Abstract
The physical modification of habitat by the rodents can create patches with altered species richness relative to adjacent, unmodified habitats. The majority of studies found a positive correlation between engineering patches and animal species richness/diversity/abundance. In this paper, we show occupancy of wheatear (Oenanthe isabellina) in engineering patches created by Mongolian marmot (Marmota sibirica) and no engineering in different habitat types of the arid steppe ecosystem. We surveyed 123 sites for Isabelline wheatear in Hustai National Park, Mongolia in 2016. We used a model selection approach to evaluate the effect of marmot burrows and other potential variables on occupancy probability. We detected Isabelline wheatear in 46.34% of the surveys (171 of 369). Our top model indicated that occupancy was strongly influenced by marmot burrows. The estimated wheatear occupancy was 0.95 at a burrow site and 0.64 at a non-burrow site. Detection was a function of temperature and highest around 24.6 ºC. Our results indicate that Isabelline wheatear distribution is strongly influenced by marmots as burrows presumably provide it with important habitat resources. The loss of marmots will probably have direct effects on the distribution of the breeding bird and the ecosystem processes they support. Our study provides one of the few examples of the role of marmots in influencing breeding birds and supports the notion of marmots as a keystone species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecosystem engineers represent species that influence the availability of resources to other species by physically modifying, maintaining, or creating habitats (Jones et al. 1994; Wright and Jones 2006). The influence of ecosystem engineers can be strong, directly affect the abundance and distribution of other species, and indirectly affect ecosystem processes (Jones et al. 1997). The loss of ecosystem engineers consequently presents challenges to the conservation and management of species and ecosystems (Roman et al. 2014).
Burrowing species often represent ecosystem engineers. For example, rodents such as pocket gophers (Geomys spp.), prairie dogs (Cynomys spp.), and zokors (Myospalax spp.), and carnivores, such as Arctic foxes (Vulpes lagopus) create subterranean burrows that provide habitat for other species, and their excavations affect nutrient dynamics, vegetation structure, diversity, productivity, and soil hydrology (Reichman and Seabloom 2002; Zhang et al. 2003; VanNimwegen et al. 2008; Gharajehdaghipour et al. 2016). These changes increase landscape heterogeneity and affect food webs (Hastings et al. 2007).
The Mongolian marmot (Marmota sibirica) is a large rodent species (ca. 6–8 kg) that ranges mainly across the steppe regions of Mongolia, but also parts of Russia and China (Clayton 2016). The impact of marmots on other species is particularly important as the species has experienced substantial declines in their population and range size due to overhunting for fur, meat, and body parts for traditional medicine (Clark et al. 2006; Kolesnikov et al. 2009). Once this species has been estimated at over 20 million in the 1990s, marmots have declined over 75% since then, and are now listed as Globally Endangered by the International Union for Conservation of Nature (IUCN, Clayton 2016).
Mongolian marmots excavate extensive burrow systems and live in colonies that have been recorded to cover several hectares and include > 90 burrow entrances (Townsend 2006, 2009). Marmot burrow system tunnels and chambers extending up to > 100 cm in depth (Kucheruk 1983). Mongolian marmots act as ecosystem engineers by digging burrows, which affect soil and vegetation conditions on colonies (Van Staalduinen and Werger 2007; Yoshihara et al. 2009; Yoshihara et al. 2010b; Sasaki et al. 2013; Todgerel and Dorzhiev 2018). Burrows also provide habitat for other species. For example, burrows are commonly used as daytime dens for carnivores, such as corsac foxes (Vulpes corsac) and Pallas’ cats (Otocolobus manul) (Murdoch et al. 2009; Ross et al. 2010). Other species have been observed using burrows as shelter, including small mammals (e.g., gerbils, Meriones spp.), birds (e.g., Common Shelducks, Tadorna tadorna), and reptiles (e.g., toad-headed agamas, Phrynocephalus versicolor, Mongolian racerunners, Eremias argus) and darkling beetles (Blaps rugosa) (Adiya 2000, 2021a, 2021b; Murdoch et al. 2013; Buyandelger et al. 2017, 2018, 2019). Mongolian marmots are often considered a keystone species for the disproportionately large impacts they have on other species (Zahler et al. 2004; Murdoch et al. 2009).
The effects of Mongolian marmots on birds are poorly studied. However, many authors noted the Isabelline wheatears regularly use rodent burrows for nesting (Smith and Foggin 1999; Collar 2005; Khanal 2007; Aspinall 2009; Li and Lu 2012). The Isabelline wheatear is a small passerine bird that is a member of the family Muscicapidae. They are considered as a migratory bird and commonly breed throughout the desert and steppe ecosystems of Mongolia (Purevsuren and Jargal 2019; Gombobaatar and Monks 2011). Breeding of the monogamous pairs occurs from late April to mid-July in Mongolia. They set deep in a rodent burrow or burrow of similar mammals or occasionally in a natural hole or crevice (Collar 2005; BirdLife International 2020). Li and Lu (2012) reported nesting success of the wheatears was greater than sympatric open-nesting passerine in Tibet. This is probably due to the rodent burrow reduces physical and biological stresses for the broods.
