Abstract
Sungai Puloh mangrove estuary supports a large diversity of macrobenthic organisms and provides social benefits to the local community. Recently, it became a major recipient of heavy metals originating from industries in the hinterland as a result of industrialization and urbanization. This study was conducted to evaluate mobility and pollution status of heavy metals (Cd, Cu, Ni, Pb, Zn, and Fe) in intertidal surface sediments of this area. Surface sediment samples were collected based on four different anthropogenic sources. Metals concentrations were analyzed using an atomic absorption spectrophotometer (AAS). Results revealed that the mean concentrations were Zn (1023.68 ± 762.93 μg/g), Pb (78.8 ± 49.61 μg/g), Cu (46.89 ± 43.79 μg/g), Ni (35.54 ± 10.75 μg/g), Cd (0.94 ± 0.29 μg/g), and Fe (7.14 ± 0.94 %). Most of the mean values of analyzed metals were below both the interim sediment quality guidelines (ISQG-low and ISQG-high), except for Pb concentration (above ISQG-low) and Zn concentration (above ISQG-high), thus suggesting that Pb and Zn may pose some environmental concern. Cadmium, Pb, and Zn concentrations were above the threshold effect level (TEL), indicating seldom adverse effect of these metals on macrobenthic organisms. Pollution load index (PLI) indicated deterioration and other indices revealed the intertidal surface sediment is moderately polluted with Cd, Pb, and Zn. Therefore, this mangrove area requires urgent attention to mitigate further contamination. Finally, this study will contribute to data sources for Malaysia in establishing her own ISQG since it is a baseline study with detailed contamination assessment indices for surface sediment of intertidal mangrove area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Elemental contamination in coastal areas arising from rapid urbanization and industrialization has become an increasing worldwide concern because this ecosystem receives anthropogenic and industrial wastes. These wastes which are bioaccumulative, toxic, and persistent include heavy metals. Heavy metals are introduced to the aquatic environment and accumulate in sediments by several pathways via natural and anthropogenic processes (Akoto et al. 2008). These processes include disposal of liquid effluents, runoffs and leachates, surface soil erosion, bioturbation, dredging, water drainage, atmospheric deposition, and indiscriminate use of heavy metal-bearing fertilizer and pesticides in agricultural fields (Soares et al. 1999; Yang and Rose 2005; Nouri et al. 2008; Sow et al. 2013). One of the most affected ecosystems in coastal areas is the mangrove ecosystem. The surface sediments of mangrove ecosystem could become significantly polluted with heavy metals, especially in coastal regions near urban areas and harbors (Loring and Rantala 1992). Mangrove ecosystem is important for wild life, fisheries, and aquacultural initiatives. This unique ecosystem has been known as nursery areas for juvenile fishes, crustaceans, and marine life (Sasekumar et al. 1992); they also provide refuge to numerous macrobenthic organisms from their predators during low tide. The tropical coastal mangrove serves more importantly as feeding ground than nursery ground for juveniles of commercially important fish (Chong et al. 1990). The mangrove ecosystem being an ecotone between terrestrial/freshwater and marine environment (Vane et al. 2009) makes it a good trap zone against sea and land debris.
However, today’s mangrove ecosystem has been abused through human impacts. Despite their importance, mangrove ecosystems have been exploited and subjected to inappropriate management practices in terms of land reclamation and unsustainable forestry, as well as aquaculture initiatives (Ong 1995). Among the major pollutants from anthropogenic inputs are heavy metals (MacFarlane 2002). Mangrove sediments serve as a sink for heavy metals that may be released back to the water column by various remobilization processes (Daskalakis and O’Connor 1995; Long et al. 1995; Argese et al. 1997; Zulkifli et al. 2010a) as a consequence of equilibrium disruption. Heavy metals can be mobilized from sediment if there is a change in physicochemical properties (Kelderman et al. 2000) and consequently result in trophic transfer to detritivores and other higher level trophic organisms in the food web. In addition, the accumulation of heavy metals in sediments can bioaccumulate and also produce harmful effects on the biota living in them (Harikumar and Nasir 2010; Joksimovic et al. 2011). This implies that the consumption of commercial fishes feeding on contaminated benthic organism poses a health concern to the local community and the general public. In this environment, therefore, sediments can play a double role, both as a carrier and as a possible source of contaminants. As a result of the ubiquitous presence of heavy metals, their persistence and their ability to create hazardous conditions in the environment, a number of studies (Mackey et al. 1992; Perdomo et al. 1999; Shriadah 1999; Tam and Wong 2000; Mremi and Machiwa 2004; Defew et al. 2005; Otero et al. 2009; Marchand et al. 2011) have been carried out all over the world to assess the levels of different elements in mangrove sediments.
Malaysia being one of the fastest developing countries in the world with diverse and numerous species of fishes and benthic organisms being harvested by fishermen in mangrove estuaries (Chong et al. 1990) is threatened with intense anthropogenic activities. Malaysia mangrove ecosystem has been endangered since past decades as a result of rapid development in coastal areas, over logging, and clear-cutting of mangroves along the coastlines and increased reliance on mangroves for aquaculture and wood products (Hashim et al. 2010). In order to protect and conserve this fragile mangrove, a complete understanding of mangrove environment and evaluation of anthropogenic inputs of various heavy metals is essential. The examination and study of sediment quality can reveal the pollutant variations, degradations, and cycles, and their chronic effects on water pollution. Sediment quality has been recognized as an important indicator of water pollution, since they act as pool for contaminants (Santos Bermejo et al. 2003).
To assess anthropogenic influences and the extent of contamination by heavy metals, numerous indices (e.g., enrichment factor (EF), contamination factor (CF), pollution load index (PLI), geoaccumulation index (Igeo), and statistical tools are employed worldwide (Sekabira et al. 2010). There are no studies assessing anthropogenic influences on sediment chemical composition using such indices in Sungai Puloh mangrove which is one of the major productive mangrove ecosystems with great biodiversity in Malaysia. The present study was aimed to determine the concentrations, distribution, mobility, and sources of heavy metals (Cd, Cu, Ni, Pb Zn, and Fe) in surface sediments of Sungai Puloh intertidal mangrove ecosystem and to assess the pollution status as well as the possible influence of anthropogenic activities. The following contamination indices were employed: CF, EF, Igeo, PLI, effective range low (ERL), and effective range median (ERM).
