 67–84
Chan K, Van Zwieten L, Meszaros I, Downie A, Joseph S (2008a) Agronomic values of greenwaste biochar as a soil amendment. Soil Res 45(8):629–634
Chan K, Van Zwieten L, Meszaros I, Downie A, Joseph S (2008b) Using poultry litter biochars as soil amendments. Soil Res 46(5):437–444
Chen B, Chen Z (2009) Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 76(1):127–133. doi:10.1016/j.chemosphere.2009.02.004
Chen B, Huang W (2011) Effects of compositional heterogeneity and nanoporosity of raw and treated biomass-generated soot on adsorption and absorption of organic contaminants. Environ Pollut 159(2):550–556
Chen B, Yuan M (2011) Enhanced sorption of polycyclic aromatic hydrocarbons by soil amended with biochar. J Soils Sediments 11(1):62–71. doi:10.1007/s11368-010-0266-7
Chen B, Xuan X, Zhu L, Wang J, Gao Y, Yang K, Shen X, Lou B (2004) Distributions of polycyclic aromatic hydrocarbons in surface waters, sediments and soils of Hangzhou City, China. Water Res 38(16):3558–3568
Chen B, Zhou D, Zhu L (2008) Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol 42(14):5137–5143. doi:10.1021/es8002684
Chen H, Yao J, Wang F, Choi MM, Bramanti E, Zaray G (2009) Study on the toxic effects of diphenol compounds on soil microbial activity by a combination of methods. J Hazard Mater 167(1):846–851
Chen B, Chen Z, Lv S (2011) A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour Technol 102(2):716–723
Chen B, Yuan M, Qian L (2012a) Enhanced bioremediation of PAH-contaminated soil by immobilized bacteria with plant residue and biochar as carriers. J Soils Sediments 12(9):1350–1359
Chen Z, Chen B, Chiou CT (2012b) Fast and slow rates of naphthalene sorption to biochars produced at different temperatures. Environ Sci Technol 46(20):11104–11111
Chen Z, Chen B, Zhou D, Chen W (2012c) Bisolute sorption and thermodynamic behavior of organic pollutants to biomass-derived biochars at two pyrolytic temperatures. Environ Sci Technol 46(22):12476–12483
Cheng C-H, Lehmann J, Thies JE, Burton SD, Engelhard MH (2006) Oxidation of black carbon by biotic and abiotic processes. Org Geochem 37(11):1477–1488. doi:10.1016/j.orggeochem.2006.06.022
Cheng C-H, Lehmann J, Engelhard MH (2008) Natural oxidation of black carbon in soils: changes in molecular form and surface charge along a climosequence. Geochim Cosmochim Acta 72(6):1598–1610. doi:10.1016/j.gca.2008.01.010
Cho Y-M, Werner D, Choi Y, Luthy RG (2012) Long-term monitoring and modeling of the mass transfer of polychlorinated biphenyls in sediment following pilot-scale in-situ amendment with activated carbon. J Contam Hydrol 129–130:25–37. doi:10.1016/j.jconhyd.2011.09.009
Chun Y, Sheng G, Chiou CT, Xing B (2004) Compositions and sorptive properties of crop residue-derived chars. Environ Sci Technol 38(17):4649–4655. doi:10.1021/es035034w
Cimò G, Kucerik J, Berns AE, Schaumann GE, Alonzo G, Conte P (2014) Effect of heating time and temperature on the chemical characteristics of biochar from poultry manure. J Agric Food Chem 62(8):1912–1918
Cohen-Ofri I, Popovitz-Biro R, Weiner S (2007) Structural characterization of modern and fossilized charcoal produced in natural fires as determined by using electron energy loss spectroscopy. Chem Eur J 13(8):2306–2310
Cornelissen G, Rigterink H, Ferdinandy MMA, van Noort PCM (1998) Rapidly desorbing fractions of PAHs in contaminated sediments as a predictor of the extent of bioremediation. Environ Sci Technol 32(7):966–970. doi:10.1021/es9704038
Cornelissen G, Rigterink H, van Noort PCM, Govers HAJ (2000) Slowly and very slowly desorbing organic compounds in sediments exhibit langmuir-type sorption. Environ Toxicol Chem 19(6):1532–1539. doi:10.1002/etc.5620190609
Cornelissen G, Gustafsson Ö, Bucheli TD, Jonker MTO, Koelmans AA, van Noort PCM (2005) Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ Sci Technol 39(18):6881–6895. doi:10.1021/es050191b
Cross A, Sohi SP (2011) The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol Biochem 43(10):2127–2134