We examined the relationship between Mongolian marmots and wheatears, specifically Isabelline wheatear, to gain a better understanding of the influences of marmot ecosystem engineering on this bird in the steppe ecosystems. Our objective was to examine whether the Isabelline wheatear distribution is influenced by marmot burrows. The loss of marmots may lead to negative effects on steppe species and ecological processes and represents a conservation concern across the marmot’s range.
Materials and methods
Study area
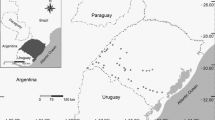
We conducted the present study in Hustai National Park, which covers approximately 60,000 ha in north-central Mongolia (Fig. 1). Hustai is located at the most southern part of the Siberian taiga in the Mongolian Dauria phytogeographical region of Mongolia (Tserendulam et al. 2018) and includes grassland and shrubland steppe (88% of area) and deciduous forest (4% of area). The region is characterized by steppes in basins and an otherwise mountainous relief, partly covered by forests. Vegetation is dominated by the bunch grass, Stipa krylovi, with Artemisia adamsii, Artemisia frigida, Agropyron cristatum, and Cymbaria dahurica as typical species. The topography is variable and includes mountains composed of granitic rocks, flat or gently rolling plains, and river valleys, and elevation varies from 1100 to 1840 m. The region is arid with ≤ 240 mm of annual precipitation, which falls mostly as summer rain (80%) between June and August, and air temperature ranges from − 40 (average winter temperature − 20 °C) to + 40 (average summer temperature + 18 °C). The study area is relatively rich with wildlife species, due to its protected status and conservation management. Marmots are highly abundant relative to other regions in Mongolia (Kolesnikov et al. 2009). The park estimated that 13,213 marmots occupied in 10,380 ha of Hustai, which would equate to ca. 1.27 individuals/ha (Todgerel 2020).
Survey sites for Isabellina wheatear (Oenanthe isabellina) occupancy in Hustai National Park, Mongolia. Population density of marmot based on an assessment of Mongolian marmot distribution and density of Hustai National Park (HNPT 2009)
Surveys
We surveyed for Isabelline wheatear presence at 123 sites, which included 82 sites at the entrance of marmot burrows (marmot sites), and 41 sites not at marmot burrow (non-marmot sites). The sites based on marmot distribution map of Hustai National Park (Todgerel 1998; HNPT 2009). We selected sites to maximize the variability of habitat types, soil types, and topography, and to ensure a minimum spacing of > 500 m for independence. We conducted a survey of each site in June, July, and August of 2016 (n = 3 total surveys per site). Each site was a circular plot with a 100 m radius, and each survey involved by two people, one researcher walking and one researcher standing and observing from the center of the survey plot. We based the survey are on the maximum marmot home range size estimated by another study in Hustai (0.81; Buuveibaatar and Yoshihara 2012). We recorded as ‘1’ if the species was detected and ‘0’ if the species was not detected to generate a capture history for each site. Possible capture histories included: 111, 110, 101, 011, 001, 010, 100, 000. At the beginning of each survey, we recorded air temperature (°C) at ground level and wind speed (m/s) at the center of each plot using a handheld weather meter (Kestrel 2500, Nielsen-Kellerman Co., Boothwyn, Pennsylvania, USA).
Modeling approach
We developed a model that predicted the occupancy probability of Isabelline wheatear based on the detection/non-detection survey data. We used an occupancy modeling approach, which uses maximum likelihood methods to estimate occupancy probability while accounting for imperfect detection (MacKenzie et al. 2002). Our approach involved developing a set of candidate models. Each model represented a hypothesis and included a parameter for occupancy probability (ψ) and detection probability (p). Some models also included parameters for the effect of a single or additive combination of covariates on occupancy and detection. Occupancy covariates included: (1) site type (marmot or non-marmot). We were primarily interested in examining the influence of marmot burrows on Isabelline wheatear occupancy and predicted that burrows would positively impact the bird distribution. (2) Habitat types included open plain, mountain, foothill and drainage. These four types represent the primary, broad-scale habitats in the study area and were defined based on vegetation community and topography. Open plains habitat represented flat and gently rolling plains dominated by grasses (~ 1200 m), mountain habitat represented topographically variable terrain characterized by mountains (up to ~ 1800 m) and mixed grasses, shrubs and trees, and foothill and drainage habitat represented narrow draws in mountain foothills between open plain and mountain habitat that drained seasonal precipitation and were dominated mainly by grasses and shrubs (Hustai National Park Trust 2016). Marmots occur in each habitat but unevenly based on previous observations. We predicted that open plains and foothill would have a stronger positive effect on occupancy over other types based on studies of marmots elsewhere suggesting that open plains represent high-quality habitat concerning foraging and burrowing resources (Buyandelger et al. 2017). Detection covariates included wind speed and temperature. We expected that Isabelline wheatear would become less active and retreat to a burrow in high winds as wind reduces mobility by blowing sand and soil at ground level. We also expected that wheatear would be least active (hidden under vegetation or in a burrow) at low and high temperatures and most active and detectable within an optimum temperature range. We modeled temperature as a polynomial based on this expectation (temperature + temperature2).