Materials and methods
Study area
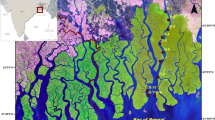
The mangrove area of Sungai Puloh (SGP, 03° 04.786′ N, 101° 23.903′ E) is located in the state of Selangor, west coast of Peninsular Malaysia. Sungai Puloh forms a small linked estuary with the Straits of Malacca. The Straits of Malacca is a semidiurnal and meso-macrotidal system, with tidal ranges approximately 1–3 m during neap tide and 3–5 m during spring tides (Polgar and Crosa 2009). Sungai Puloh mangrove area sustains a great diversity of commercial fish species and macrobenthic organisms. It stretches about 6.87 km in length and is situated in approximately less than 5 km off the major intercity road of Jalan Kapar. There are several small- and medium-sized industries (SMIs) operating in the vicinity. The industries involve various types such as scrap metal yards, recycling sectors (these factories handle a lot of scrap metals, polyethylene containers, and various nondegradable materials), automobile workshops, oil palm mills, and others. Most of their wastes are drained in this mangrove ecosystem. There is also a coal-fired power generating plant (Tenaga Nasional Berhad’s Sultan Salahuddin bin Abdul Aziz-SSA) at Kapar near to Sg. Puloh mangrove area which is the country’s largest coal-fired station generating a total of 1420 MW of electricity into the National Grid network. Also, a handful of residential houses were observed in this area, while fishing and cattle rearing are the main domestic activity close to the Puloh River.
Samples collection
Forty-two surface sediment samples were collected in March, 2012 from 14 sampling stations along the intertidal mangrove area of Puloh River (Fig. 1). In order to determine the distribution and source of metal contamination in Puloh mangrove sediments, these stations were divided into four site groups based on possible anthropogenic activities viz: site I (stations 1, 2, and 3) = factory outlet—smelting activities and recycling industries; site II (stations 4, 5, and 6) = sluice gate—urban runoff, effluent from coal-fired plant, commercial and restaurant activities, auto parts, agriculture area, and animal husbandry; site III (stations 7, 8, 9, and 10) = Jetty—fishermen activities; and site IV (stations 11, 12, 13, and 14) = beyond Jetty—sea activities. Triplicate samples of surface sediment from each station were collected using a plastic scoop. The upper 2 cm of each sample was placed in a polyethylene bag with a stainless steel spatula. All the samples were kept in an iced chest, taken to the laboratory, and frozen until further analysis.
Sediment preparation and digestion
Surface sediment samples were dried by using air-circulating oven to a constant dry weight at 80 °C. Later, the samples were ground using a clean dry pestle and ceramic mortar and sieved through a 63-μm stainless steel sieve. Heavy metals (Cd, Cu, Ni, Pb, Zn, and Fe) concentrations in surface sediment samples were analyzed using the direct aqua regia method. About 1 g of homogenized sample was weighed and digested in a mixture of concentrated nitric acid (HNO3 AnalaR grade, BDH 69 %) and perchloric acid (AnalaR grade 60 %) in a ratio 4:1. The samples were then placed in the digesting block, and the temperature was first set at a low temperature of 40 °C for 1 h and later increased to 140 °C for at least 3 h (Ismail 1993; Yap et al. 2002). After digestion, the samples were diluted to a 40-ml volume with double-distilled water and filtered using the Whatman No. 1 filter paper into acid-washed polyethylene sample bottles and stored for metal determination.
Metal determination
After digestion and filtration, the samples were analyzed by using an air-acetylene flame atomic absorption spectrophotometer (Analyst 800 model, by Perkin-Elmer) and the data are presented in dry weight basis (μg/g dry weight). In order to avoid contamination, all glass wares were soaked in acid wash (10 % HCL) for at least 24 h and later rinsed with double-distilled water and air dried before use. To ensure precision and accuracy of the analytical method, quality control calibration curves were generated by analyzing multiple-level calibration standards, and standard solutions of each metal studied were prepared from 1000 mg/l (BDH Spectrosol®) stock solution. Certified reference material (CRM) (International Atomic Energy Agency, Soil-5, Vienna, Austria) was used to check the quality of this method. The analytical results for reference material and its certified values showed satisfactory metal recovery percentages being about 109, 97, 96, 98, 95, and 104 % for Cd, Cu, Ni, Pb, Zn, and Fe, respectively. Blank was used to zero the machine and a quality control sample was analyzed in every eight samples during metal analysis.
Indices
Several indices, namely contamination factor, enrichment factor, pollution load index, and geoaccumulation index, were used to assess the metal pollution in this intertidal mangrove surface sediments.
Contamination factor
The geochemical and physiochemical characteristics of sediments are a vital tool for environmental pollution assessment, as well as identifying the contamination point sources. The contamination factor (CF) is an indicator of sediment contamination which is used to evaluate the pollution of the environment by a given toxic substance. It is expressed as the concentration of each metal with respect to the background value in sediment as follows (Turekian and Wedepohl 1961): CF = metal concentration in sediment/background value of metal.
The evaluation criteria for using CF are as follows: CF < 1 refers to low contamination, 1 < CF < 3 means moderate contamination, 3 < CF < 6 indicates considerable contamination, and CF > 6 indicates very high contamination.
In this study, the background value used is that of the world average concentration of metals reported for shale (Turekian and Wedepohl 1961).