Das K, Garcia-Perez M, Bibens B, Melear N (2008) Slow pyrolysis of poultry litter and pine woody biomass: impact of chars and bio-oils on microbial growth. J Environ Sci Health A 43(7):714–724
Day D, Evans RJ, Lee JW, Reicosky D (2004) Valuable and stable carbon co-product from fossil fuel exhaust scrubbing. Prepr Pap Am Chem Soc Div Fuel Chem 49(1):352
Deenik JL, McClellan T, Uehara G, Antal MJ, Campbell S (2010) Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci Soc Am J 74(4):1259–1270
Delmont TO, Robe P, Cecillon S, Clark IM, Constancias F, Simonet P, Hirsch PR, Vogel TM (2011) Accessing the soil metagenome for studies of microbial diversity. Appl Environ Microbiol 77(4):1315–1324
DeLuca TH, MacKenzie MD, Gundale MJ, Holben WE (2006) Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci Soc Am J 70(2):448–453. doi:10.2136/sssaj2005.0096
Denef K, Six J, Bossuyt H, Frey SD, Elliott ET, Merckx R, Paustian K (2001) Influence of dry–wet cycles on the interrelationship between aggregate, particulate organic matter, and microbial community dynamics. Soil Biol Biochem 33(12):1599–1611
Diggs DL, Huderson AC, Harris KL, Myers JN, Banks LD, Rekhadevi PV, Niaz MS, Ramesh A (2011) Polycyclic aromatic hydrocarbons and digestive tract cancers: a perspective. J Environ Sci Health C 29(4):324–357
Downie A, Crosky A, Munroe P (2009) Physical properties of biochar. In: Lehmann J, Joseph S (eds) Biochar for environmental management science and technology. Earthscan, London
Durenkamp M, Luo Y, Brookes P (2010) Impact of black carbon addition to soil on the determination of soil microbial biomass by fumigation extraction. Soil Biol Biochem 42(11):2026–2029
Eckmeier E, Gerlach R, Skjemstad J, Ehrmann O, Schmidt M (2007) Minor changes in soil organic carbon and charcoal concentrations detected in a temperate deciduous forest a year after an experimental slash-and-burn. Biogeosciences 4(3):377–383
Edwards D, Andriot M, Amoruso M, Tummey A, Bevan C, Tveit A, Hayes L, Youngren S, Nakles D (1997) Development of fraction specific reference doses (RfDs) and refernce concentrations (RfCs) for total petroleum hydrocarbons (TPH). Total Pet Hydrocarb Work Group Ser 4
Endo S, Grathwohl P, Haderlein SB, Schmidt TC (2009) Effects of native organic material and water on sorption properties of reference diesel soot. Environ Sci Technol 43(9):3187–3193
Ennis CJ, Evans AG, Islam M, Ralebitso-Senior TK, Senior E (2012) Biochar: carbon sequestration, land remediation, and impacts on soil microbiology. Crit Rev Environ Sci Technol 42(22):2311–2364
Ezawa T, Yamamoto K, Yoshida S (2002) Enhancement of the effectiveness of indigenous arbuscular mycorrhizal fungi by inorganic soil amendments. Soil Sci Plant Nutr 48(6):897–900
Fagernäs L, Kuoppala E, Simell P (2012) Polycyclic aromatic hydrocarbons in birch wood slow pyrolysis products. Energy Fuel 26(11):6960–6970
Farrell M, Kuhn TK, Macdonald LM, Maddern TM, Murphy DV, Hall PA, Singh BP, Baumann K, Krull ES, Baldock JA (2013) Microbial utilisation of biochar-derived carbon. Sci Total Environ 465:288–97
Fu PP (1990) Metabolism of nitro-polycyclic aromatic hydrocarbons. Drug Metab Rev 22(2–3):209–268
Fu PP, Xia Q, Sun X, Yu H (2012) Phototoxicity and environmental transformation of polycyclic aromatic hydrocarbons (PAHs)—light-induced reactive oxygen species, lipid peroxidation, and DNA damage. J Environ Sci Health C 30(1):1–41. doi:10.1080/10590501.2012.653887
George N, Davies JT (1988) Parameters affecting adsorption of microorganisms on activated charcoal cloth. J Chem Technol Biotechnol 43(3):173–186
Gomez-Eyles JL, Sizmur T, Collins CD, Hodson ME (2011) Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ Pollut 159(2):616–622. doi:10.1016/j.envpol.2010.09.037
Grossman JM, O’Neill BE, Tsai SM, Liang B, Neves E, Lehmann J, Thies JE (2010) Amazonian anthrosols support similar microbial communities that differ distinctly from those extant in adjacent, unmodified soils of the same mineralogy. Microb Ecol 60(1):192–205
Guerin WF, Boyd SA (1997) Bioavailability of naphthalene associated with natural and synthetic sorbents. Water Res 31(6):1504–1512. doi:10.1016/s0043-1354(96)00402-2