Enrichment factor
Enrichment factor (EF) has been widely reported as an important tool to differentiate between anthropogenic and natural occurring metal sources in the sediment (Selvaraj et al. 2004; Naji and Ismail 2011). Aluminum (Al) and iron (Fe) are two main elements used as normalizers for EF computation. However, Fe is used as a normalizer in this study because it is the fourth major element in the earth’s crust and most often has little or no adverse environmental concerns (Naji and Ismail 2011). Moreover, the following unique properties of Fe make it a better normalizer: (1) its geochemistry is close to that of trace metals, (2) it is associated with fine solid surfaces, and (3) its natural sediment concentration tends to be uniform (Daskalakis 1995; Naji and Ismail 2011). The enrichment factor for Fe-normalized data was calculated using the following equation:
Where (Cx/Fe) sample and (Cx/Fe) average shale values, respectively, are the metal concentrations (μg/g) dry weight in relation to Fe levels (dry weight) in sediment samples and average shale values taken from Krauskopf and Bird (1995). EF values were interpreted as suggested by Wang et al. (2008). If 0.5 ≤ EF ≤ 1.5, then it indicates that metal could be mainly from natural weathering process, and if EF > 1.5, then it indicates that metal is from anthropogenic sources or a greater percentage of the metal is from non-natural weathering process. However, the degree of enrichment was interpreted as proposed by Birch (2003). EF < 1 indicates no enrichment, 1 < EF < 3 indicates minor enrichment, 3 ≤ EF ≤ 5 indicates moderate enrichment, 5 ≤ EF ≤ 10 indicates moderate to severe enrichment, 10 ≤ EF ≤ 25 indicates severe enrichment, 25 ≤ EF ≤ 50 indicates very severe enrichment, and EF > 50 suggests extremely severe enrichment.
Pollution load index
The pollution load index (PLI) is the estimated geometric mean of relative concentration factors of selected heavy metal of a seemingly polluted site. According to Daud et al. (2009), PLI is an integrated tool used to assess the extent of pollution at a site for a selected number of metals. The PLI value equal to 1 suggests the absence of pollution, whereas PLI > 1 suggests that the site is considered polluted (Cabrera et al. 1999). The level of pollution has been assessed by using the method proposed by Tomlinson et al. (1980), i.e., the concentration factor of each heavy metal with respect to the background values in the sediment. The parameter is shown as follows:
Where n = number of metals and CF = contamination factors.
Geoaccumulation index
This index determines the sediment contamination by organic and inorganic substances by comparing present metal concentration with preindustrial levels. Geoaccumulation index (Igeo) was originally defined by Muller (1969) in order to determine and define metal contamination in sediments by comparing current concentration with preindustrial levels as follows:
Where C n is the measured concentration of the examined element n in the sediment sample and B n is geochemical background value of the element n of average shale. The seven different classes of geoaccumulation index along with associated sediment pollution extent as proposed by Muller (1981) are given in the Table 1.
Statistical analysis
The statistical analyses were performed using Statistical package for Social Science (SPSS) version 20. The nonparametric analysis (Kruskal-Wallis) with a probability of 95 % was employed to compare the distribution of metal concentrations in surface sediments from different sites. One-way ANOVA was used to compare the means and Tukey’s HSD multiple comparisons was adopted for the post hoc test of significant difference, while the possible relationship between metals was estimated by determining the correlation coefficient.
Results and discussion
The levels and distribution of heavy metals
The average and range of heavy metal concentrations in surface sediment for all stations are presented in Table 2. The ranges for heavy metal levels in the surface sediment were 16.45–132.91, 22.79–55.54, 0.60–1.55, 35.51–167.38, and 291.96–2584.34 μg/g and 5.57–8.68 % for Cu, Cd, Pb, Zn, and Fe, respectively. It was found that Zn has the highest mean concentration Zn (1023.68 ± 762.93 μg/g) followed by Pb (78.84 ± 49.61 μg/g), Cu (46.89 ± 43.79 μg/g), Ni (35.54 ± 10.75 μg/g), and Cd (0.94 ± 0.29 μg/g) (Table 2). Most of the heavy metals have highest concentrations in stations of site II, which receives effluents from coal-fired power plant and urban runoffs from the inner city of Klang. Anthropogenic activities resulting from rapid development of factories, industrial activities, coal-generated power plants, transportation, and agricultural-related activities make the main source of Cu, Zn, Cd, Ni, and Pb contaminants in this area (Zulkifli et al. 2010b). However, the highest mean concentration of Fe (8.68 %) was observed in site I, close to smelting industry. This could be a result of deposition of ferrous materials directly from the recycling factory. The higher metal concentrations found in site II could probably be a result of the intense anthropogenic activities along Jalan Kapar, Klang, and effluents from the nearby coal-fired gas plant. Effluents from coal-fired plants have been found to be responsible for altering the chemical composition of the soil. Fly ash, a by-product of coal-fired plant, is usually rich in trace metals. If the fly ash is heaped outside peradventure it comes in contact with rain or other water, the heavy metals will leach from the fly ash and contaminate the soil, groundwater, surface water, and even the mangrove area. Agrawal et al. (2010) reported that samples of sediments surrounding a coal-fired power plant contained higher concentrations of lead, cadmium, and nickel after rain events. Sulfur dioxide and nitrogen oxides both combine with water in the atmosphere to create acid rain which acidifies the soils and water, killing off plants, fish, and the animals that depend on them. Also, Wang et al. (2008) has elaborated on the leaching behavior and environmental impact of the potentially hazardous elements found in coal combustion residues. It has been widely reported by many authors (Sato and Sada 1992; Stalikas et al. 1997; Meij and te Winkel 2007; Karamanis et al. 2009; Lu et al. 2012) that coal-fired power plants are one of the main sources of pollution affecting the general environment in terms of health hazards, air, soil, and water contamination, and land use. Effluents from coal burning contain large amounts of solids and have high biochemical and chemical oxygen demands. The high organic matter contained in coal burning effluents could aid the retention of heavy metals in sediments. Coal burning results in the emission of fine particulate matter into the atmosphere; smog formed by nitrogen oxide and reactive organic gases causes crop, forest, and property damage. This coal combustion waste consists of radionuclides, lead, mercury, arsenic, and other heavy metals, which can be extremely toxic to human health. These wastes may leach into downstream water supplies or soils where they can enter the food chain and potentially induce chronic toxic effects in humans and other organisms. As these power plants seek to reduce their air emissions, more pollutants are left behind in solid waste residues in the form of coal ash, sludge from desulfurization systems, and particles trapped by other control systems. A typical 1000-MW power plant consumes 12,000 t of coal per day and produces an alarming amount of 1,000,000 t of wastes per year (Agrawal et al. 2010). This means that more than 1,000,000 t of wastes annually are produced from the power plant near the mangrove area.
The distribution of heavy metals among the four source groups can be seen in Fig. 2a–f.
The concentrations of heavy metals in sediment followed the order of sites II > IV > III > I for Ni and Cd. Concentrations of Cu showed similar trend except for the sites I > III. A common pattern was observed for Zn and Pb concentrations with the order II > I > IV > III, while Fe showed a different trend as for other metals. The average distribution of concentrations of heavy metals (Cd, Cu, Ni, Pb, Zn, and Fe) in sediments from different sites were compared by using a nonparametric Kruskal-Wallis test and they were significantly different (p < 0.05).
One-way ANOVA was employed to compare the mean metal concentrations among the sites, and the result (Table 3) showed that most of the examined metals except Fe concentrations in sediments of site II were significantly different (p < 0.05) from other sites. However, Cd and Cu concentrations in sediments of site I with site III and as well as between sites I and IV for Cu and between sites III and IV for Cd were not significantly different (p > 0.05). Concentrations of Pb, Ni, Zn, and Fe in sediments of sites I and II were significantly different p < 0.05) from other sites except between sites I and site III for Ni; between sites I and IV for Zn, and sites II and IV for Fe were not significantly different (p > 0.05). There were significant differences (p < 0.05) for Cu, Pb, Ni, Fe, and Zn concentrations in sediments between sites III and IV while there was no significant difference (p > 0.05) for Cd. The very wide range of differences between metals in sites II and III could be attributed to the immobilization of heavy metals by the closure of the sluice gate. Sluice gate is a barrier that functions to prevent the incursion of tidal water into the developed areas. When the sluice gate in site II is closed, the effluents which are responsible for the transfer of sediment is contained for a long time and thereby favor the heavy metals to be precipitated and trapped within the sediment, only to be remobilized during low tide when the gate is opened for water passage to site III. During this process, very few metals (mostly the newly precipitated metals) can be remobilized leaving a greater quantity locked within the sediment of site II. This phenomenon can open up a research frontier on how sluice gates affect the mobility of heavy metals.
The relationships among the examined heavy metals were tested using Spearman’s correlation analysis. The correlation matrix revealed that Cu, Cd, Pb, Ni, and Zn in all the sampled stations were significantly correlated with one another showing strong positive relationships (r = 0.452–0.960; p < 0.01) (Table 4). It is only Cd that negatively correlated with Fe (r = −0.425; p > 0.05). However, Cu, Pb, and Zn showed strong positive association with Fe (p < 0.05), while Ni was weakly correlated with Fe (r = 0.22; p < 0.05). The strong positive correlation obtained among Cu, Cd, Pb, Ni, and Zn in this intertidal mangrove sediment of Sungai Puloh is suggestive that there is a uniform anthropogenic source and a similar behavior during mobilization (Alagarsamy 2006). On the contrary, the weak and lack of correlation with Fe underlines the fact that certain metals may behave differently during transport. The significant positive correlations of these examined metals (except Fe, Cd, and Ni) could be because the sources of these metal inputs both anthropogenic and lithogenic are similar. Gerringa (1990, 1991) demonstrated in his studies that Cu, Cd, and Pb show similar mobility and can be released by aerobic degradation of organic matter. Furthermore, Broman et al. (1991) also suggested that a common uptake and release mechanism by organisms living within the sediment can cause a similar mobility of heavy metals, while Ramessur (2004) and Yap et al. (2005) reported in their studies that Cu, Pb, and Zn were positively correlated, suggesting a common source of these metals in their studied areas.
In each site group, almost all the heavy metals had a strong correlation with other metals in each site group except for those in sites I and IV for Cd, which was negatively correlated with other metals. The negative correlation of Cd with all the metals in sites I and IV reflects that the anthropogenic inputs of these heavy metals come from site II which has higher concentrations of heavy metals and then are transported to site III when the sluice gate is opened or during tidal actions.
Contamination assessment
Contamination factor, effective range low, effective range media, and enrichment factor
Based on the average shale (Turekian and Wedepohl 1961), the mean contamination factor (CF) values of metals studied are shown in Table 5. CF value for Ni was found to be low (<1); CFs for Cu in site II and Fe in all the sites revealed moderate contamination (1 ≥ CF ≥ 3), while CFs for Cd and Pb indicated moderate to considerable contamination in all the sites (3 ≥ CF ≥ 6) except for site II where Pb showed very high contamination (CF > 6). However, only Zn CFs showed very high contamination in all the studied sites (CF > 6) (Table 5, mean CF values of heavy metal in Sungai Puloh intertidal mangrove area). The high CFs for Cd and Pb in all sites could be from urban runoffs that comprise wastewater discharges from automobile repair shops and service stations (Sheela et al. 2012), scrap yards, effluents from the coal-fired plant, and recycling industry. Moreover, according to Abu-Hilal (1987), the high Pb CFs in all the sites could be a result of some specified sources like scavenging and agricultural runoff. The high Zn CFs may be due to shipping activities; ships loaded with crude (with occasional spills) ply the Straits of Malacca which is one of the busiest shipping lanes in Southeast Asia, and antifouling paints that contain huge amount of zinc oxide, and from dissolution of pure zinc used in anticorrosion galvanized product (Rahman and Ishiga 2012). The very high CFs for Zn could also arise from numerous paint and fertilizer industries located within the vicinity of this mangrove area.
The sediment quality indices, effective range low (ERL), and effective range median (ERM) (Table 2) proposed by Long et al. (2009) was employed to predict the environmental consequences of these analyzed elements. Concentrations of Ni, Pb, and Zn (20.90, 46.70, and 150 μg/g, respectively) where higher than the ERL in all stations except for Pb concentration that is lower than ERL in few stations of site III. However, Cd and Cu concentrations (1.2 and 34 μg/g) in almost all the stations were below the ERL except for few stations in site II. Zn concentrations in all the stations were above the ERM 410 μg/g, while Ni concentrations were below the ERM 51.60 μg/g in most of the stations except for few stations in site II. The comparison of the metals with the average shale (Turekian and Wedepohl 1961) revealed that Cd, Pb, and Zn concentrations exceeded the average shale levels 0.30, 20.00, and 95.00 μg/g, respectively, while Cu and Ni concentrations were below average shale 45.00 and 68.00 μg/g, respectively, in all the stations except for Cu which showed higher levels in few stations of site II. According to MacDonald et al. (2000), ERL values are concentrations below which adverse effect on sediment dwelling organisms are not frequently expected, while the ERM represents concentration above which adverse effect are more likely to occur. Therefore, the sediment of Sungai Puloh intertidal mangrove might probably have adverse effects on macrobenthic organisms since two metals exceeded the ERM levels following the proposition of Yap and Pang (2011) that adverse biological effect might occur if one metal exceeds the ERM level.