Gundale MJ, DeLuca TH (2006) Temperature and source material influence ecological attributes of ponderosa pine and Douglas-fir charcoal. For Ecol Manag 231(1–3):86–93. doi:10.1016/j.foreco.2006.05.004
Gupta V, Carrott P, Ribeiro Carrott M, Suhas (2009) Low-cost adsorbents: growing approach to wastewater treatment—a review. Crit Rev Environ Sci Technol 39(10):783–842
Haefele S, Knoblauch C, Gummert M, Konboon Y, Koyama S (2009) Black carbon (biochar) in rice-based systems: characteristics and opportunities. In: Amazonian Dark Earths: Wim Sombroek's Vision. Springer, pp 445–463
Hale S, Hanley K, Lehmann J, Zimmerman A, Cornelissen G (2011) Effects of chemical, biological, and physical aging as well as soil addition on the sorption of pyrene to activated carbon and biochar. Environ Sci Technol 45(24):10445–10453
Hamer U, Marschner B, Brodowski S, Amelung W (2004) Interactive priming of black carbon and glucose mineralisation. Org Geochem 35(7):823–830. doi:10.1016/j.orggeochem.2004.03.003
Haritash A, Kaushik C (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169(1):1–15
Harvey RG (1991) Polycyclic aromatic hydrocarbons: chemistry and carcinogenicity. CUP Archive
Hass A, Gonzalez JM, Lima IM, Godwin HW, Halvorson JJ, Boyer DG (2012) Chicken manure biochar as liming and nutrient source for acid Appalachian soil. J Environ Qual 41(4):1096–1106
Hawthorne SB, Grabanski CB (2000) Correlating selective supercritical fluid extraction with bioremediation behavior of PAHs in a field treatment plot. Environ Sci Technol 34(19):4103–4110. doi:10.1021/es001178o
Hawthorne SB, Poppendieck DG, Grabanski CB, Loehr RC (2001) PAH release during water desorption, supercritical carbon dioxide extraction, and field bioremediation. Environ Sci Technol 35(22):4577–4583
Hawthorne SB, Poppendieck DG, Grabanski CB, Loehr RC (2002) Comparing PAH availability from manufactured gas plant soils and sediments with chemical and biological tests. 1. PAH release during water desorption and supercritical carbon dioxide extraction. Environ Sci Technol 36(22):4795–4803. doi:10.1021/es020626k
Hilber I, Wyss GS, Mader P, Bucheli TD, Meier I, Vogt L, Schulin R (2009) Influence of activated charcoal amendment to contaminated soil on dieldrin and nutrient uptake by cucumbers. Environ Pollut 157(8–9):2224–2230
Hilber I, Blum F, Leifeld J, Schmidt H-P, Bucheli TD (2012) Quantitative determination of PAHs in biochar: a prerequisite to ensure its quality and safe application. J Agric Food Chem 60(12):3042–3050
Hilscher A, Knicker H (2011) Carbon and nitrogen degradation on molecular scale of grass-derived pyrogenic organic material during 28 months of incubation in soil. Soil Biol Biochem 43(2):261–270
Hockaday WC (2006) The organic geochemistry of charcoal black carbon in the soils of the University of Michigan Biological Station. Ohio State University
Hockaday WC, Grannas AM, Kim S, Hatcher PG (2007) The transformation and mobility of charcoal in a fire-impacted watershed. Geochim Cosmochim Acta 71(14):3432–3445
Huang W, Chen B (2010) Interaction mechanisms of organic contaminants with burned straw ash charcoal. J Environ Sci 22(10):1586–1594
International-Biochar-Initiative (2012) Standardized product definition and product testing guidelines for biochar that is used in soil. IBI biochar standards
Ippolito J, Novak J, Busscher W, Ahmedna M, Rehrah D, Watts D (2012) Switchgrass biochar affects two Aridisols. J Environ Qual 41(4):1123–1130
Jin H (2010) Characterization of microbial life colonizing biochar and biochar-amended soils
Jindo K, Sánchez-Monedero MA, Hernández T, García C, Furukawa T, Matsumoto K, Sonoki T, Bastida F (2012) Biochar influences the microbial community structure during manure composting with agricultural wastes. Sci Total Environ 416:476–481
Johnsen AR, Wick LY, Harms H (2005) Principles of microbial PAH-degradation in soil. Environ Pollut 133(1):71–84
Jones KC, De Voogt P (1999) Persistent organic pollutants (POPs): state of the science. Environ Pollut 100(1):209–221