Contamination and possible anthropogenic impacts on the surface sediment in Sungai Puloh was also assessed using enrichment factor (EF). The mean EFs were calculated with respect to average shale (Turekian and Wedepohl 1961) and are shown in Table 6. Cu and Ni EFs showed no enrichment in almost all the stations except for stations in site II which revealed minor enrichment for Cu (1.75–1.80). And thus suggests that Cu in site II is mainly from anthropogenic sources. The mean EF values for Cu and Ni (0.67 and 0.34, respectively) of all the stations were ≤1, indicating there is no enrichment of these metals in the studied mangrove area. Cd and Pb with mean EF values of 2.05 and 2.49, respectively, indicated minor enrichment, while mean EF value of 6.84 for Zn is suggestive of moderate to severe enrichment. The highest EF values for all the metals studied, namely Cu: 1.80, Ni: 0.50, Cd: 3.15, Pb: 5.31, and Zn: 16.61, were found in the site which receives effluents from the coal-fired plant, agriculture, and urban runoffs. Among all the analyzed metals, Cu and Ni had an average EF value (<1), indicating that these metals originated mainly from natural weathering processes. However, Cd, Pb, and Zn with mean EF value (>1.5) suggested that these metals were of anthropogenic sources according to Wang et al. (2008).
EF values for all the metals were highest in site II and lowest in site I when compared with other sites. Higher values of Pb and Zn were recorded in site I which has higher EF values than sites III and IV (Fig. 3). According to the proposition of Birch (2003) for the interpretation of the degree of enrichment factor, Cd showed minor (1 < EF < 3) enrichment in almost all the sites. There was no enrichment (EF < 1) for Cu and Ni for all the sites except for site II with minor enrichment for Cu, while Pb showed minor enrichment for almost all the sites except for site II with moderate to severe (5 ≤ EF ≤ 10) enrichment. However, Zn was moderately enriched for all sites except for site II with severe (10 ≥ EF ≤ 25) enrichment. Site II showed the highest EF values of the analyzed metals indicating a higher degree of anthropogenic inputs in this site. This is in agreement with the observation of González and Brügmann (1991) that municipal and/or industrial waste water discharges into coastal areas are the most important sources of contamination of water and sediment with heavy metals. The wash water from auto repair shops dotted along Kapar road, the motor panels, engines and gearbox waste oil, and vehicle emissions contribute to the enrichment of Cd, Pb, and Zn in the surface sediment of this mangrove. Also, the enrichment of Zn may stem from anthropogenic sources which include vehicle tires, galvanized building materials, paints, and industrial activities (electrical and electronic factories); the main sources of Cu are from vehicle brake pads, plumbing, active coal-based power plant, and industrial activities, while Pb major sources are paints, batteries, cables, alloys, and gasoline (heavy traffic in the nearby city), which can be made more sever by the use of leaded petrol (Ismail et al. 1993; Yap et al. 2003; Zulkifli et al. 2010a, b; Sheela et al. 2012). Kamaruzzaman et al. (2011) also reported that Pb in sediments of coastal area in Malaysia is a result of anthropogenic activities. The average EF values of heavy metals in sediment from Sungai Puloh were lower (for Cu and Pb) and higher (for Zn) than those in sediments from the north western Peninsular Malaysia as reported by Yap and Pang (2011). Sany et al. (2013a) reported significant enrichment of Cd and Pb in Klang straits, west Peninsular Malaysia compared with the minor to moderate enrichment of Cd and Pb in this study; this could be a result of more anthropogenic activities in the Klang River than in Sg. Puloh. However, these reports underscore the fact that there is a threat of heavy metal especially Cd and Pb contamination in the west coast of Peninsular Malaysia originating from anthropogenic activities.
Pollution assessment
Pollution load index and index of geoaccumulation
The pollution load index (PLI) and the calculated index of geoaccumulation (Igeo) values of the studied metals in all the stations are presented in Table 7 (mean Igeo, its classes, and PLI of the studied metals in surface sediments for all stations). PLI values were found to be high (PLI > 1) in all the stations, indicating that there is presence of pollutants and therefore unavoidable progressive deterioration of sediment quality (Tomlinson et al. 1980). The main contributors to each PLI are Cd, Pb, and Zn as it is revealed by EFs. The highest PLI among the sites were found in site II (Fig. 4). However, all the sites are considered polluted according to Cabrera et al. (1999) since PLI is >1 for all sites. The average Igeo revealed that the surface sediment of this mangrove area is moderately polluted with Cd and Pb, and moderately polluted to strongly polluted by Zn. Using the classification of Muller (1981) for the degree of pollution among the sites (Table 8) revealed that site I is unpolluted to moderately polluted with Cd, moderately polluted with Pb, and moderately polluted to strongly polluted with Zn.
Only site II showed unpolluted to moderately polluted with Cu (and no pollution with Cu in other sites), moderately polluted with Cd, moderately to strongly polluted with Pb, and strongly to extremely polluted with Zn. In site III which appears to be the least polluted among the sites revealed unpolluted to moderately polluted with Cd and Pb, and moderately polluted with Zn. All the sites revealed no pollution with Ni. Considering the mean PLI and Igeo values of heavy metals obtained in the surface sediments on this mangrove, it could be reported that the Sungai Puloh mangrove surface sediments is moderately polluted with Cd, Pb, and Zn and therefore require urgent action from appropriate quarters.
The ranges of heavy metal concentrations of this study were compared with sediment quality guidelines and other studies reported within Malaysia and other parts of the world (Table 9). In comparison with Malaysia studies, Cu is found to be comparable with that reported by Yap et al. (2003) and Zulkifli et al. (2010b) but higher than those reported by Ismail et al. (1993), Ismail and Asmah (1999), Kamaruzzaman et al. (2011), and Sany et al. (2013b). Also, Cd and Pb in this study are comparable with the recent study of Sany et al. (2013b), while Ni, Cd, Pb, and Zn are higher than earlier studies reported for Peninsular Malaysia, Hong Kong, and Singapore. However, when this study is compared to studies in other parts of the world, Cu ranges are lower than those of South west coast of Spain, Port Jackson Australia, Guanabara Bay, Brazil, Kaoshiung Habour, Taiwan, and Red Sea, Egypt. Cadmium and Pb are comparable with those of South Coast of pain, and Guanabara Bay, Brazil, while Ni was found to be higher in that reported in Guanabara Bay, Brazil than that of this study. Furthermore, this study revealed that Ni and Pb are higher in the present study than those of Dumai coast, Indonesia. The Zn concentrations in this study is comparable with those reported by Ismail et al. (1993) and Chen et al. (2007), but higher than other reported studies. The mean concentrations of Cu, Ni, Cd, Pb, and Zn in this study were also compared with the Hong Kong interim sediment quality guidelines (ISQG) reported by Chapman et al. (1999). The comparison revealed that all other studied metals except Zn (1023.68 ± 762.93) μg/g were below both the ISQG-low and ISQG-high, except for Pb (78.84 ± 49.61) μg/g that was a bit higher than the ISQG-low. When the mean concentrations of Cu, Cd, and Pb were compared with those of Canadian sediment quality guidelines, it was observed that Cu, Cd, and Pb concentrations were above the threshold effect level (TEL)—a level below which adverse biological effects are not expected to occur frequently, and were below the probable effect level (PEL)—a level above which adverse biological effect is expected to occur very often. Since this two levels (TEL and PEL) have been commonly used as screening tools for sediment quality by different stakeholders involved in sediment management activities (Canadian Councils of Minister of environment 2002), and Cd and Pb which are not known to have any beneficial value to living organisms were above the thresh hold effect level (TEL). Therefore, it is advised that further investigation of these two metals Cd and Pb within this mangrove area be conducted and anthropogenic mitigation measures be implemented.
Conclusion
The anthropogenic inputs of heavy metals in intertidal surface sediments of Sungai Puloh is revealed by the EF with respect to Cd, Pb, and Zn, which are mainly from the urban runoffs, and coal-fired plants/industrial effluents. Based on the PLI and Igeo, the quality of the sediment could be said to be moderately polluted and continues to deteriorate and this may impact on the macrobenthic organisms living within the study area. Therefore, this mangrove area requires urgent attention to mitigate further contamination. Finally, this study will contribute to data sources for Malaysia in establishing her own ISQG since it is a baseline study with detailed contamination assessment indices for surface sediment of this intertidal mangrove area.
References
Abu-Hilal AH (1987) Distribution of trace elements in nearshore surface sediments from the Jordan Gulf of Aqaba (Red Sea). Mar Pollut Bull 18(4):190–193
Agrawal P, Mittal A, Prakash R, Kumar M, Singh T, Tripathi S (2010) Assessment of contamination of soil due to heavy metals around coal fired thermal power plants at Singrauli region of India. Bull Environ Contam Toxicol 85(2):219–223
Akoto O, Bruce T, Darko G (2008) Heavy metals pollution profiles in streams serving the Owabi reservoir. Afr J Environ Sci Technol 2(11):354–359
Alagarsamy R (2006) Distribution and seasonal variation of trace metals in surface sediments of the Mandovi estuary, west coast of India. Estuar Coast Shelf Sci 67(1):333–339
Amin B, Ismail A, Arshad A, Yap CK, Kamarudin MS (2009) Anthropogenic impacts on heavy metal concentrations in the coastal sediments of Dumai, Indonesia. Environ Monit Assess 148(1):291–305
Argese E, Ramieri E, Bettiol C, Pavoni B, Chiozzotto E, Sfriso A (1997) Pollutant exchange at the water/sediment interface in the Venice canals. Water Air Soil Pollut 99(1):255–263
Bayen S, Wurl O, Subramanian K, Kae Shing Wong K, Sivasothi N, Obbard JP (2005) Heavy metal contamination in mangrove habitats of Singapore. Mar Pollut Bull 50(12):1732–1738
Birch G. (2003) A scheme for assessing human impacts on coastal aquatic environments using sediments. In C. D. Woodcoffe, & R. A. Furness (Eds.). Coastal GIS, Wollongong University Papers in Center for Marinetime Pollicy,14, Austrailia. Retrieved from http://www.ozestuaries.org/indicators/DEF_sedement_scheme.html
Broman D, Lindqvist L, Lundbergh I (1991) Cadmium and zinc in < i > Mytilus edulis</i > L. from the Bothnian sea and the northern baltic proper. Environ Pollut 74(3):227–244
Cabrera F, Clemente L, Dıaz Barrientos E, López R, Murillo J (1999) Heavy metal pollution of soils affected by the Guadiamar toxic flood. Sci Total Environ 242(1):117–129
Canadian, M. O. E (2002) Canadian sediment quality guidelines for the protection of aquatic life: summary tables. updated. in: Canadian environmental quality guidelines, 1999. Canadian Council of Ministers of Environment, Winnipeg
Chapman PM, Allard PJ, Vigers GA (1999) Development of sediment quality values for Hong Kong special administrative region: a possible model for other jurisdictions. Mar Pollut Bull 38(3):161–169