Jonker MTO, Koelmans AA (2002) Sorption of polycyclic aromatic hydrocarbons and polychlorinated biphenyls to soot and soot-like materials in the aqueous environment: mechanistic considerations. Environ Sci Technol 36(17):3725–3734. doi:10.1021/es020019x
Jonker MTO, Hawthorne SB, Koelmans AA (2005) Extremely slowly desorbing polycyclic aromatic hydrocarbons from soot and soot-like materials: evidence by supercritical fluid extraction. Environ Sci Technol 39(20):7889–7895. doi:10.1021/es0505191
Joseph S, Camps-Arbestain M, Lin Y, Munroe P, Chia C, Hook J, Van Zwieten L, Kimber S, Cowie A, Singh B (2010) An investigation into the reactions of biochar in soil. Soil Res 48(7):501–515
Kasozi GN, Zimmerman AR, Nkedi-Kizza P, Gao B (2010) Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ Sci Technol 44(16):6189–6195
Kawamoto K, Ishimaru K, Imamura Y (2005) Reactivity of wood charcoal with ozone. J Wood Sci 51(1):66–72
Keck J, Sims RC, Coover M, Park K, Symons B (1989) Evidence for cooxidation of polynuclear aromatic hydrocarbons in soil. Water Res 23(12):1467–1476
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44(4):1247–1253. doi:10.1021/es9031419
Keith A, Singh B, Singh BP (2011) Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil. Environ Sci Technol 45(22):9611–9618
Kelsey JW, Kottler BD, Alexander M (1997) Selective chemical extractants to predict bioavailability of soil-aged organic chemicals. Environ Sci Technol 31(1):214–217. doi:10.1021/es960354j
Khodadad CL, Zimmerman AR, Green SJ, Uthandi S, Foster JS (2011) Taxa-specific changes in soil microbial community composition induced by pyrogenic carbon amendments. Soil Biol Biochem 43(2):385–392
Kimetu JM, Lehmann J (2010) Stability and stabilisation of biochar and green manure in soil with different organic carbon contents. Soil Res 48(7):577–585
Kinney T, Masiello C, Dugan B, Hockaday W, Dean M, Zygourakis K, Barnes R (2012) Hydrologic properties of biochars produced at different temperatures. Biomass Bioenergy 41:34–43
Kolb SE, Fermanich KJ, Dornbush ME (2009) Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci Soc Am J 73(4):1173–1181. doi:10.2136/sssaj2008.0232
Kramer C, Gleixner G (2008) Soil organic matter in soil depth profiles: distinct carbon preferences of microbial groups during carbon transformation. Soil Biol Biochem 40(2):425–433
Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem 41:210–219
Kwon S, Pignatello JJ (2005) Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): pseudo pore blockage by model lipid components and its implications for N2-probed surface properties of natural sorbents. Environ Sci Technol 39(20):7932–7939
Laborda F, Monistrol I, Luna N, Fernandez M (1999) Processes of liquefaction/solubilization of Spanish coals by microorganisms. Appl Microbiol Biotechnol 52(1):49–56
Lehannes J, Joseph S (2009) Biochar for environmental management: an introduction. biochar for environmental management: science and technology. Earthscan, London, pp 1–12
Lehmann J (2007) Bio-energy in the black. Front Ecol Environ 5(7):381–387. doi:10.1890/1540-9295(2007)5[381:bitb]2.0.co;2
Lehmann J, Czimczik C, Laird D, Sohi S (2009) Stability of biochar in the soil. In: Joseph JLS (ed) Biochar for environmental management. Earthscan, London, pp 183–206
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43(9):1812–1836
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O'Neill B, Skjemstad J, Thies J, Luizao F, Petersen J (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70(5):1719–1730
Luo Y, Durenkamp M, De Nobili M, Lin Q, Brookes P (2011) Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol Biochem 43(11):2304–2314
Luthy RG, Aiken GR, Brusseau ML, Cunningham SD, Gschwend PM, Pignatello JJ, Reinhard M, Traina SJ, Weber WJ, Westall JC (1997) Sequestration of hydrophobic organic contaminants by geosorbents. Environ Sci Techn 31(12):3341–3347. doi:10.1021/es970512m
MacKenzie M, DeLuca T (2006) Charcoal and shrubs modify soil processes in ponderosa pine forests of western Montana. Plant Soil 287(1–2):257–266