Chen C-W, Kao C-M, Chen C-F, Dong C-D (2007) Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere 66(8):1431–1440
Chong VC, Sasekumar A, Leh MUC, D’Cruz R (1990) The fish and prawn communities of a Malaysian coastal mangrove system, with comparisons to adjacent mud flats and inshore waters. Estuar Coast Shelf Sci 31:703–722
Daskalakis KD, O’Connor TP (1995) Normalization and elemental sediment contamination in the coastal United States. Environ Sci Technol 29(2):470–477
Daud M, Wasim M, Khalid N, Zaidi JH, Iqbal J (2009) Assessment of elemental pollution in soil of Islamabad city using instrumental neutron activation analysis and atomic absorption spectrometry techniques. Radiochim Acta 97(2):117–121
Defew LH, Mair JM, Guzman HM (2005) An assessment of metal contamination in mangrove sediments and leaves from Punta Mala Bay, Pacific Panama. Mar Pollut Bull 50(5):547–552
El-Said GF, Youssef DH (2012) Ecotoxicological impact assessment of some heavy metals and their distribution in some fractions of mangrove sediments from Red Sea. Egypt Environ Monitor Assess 1–12
Gerringa L (1991) Mobility of Cu, Cd, Ni, Pb, Zn, Fe and Mn in marine sediment slurries under anaerobic conditions and at 20 % air saturation. Neth J Sea Res 27(2):145–156
González H, Brügmann L (1991) Heavy metals in littoral deposits off Havana City, Cuba. Chem Ecol 5(3):171–179
Harikumar P, Nasir U (2010) Ecotoxicological impact assessment of heavy metals in core sediments of a tropical estuary. Ecotoxicol Environ Saf 73(7):1742–1747
Hashim R, Kamali B, Tamin NM, Zakaria R (2010) An integrated approach to coastal rehabilitation: mangrove restoration in Sungai Haji Dorani, Malaysia. Estuar Coast Shelf Sci 86(1):118–124
Ismail A (1993) Heavy metal concentrations in sediments off Bintulu, Malaysia. Mar Pollut Bull 26:706–707
Ismail A, Asmah M (1999) Copper, zinc, lead and cadmium in intertidal molluscs and sediment off Seberang Perai coastline, Malaysia. Paper presented at the fourth Princess Chulabhorn International Science Congress, Bangkok
Ismail A, Badri M, Noor Ramlan M (1993) The background levels of heavy metal concentration in sediments of the west coast of Peninsular Malaysia. Sci Total Environ 134:315–323
Joksimovic D, Tomic I, Stankovic AR, Jovic M, Stankovic S (2011) Trace metal concentrations in Mediterranean blue mussel and surface sediments and evaluation of the mussels quality and possible risks of high human consumption. Food Chem 127(2):632–637
Kamaruzzaman BY, Akbar b SNT, Shahbudin S, Jalal KCA, Ong MC, Al-Barwani SM, Goddard JS (2011) Spatial concentrations of lead and copper in bottom sediments of Langkawi coastal area, Malaysia. Res J Environ Sci 5(2):179–186
Karamanis D, Ioannides K, Stamoulis K (2009) Environmental assessment of natural radionuclides and heavy metals in waters discharged from a lignite-fired power plant. Fuel 88(10):2046–2052
Kelderman P, Drossaert W, Min Z, Galione L, Okonkwo L, Clarisse I (2000) Pollution assessment of the canal sediments in the city of Delft (the Netherlands). Water Res 34(3):936–944
Krauskopf KB, Bird DK (1995) Introduction to geochemistry. McGraw-Hill Inc, New York
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19(1):81–97
Long ER, Field LJ, MacDonald DD (2009) Predicting toxicity in marine sediments with numerical sediment quality guidelines. Environ Toxicol Chem 17(4):714–727
Loring DH, Rantala R (1992) Manual for the geochemical analyses of marine sediments and suspended particulate matter. Earth Sci Rev 32(4):235–283
Lu X., Liu W., Zhao C., & Chen, C. Environmental assessment of heavy metal and natural radioactivity in soil around a coal-fired power plant in China. J Radioanal Nucl Chem, 1-10
MacDonald DD, Ingersoll C, Berger T (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39(1):20–31
MacFarlane G (2002) Leaf biochemical parameters in < i > Avicennia marina</i > (Forsk.) Vierh as potential biomarkers of heavy metal stress in estuarine ecosystems. Mar Pollut Bull 44(3):244–256
Mackey A, Hodgkinson M, Nardella R (1992) Nutrient levels and heavy metals in mangrove sediments from the Brisbane River, Australia. Mar Pollut Bull 24(8):418–420
Marchand C, Allenbach M, Lallier-Vergès E (2011) Relationships between heavy metals distribution and organic matter cycling in mangrove sediments (Conception Bay, New Caledonia). Geoderma 160(3):444–456
Meij R, te Winkel H (2007) The emissions of heavy metals and persistent organic pollutants from modern coal-fired power stations. Atmos Environ 41(40):9262–9272
Morillo J, Usero J, Gracia I (2004) Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere 55(3):431–442
Mremi S, Machiwa J (2004) Heavy metal contamination of mangrove sediments and the associated biota in Dar es Salaam, Tanzania. Tanzania J Sci 29(1):61–76
Muller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geojournal 2(3):108–118
Muller G (1981) The heavy metal pollution of the sediments of Neckars and its tributary: a stocktaking. Chemiker- Zeitung 105:157–164
Naji A, Ismail A (2011) Assessment of metals contamination in Klang River surface sediments by using different indexes. Environ Asia 4(1):30–38
Neto JAB, Gingele FX, Leipe T, Brehme I (2006) Spatial distribution of heavy metals in surficial sediments from Guanabara Bay: Rio de Janeiro, Brazil. Environ Geol 49(7):1051–1063
Nouri J, Mahvi A, Jahed G, Babaei A (2008) Regional distribution pattern of groundwater heavy metals resulting from agricultural activities. Environ Geol 55(6):1337–1343
Ong J (1995) The ecology of mangrove conservation & management. Hydrobiologia 295(1):343–351
Otero X, Ferreira T, Huerta-Díaz M, Partiti C, Souza V, Vidal-Torrado P, Macías F (2009) Geochemistry of iron and manganese in soils and sediments of a mangrove system, Island of Pai Matos (Cananeia—SP, Brazil). Geoderma 148(3):318–335
Perdomo L, Ensminger I, Elster C, Wallner-kersanach M, Schnetter ML (1999) The mangrove ecosystem of the Ciénaga Grande de Santa Marta (Colombia): observations on regeneration and trace metals in sediment. Mar Pollut Bull 37(8):393–403
Polgar G, Crosa G (2009) Multivariate characterisation of the habitats of seven species of Malayan mudskippers (Gobiidae: Oxudercinae). Mar Biol 156(7):1475–1486
Rahman MA, Ishiga H (2012) Trace metal concentrations in tidal flat coastal sediments, Yamaguchi Prefecture, southwest Japan. Environ Monit Assess 1–17
Ramessur RT (2004) Statistical comparison and correlation of zinc and lead in estuarine sediments along the western coast of Mauritius. Environ Int 30(8):1039–1044
Santos Bermejo J, Beltrán R, Gómez Ariza J (2003) Spatial variations of heavy metals contamination in sediments from Odiel river (Southwest Spain). Environ Int 29(1):69–77
Sany SBT, Salleh A, Rezayi M, Saadati N, Narimany L, Tehrani GM (2013a) Distribution and contamination of heavy metal in the coastal sediments of Port Klang, Selangor, Malaysia. Water Air Soil Pollut 224:1476. doi:10.1007/s11270-013-1476-6
Sany SBT, Salleh A, Sulaiman AH, Sasekumar A, Rezayi M, Tehrani GM (2013b) Heavy metal contamination in water and sediment of the Port Klang coastal area, Selangor, Malaysia. Environ Earth Sci 69:2013–2025. doi:10.1007/s12665-012-2038-8
Sasekumar A, Chong VC, Leh MU, D’Cruz R (1992) Mangroves as habitats for fish and prawns. Hydrobiologia 247:195–207
Sato K, Sada K (1992) Effects of emissions from a coal-fired power plant on surface soil trace element concentrations. Atmos Environ A Gen Topics 26(2):325–331
Sekabira K, Oryem Origa H, Basamba T, Mutumba G, Kakudidi E (2010) Assessment of heavy metal pollution in the urban stream sediments and its tributaries. Int J Environ Sci Technol 7(3):435–446
Selvaraj K, Ram Mohan V, Szefer P (2004) Evaluation of metal contamination in coastal sediments of the Bay of Bengal, India: geochemical and statistical approaches. Mar Pollut Bull 49(3):174–185
Sheela A, Letha J, Joseph S, Thomas J (2012) Assessment of heavy metal contamination in coastal lake sediments associated with urbanization: Southern Kerala, India. Lakes Reserv Res Manag 17(2):97–112
Shriadah M (1999) Heavy metals in mangrove sediments of the United Arab Emirates shoreline (Arabian Gulf). Water Air Soil Pollut 116(3):523–534
Soares H, Boaventura R, Machado A, Esteves da Silva J (1999) Sediments as monitors of heavy metal contamination in the Ave river basin (Portugal): multivariate analysis of data. Environ Pollut 105(3):311–323
Sow A. Y., Ismail A. & Zulkifli S. Z. (2013) An assessment of heavy metal bioaccumulation in Asian swamp eel, Monopterus albus, during plowing stages of a paddy cycle. Bull Environment Toxicol, 1-7
Stalikas CD, Chaidou CI, Pilidis GA (1997) Enrichment of PAHs and heavy metals in soils in the vicinity of the lignite-fired power plants of West Macedonia (Greece). Sci Total Environ 204(2):135–146
Tam N, Wong Y (2000) Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ Pollut 110(2):195–205
Tomlinson D, Wilson J, Harris C, Jeffrey D (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol Mar Res 33(1):566–575
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72(2):175–192
Vane CH, Harrison I, Kim A, Moss-Hayes V, Vickers B, Hong K (2009) Organic and metal contamination in surface mangrove sediments of South China. Mar Pollut Bull 58(1):134–144
Wang W, Qin Y, Song D, Wang K (2008) Column leaching of coal and its combustion residues, Shizuishan, China. Int J Coal Geol 75(2):81–87
Yang H, Rose N (2005) Trace element pollution records in some UK lake sediments, their history, influence factors and regional differences. Environ Int 31(1):63–75
Yap C, Pang B (2011) Assessment of Cu, Pb, and Zn contamination in sediment of north western Peninsular Malaysia by using sediment quality values and different geochemical indices. Environ Monit Assess 183(1):23–39
Yap C, Ismail A, Tan S, Omar H (2002) Concentrations of Cu and Pb in the offshore and intertidal sediments of the west coast of Peninsular Malaysia. Environ Int 28(6):467–479
Yap CK, Ismail A, Tan SG (2003) Cd and Zn concentrations in the straits of Malacca and intertidal sediments of the west coast of Peninsular Malaysia. Mar Pollut Bull 46(10):1349–1353
Yap C, Rahim-Ismail A, Ismail A, Tan S (2005) Analysis of heavy metal concentration data (Cd, Cu, Pb and Zn) in different geochemical fractions of the surface sediments in the Straits of Malacca by the use of correlation and multiple linear stepwise regression analyses. Malays Appl Biol 34(2):51
Yap CK, Choh MS, Berandah FE, Ismail A, Tan SG (2006) Comparison of heavy metal concentrations in surface sediment of Tanjung Piai wetland with other sites receiving anthropogenic inputs along the southwestern coast of Peninsular Malaysia. Wetl Sci 4(1)
Zulkifli SZ, Ismail A, Mohamat-Yusuff F, Arai T, Miyazaki N (2010a) Johor Strait as a hotspot for trace elements contamination in Peninsular Malaysia. Bull Environ Contam Toxicol 84(5):568–573
Zulkifli SZ, Mohamat-Yusuff F, Arai T, Ismail A, Miyazaki N (2010b) An assessment of selected trace elements in intertidal surface sediments collected from the Peninsular Malaysia. Environ Monit Assess 169(1):457–472
Acknowledgments
The authors express their gratitude to the Universiti Putra Malaysia for providing infrastructural facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Udechukwu, B.E., Ismail, A., Zulkifli, S.Z. et al. Distribution, mobility, and pollution assessment of Cd, Cu, Ni, Pb, Zn, and Fe in intertidal surface sediments of Sg. Puloh mangrove estuary, Malaysia. Environ Sci Pollut Res 22, 4242–4255 (2015). https://doi.org/10.1007/s11356-014-3663-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3663-4