Major J, Steiner C, Downie A, Lehmann J (2009) Biochar effects on nutrient leaching. biochar for environmental management: science and technology. Earthscan, London, pp 271–282
Major J, Lehmann J, Rondon M, Goodale C (2010) Fate of soil‐applied black carbon: downward migration, leaching and soil respiration. Glob Chang Biol 16(4):1366–1379
Mannino A, Harvey HR (2004) Black carbon in estuarine and coastal ocean dissolved organic matter. Limnol Oceanogr 49(3):735–740
Manyà JJ (2012) Pyrolysis for biochar purposes: a review to establish current knowledge gaps and research needs. Environ Sci Technol 46(15):7939–7954
Masiello CA, Druffel ER (2001) Carbon isotope geochemistry of the Santa Clara River. Glob Biogeochem Cycles 15(2):407–416
Matlack GR (2001) Factors determining the distribution of soil nematodes in a commercial forest landscape. For Ecol Manag 146(1):129–143
McBeath AV, Smernik RJ (2009) Variation in the degree of aromatic condensation of chars. Org Geochem 40(12):1161–1168
McLaughlin H, Anderson PS, Shields FE, Reed TB (2009) All biochars are not created equal, and how to tell them apart. North American Biochars Conference, Boulder, pp 9–12
McVeety BD, Hites RA (1988) Atmospheric deposition of polycyclic aromatic hydrocarbons to water surfaces: a mass balance approach. Atmos Environ (1967) 22(3):511–536
Meckenstock RU, Safinowski M, Griebler C (2004) Anaerobic degradation of polycyclic aromatic hydrocarbons. FEMS Microbiol Ecol 49(1):27–36
Meynet P, Hale SE, Davenport RJ, Cornelissen G, Breedveld GD, Werner D (2012) Effect of activated carbon amendment on bacterial community structure and functions in a PAH impacted urban soil. Environ Sci Technol 46(9):5057–5066. doi:10.1021/es2043905
Meynet P, Moliterni E, Davenport RJ, Sloan WT, Camacho JV, Werner D (2014) Predicting the effects of biochar on volatile petroleum hydrocarbon biodegradation and emanation from soil: a bacterial community finger-print analysis inferred modelling approach. Soil Biol Biochem 68:20–30
Moreno-Castilla C, Lopez-Ramon M, Carrasco-Marın F (2000) Changes in surface chemistry of activated carbons by wet oxidation. Carbon 38(14):1995–2001
Morris D, Gilbert R, Reicosky D, Gesch R (2004) Oxidation potentials of soil organic matter in histosols under different tillage methods. Soil Sci Soc Am J 68(3):817–826
Mukome FN, Zhang X, Silva LC, Six J, Parikh SJ (2013) Use of chemical and physical characteristics to investigate trends in biochar feedstocks. J Agric Food Chem 61(9):2196–2204
Murage EW, Voroney P, Beyaert RP (2007) Turnover of carbon in the free light fraction with and without charcoal as determined using the 13C natural abundance method. Geoderma 138(1):133–143
Nguyen TH, Ball WP (2006) Absorption and adsorption of hydrophobic organic contaminants to diesel and hexane soot. Environ Sci Technol 40(9):2958–2964
Nguyen BT, Lehmann J (2009) Black carbon decomposition under varying water regimes. Org Geochem 40(8):846–853
Nguyen TH, Cho H-H, Poster DL, Ball WP (2007) Evidence for a pore-filling mechanism in the adsorption of aromatic hydrocarbons to a natural wood char. Environ Sci Technol 41(4):1212–1217
Nguyen BT, Lehmann J, Hockaday WC, Joseph S, Masiello CA (2010) Temperature sensitivity of black carbon decomposition and oxidation. Environ Sci Technol 44(9):3324–3331
Noguera D, Rondón M, Laossi K-R, Hoyos V, Lavelle P, Cruz de Carvalho MH, Barot S (2010) Contrasted effect of biochar and earthworms on rice growth and resource allocation in different soils. Soil Biol Biochem 42(7):1017–1027
Noordkamp E, Grotenhuis J, Rulkens W (1997) Selection of an efficient extraction method for the determination of polycyclic aromatic hydrocarbons in contaminated soil and sediment. Chemosphere 35(9):1907–1917
O’Neill B, Grossman J, Tsai M, Gomes J, Lehmann J, Peterson J, Neves E, Thies J (2009) Bacterial community composition in Brazilian anthrosols and adjacent soils characterized using culturing and molecular identification. Microb Ecol 58(1):23–35
Ogawa M (1994) Symbiosis of people and nature in the tropics. Farming Jpn 28(5):10–34
Oleszczuk P, Hale SE, Lehmann J, Cornelissen G (2012) Activated carbon and biochar amendments decrease pore-water concentrations of polycyclic aromatic hydrocarbons (PAHs) in sewage sludge. Bioresour Technol 111:84–91
Ortega-Calvo JJ, Saiz-Jimenez C (1998) Effect of humic fractions and clay on biodegradation of phenanthrene by a Pseudomonas fluorescens strain isolated from soil. Appl Environ Microbiol 64(8):3123–3126
Painter TJ (1998) Carbohydrate polymers in food preservation: an integrated view of the Maillard reaction with special reference to discoveries of preserved foods in Sphagnum-dominated peat bogs. Carbohydr Polym 36(4):335–347
Paul EA (2006) Soil microbiology, ecology and biochemistry. Academic press
Phillips DH (1999) Polycyclic aromatic hydrocarbons in the diet. Mutat Res Genet Toxicol Environ Mutagen 443(1):139–147
Phillips D, Foss J, Buckner E, Evans R, FitzPatrick E (2000) Response of surface horizons in an oak forest to prescribed burning. Soil Sci Soc Am J 64(2):754–760
Pietikäinen J, Kiikkilä O, Fritze H (2000) Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos 89(2):231–242. doi:10.1034/j.1600-0706.2000.890203.x
Pignatello JJ, Xing B (1995) Mechanisms of slow sorption of organic chemicals to natural particles. Environ Sci Technol 30(1):1–11. doi:10.1021/es940683g
Pignatello JJ, Kwon S, Lu Y (2006) Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): attenuation of surface activity by humic and fulvic acids. Environ Sci Technol 40(24):7757–7763. doi:10.1021/es061307m
Pollock M (1947) The growth of H. pertussis on media without blood. Br J Exp Pathol 28(4):295
Qin G, Gong D, Fan M-Y (2013) Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. Int Biodeterior Biodegrad 85:150–155
Raza M, Zakaria MP, Hashim NR, Yim UH, Kannan N, Ha SY (2013) Composition and source identification of polycyclic aromatic hydrocarbons in mangrove sediments of Peninsular Malaysia: indication of anthropogenic input. Environ Earth Sci 1-12
Rhodes AH, Carlin A, Semple KT (2008) Impact of black carbon in the extraction and mineralization of phenanthrene in soil. Environ Sci Technol 42(3):740–745. doi:10.1021/es071451n
Rondon MA, Lehmann J, Ramírez J, Hurtado M (2007) Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fertil Soils 43(6):699–708
Rouquerol J, Avnir D, Fairbridge C, Everett D, Haynes J, Pernicone N, Ramsay J, Sing K, Unger K (1994) Recommendations for the characterization of porous solids (technical report). Pure Appl Chem 66(8):1739–1758
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4(10):1340–1351
Rügner H, Kleineidam S, Grathwohl P (1999) Long term sorption kinetics of phenanthrene in aquifer materials. Environ Sci Technol 33(10):1645–1651. doi:10.1021/es980664x
Saito M, Marumoto T (2002) Inoculation with arbuscular mycorrhizal fungi: the status quo in Japan and the future prospects. Plant Soil 244(1–2):273–279
Samonin V, Elikova E (2004) A study of the adsorption of bacterial cells on porous materials. Microbiology 73(6):696–701
Sander M, Pignatello JJ (2005) Characterization of charcoal adsorption sites for aromatic compounds: insights drawn from single-solute and bi-solute competitive experiments. Environ Sci Technol 39(6):1606–1615
Santos F, Torn MS, Bird JA (2012) Biological degradation of pyrogenic organic matter in temperate forest soils. Soil Biol Biochem 51:115–124
Schmidt MWI, Noack AG (2000) Black carbon in soils and sediments: analysis, distribution, implications, and current challenges. Glob Biogeochem Cycles 14(3):777–793. doi:10.1029/1999gb001208
Sims RC, Overcash M (1983) Fate of polynuclear aromatic compounds (PNAs) in soil–plant systems. In: Residue reviews. Springer, pp 1–68
Sinha R, Kulldorff M, Gunter MJ, Strickland P, Rothman N (2005) Dietary benzo [a] pyrene intake and risk of colorectal adenoma. Cancer Epidemiol Biomark Prev 14(8):2030–2034
Smernik RJ (2009) Biochar and sorption of organic compounds. In: Lehmann J, Joseph S (eds) Biochar for environmental management science and technology. Earthscan, London, pp 289–300
Smith S, Ainsworth C, Traina S, Hicks R (1992) Effect of sorption on the biodegradation of quinoline. Soil Sci Soc Am J 56(3):737–746
Smith JL, Collins HP, Bailey VL (2010) The effect of young biochar on soil respiration. Soil Biol Biochem 42(12):2345–2347
Sparrevik M, Saloranta T, Cornelissen G, Eek E, Fet AM, Breedveld GD, Linkov I (2011) Use of life cycle assessments to evaluate the environmental footprint of contaminated sediment remediation. Environ Sci Technol 45(10):4235–4241
Spokas KA, Reicosky DC (2009) Impacts of sixteen different biochars on soil greenhouse gas production. Ann Environ Sci 3(1):4
Spokas KA, Novak JM, Stewart CE, Cantrell KB, Uchimiya M, DuSaire MG, Ro KS (2011) Qualitative analysis of volatile organic compounds on biochar. Chemosphere 85(5):869–882
Steinbeiss S, Gleixner G, Antonietti M (2009) Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol Biochem 41(6):1301–1310. doi:10.1016/j.soilbio.2009.03.016
Steiner C, TEiXEiRA WG, Lehmann J, Zech W (2004) Microbial response to charcoal amendments of highly weathered soils and Amazonian Dark Earths in Central Amazonia—preliminary results. Amazonian Dark Earths: Explorations in space and time 195–212
Steiner C, Das KC, Garcia M, Förster B, Zech W (2008) Charcoal and smoke extract stimulate the soil microbial community in a highly weathered xanthic Ferralsol. Pedobiologia 51(5–6):359–366. doi:10.1016/j.pedobi.2007.08.002
Steiner C, Garcia M, Zech W (2009) Effects of charcoal as slow release nutrient carrier on NPK dynamics and soil microbial population: pot experiments with ferralsol substrate. In: Amazonian Dark Earths: Wim Sombroek's Vision. Springer, pp 325–338
Tang J, Robertson BK, Alexander M (1999) Chemical-extraction methods to estimate bioavailability of DDT, DDE, and DDD in soil. Environ Sci Technol 33(23):4346–4351. doi:10.1021/es990581w
Thanh Nguyen B, Marschner P (2005) Effect of drying and rewetting on phosphorus transformations in red brown soils with different soil organic matter content. Soil Biol Biochem 37(8):1573–1576
Thies JE, Rillig MC (2009) Characteristics of biochar—biological properties. In: Lehmann JSJ (ed) Biochar for environmental management: science and technology. Earthscan, London
Titirici MM, Thomas A, Yu S-H, Müller J-O, Antonietti M (2007) A direct synthesis of mesoporous carbons with bicontinuous pore morphology from crude plant material by hydrothermal carbonization. Chem Mater 19(17):4205–4212
Tsai WT, Lee MK, Chang YM (2006) Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J Anal Appl Pyrolysis 76(1–2):230–237. doi:10.1016/j.jaap.2005.11.007
van Noort PCM, Cornelissen G, ten Hulscher TEM, Vrind BA, Rigterink H, Belfroid A (2003) Slow and very slow desorption of organic compounds from sediment: influence of sorbate planarity. Water Res 37(10):2317–2322. doi:10.1016/s0043-1354(02)00628-0
Van Zwieten L, Kimber S, Morris S, Chan K, Downie A, Rust J, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327(1–2):235–246
Vasilyeva GK, Strijakova ER, Nikolaeva SN, Lebedev AT, Shea PJ (2010) Dynamics of PCB removal and detoxification in historically contaminated soils amended with activated carbon. Environ Pollut 158(3):770–777
Vazquez-Duhalt R (1989) Environmental impact of used motor oil. Sci Total Environ 79(1):1–23. doi:10.1016/0048-9697(89)90049-1
Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald GF, Chater KF, van Sinderen D (2007) Genomics of Actinobacteria: tracing the evolutionary history of an ancient phylum. Microbiol Mol Biol Rev 71(3):495–548
Verstraete W, Wittebolle L, Heylen K, Vanparys B, de Vos P, van de Wiele T, Boon N (2007) Microbial resource management: the road to go for environmental biotechnology. Eng Life Sci 7(2):117–126. doi:10.1002/elsc.200620176
Wang H, Lin K, Hou Z, Richardson B, Gan J (2010) Sorption of the herbicide terbuthylazine in two New Zealand forest soils amended with biosolids and biochars. J Soils Sediments 10(2):283–289. doi:10.1007/s11368-009-0111-z
Wardle DA (1998) Controls of temporal variability of the soil microbial biomass: a global-scale synthesis. Soil Biol Biochem 30(13):1627–1637. doi:10.1016/s0038-0717(97)00201-0
Wardle DA, Zackrisson O, Nilsson MC (1998) The charcoal effect in Boreal forests: mechanisms and ecological consequences. Oecologia 115(3):419–426. doi:10.1007/s004420050536
Wardle DA, Nilsson M-C, Zackrisson O (2008) Fire-derived charcoal causes loss of forest humus. Science 320(5876):629–629
Warnock DD, Lehmann J, Kuyper TW, Rillig MC (2007) Mycorrhizal responses to biochar in soil—concepts and mechanisms. Plant Soil 300(1–2):9–20
Wengel M, Kothe E, Schmidt CM, Heide K, Gleixner G (2006) Degradation of organic matter from black shales and charcoal by the wood-rotting fungus Schizophyllum commune and release of DOC and heavy metals in the aqueous phase. Sci Total Environ 367(1):383–393
Wessels JG (1999) Fungi in their own right. Fungal Genet Biol 27(2):134–145
White JC, Kelsey JW, Hatzinger PB, Alexander M (1997) Factors affecting sequestration and bioavailability of phenanthrene in soils. Environ Toxicol Chem 16(10):2040–2045. doi:10.1002/etc.5620161008
Wilson SC, Jones KC (1993) Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): a review. Environ Pollut 81(3):229–249
Wu J, Brookes P (2005) The proportional mineralisation of microbial biomass and organic matter caused by air-drying and rewetting of a grassland soil. Soil Biol Biochem 37(3):507–515
Xing B, Pignatello JJ (1997) Dual-mode sorption of low-polarity compounds in glassy poly (vinyl chloride) and soil organic matter. Environ Sci Technol 31(3):792–799
Yamato M, Okimori Y, Wibowo IF, Anshori S, Ogawa M (2006) Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci Plant Nutr 52(4):489–495
Yip K, Xu M, Li C-Z, Jiang SP, Wu H (2010) Biochar as a fuel: 3. mechanistic understanding on biochar thermal annealing at mild temperatures and its effect on biochar reactivity. Energy Fuel 25(1):406–414
Young DF, Ball WP (1994) A priori simulation of tetrachloroethene transport through aquifer material using an intraparticle diffusion model. Environ Prog 13(1):9–20. doi:10.1002/ep.670130112
Yuan J-H, Xu R-K, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102(3):3488–3497
Zackrisson O, Nilsson M-C, Wardle DA (1996) Key ecological function of charcoal from wildfire in the Boreal forest. Oikos 10-19
Zavalloni C, Alberti G, Biasiol S, Vedove GD, Fornasier F, Liu J, Peressotti A (2011) Microbial mineralization of biochar and wheat straw mixture in soil: a short-term study. Appl Soil Ecol 50:45–51
Zhang T, Walawender WP, Fan L, Fan M, Daugaard D, Brown R (2004) Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem Eng J 105(1):53–59
Zhang H, Lin K, Wang H, Gan J (2010) Effect of Pinus radiata derived biochars on soil sorption and desorption of phenanthrene. Environ Pollut 158(9):2821–2825
Zhang W, Sun H, Wang L (2013) Influence of the interactions between black carbon and soil constituents on the sorption of pyrene. Soil Sediment Contam Int J 22(4):469–482
Zhou J, He Z, Van Nostrand JD, Wu L, Deng Y (2010) Applying GeoChip analysis to disparate microbial communities. Microbe 5:60–65
Zhu D, Kwon S, Pignatello JJ (2005) Adsorption of single-ring organic compounds to wood charcoals prepared under different thermochemical conditions. Environ Sci Technol 39(11):3990–3998. doi:10.1021/es050129e
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44(4):1295–1301. doi:10.1021/es903140c
Zimmerman AR, Gao B, Ahn M-Y (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43(6):1169–1179
Zimmermann M, Bird MI, Wurster C, Saiz G, Goodrick I, Barta J, Capek P, Santruckova H, Smernik R (2012) Rapid degradation of pyrogenic carbon. Glob Chang Biol 18(11):3306–3316
Acknowledgments
This work was supported by Exploratory Research Grant Scheme L4077 of the Universiti Teknologi Malaysia. The comments made by reviewers are gratefully acknowledged for improving this review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Anyika, C., Abdul Majid, Z., Ibrahim, Z. et al. The impact of biochars on sorption and biodegradation of polycyclic aromatic hydrocarbons in soils—a review. Environ Sci Pollut Res 22, 3314–3341 (2015). https://doi.org/10.1007/s11356-014-3719-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3719-5