Our model set included 12 models, which represented all combinations of occupancy and detection covariates. We also included a null model that only included the intercepts for ψ and p. We ranked the relative support of each model using Akaike’s Information Criterion corrected for small sample size (AICc), and considered models with < 2 ΔAICc to have strong empirical support (Burnham and Anderson 2002). We also calculated variable importance scores for each occupancy covariate as they were equally represented in the model set (score = sum of the weights for each model that included a given covariate), and evidence ratios for each model (ratio = weight of a given model to the top model) (Burnham and Anderson 2002).
Model fit
We tested the fit of the data to the most parameterized model in the set as AICc only provides a relative measure of model support. We tested model fit using a bootstrapping technique described by MacKenzie and Bailey (2004). Briefly, the technique involved estimating a χ2 statistic from the observed and expected capture histories under a given model. Then, the model was used to generate a new set of capture data and new χ2 statistic. This process was then repeated many times (100 in our case) to create a distribution of χ2 values. The value from the actual data was then compared to the distribution. If the value was less than the 95th percentile of the distribution, then the data were considered to have a good fit to the model.
Results
We conducted 369 surveys and detected Isabelline wheatear during 171 of those surveys (46.34%) at 57 sites (46.34% of all sites). Most sites with detections (37.4%) were at marmot burrows and occurred in drainage habitat (36.4%), followed by foothill (31.8%), open plain (18.2%), and then by mountain habitat (13.6%). In contrast, site with non-detection were primarily non-marmot (91.8%) and occurred in drainage habitat (32.7%), followed by open plain (30.1%), foothill (24.8%), and then by mountain habitat (12.4%). Mean wind speed was 3.17 ± 0.43 SE m/s for all surveys, 3.66 ± 0.56 SE m/s for surveys with detections, and 2.78 ± 0.26 SE m/s for surveys without detections. Mean temperature was + 24.82 ± 0.50 °C for all surveys, + 24.63 ± 0.53 °C for surveys with detections, and 24.97 ± 0.43 °C for surveys without detections.
The top-ranking model indicated that occupancy was a function of marmot burrow and detection was a function of temperature (Table 1). The weight of evidence that this was the best model in the set was over twice the weight of the next ranking model (Table 1). However, other model had strong empirical support, which indicated that occupancy was a function of the additive combination of marmot and habitat (Table 1). All other models had > 3 ΔAIC. We considered the top model to not only best represent the data and present results from that model, but also report model average estimates given the relative support of other models (Table 2).
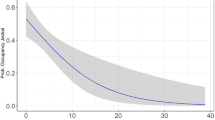
Parameter estimates from the top model indicated that occupancy was considerably higher at marmot sites than non-marmot sites (Table 2; Fig. 2). The odds ratio, which represents the predicted change in the odds of occupancy in response to a one-unit increase in a given covariate, was 20.69 (95% CI 0.09–4626.71) for marmot burrow. Detection probability predicted from the model was highest around + 25 °C, but below 0.10 at temperatures < + 13 °C and > + 37 °C (Fig. 3). Confidence intervals (95%) around covariates did not cross zero, suggesting that their effects were meaningful (Table 2). Variable importance scores across the model set were 0.95 for the marmot burrow covariate and 0.64 for the habitat covariate. Among detection covariates, variable importance scores were 0.79 for temperature and 0.06 for wind. The data fit the most parameterized model in the set (χ2 = 23.37, p = 0.01).
Isabelline wheatear (Oenanthe isabellina) occupancy probability as a function of whether a given site in the landscape was at a Mongolian marmot (Marmota sibirica) burrow. Probabilities estimated from a top-ranking model of wheatear occupancy based on detection/non-detection data collected in Hustai National Park, Mongolia
Isabelline wheatear (Oenanthe isabellina) detection probability as a function of temperature. Probabilities estimated from a top-ranking model of wheatear occupancy that accounted for imperfect detection based on detection/non-detection data collected in Hustai National Park, Mongolia. Temperature was modeled as a polynomial
Discussion
We found that burrows and surrounding topographic features exerted a strong positive impact on the Isabelline wheatear occupancy based on our model selection results and related estimates (i.e. variable importance score, evidence ratio, and odds ratio). The strong influence of burrows on the Isabelline wheatear occupancy suggests burrows provide valuable resources for this species and represent a landscape characteristic more important than the four broader landscape habitat types considered in this study (i.e., open plain, mountain, foothill and drainage), which were defined by vegetation community and topography. The marmot colonies dominate by ceaspitose Stipa Krylovii, Agropyron cristatum; and rhizomatous species such as Leymus chinensis, Artemisia Adamsii, Carex duriuscula; and annual species Dontostemon integrifolius and Salsola collina (Todgerel and Dorzhiev 2018). But the rhizomatous species does not dominant in the control site. Marmot mound is initially covered by bare soil and it helps distribute plant species which easiest colonized species, perennial forbs generally occurring in the steppe vegetation (Todgerel and Dorzhiev 2018). Moreover, marmot colony indicates that abundance and species richness of arthropods which might be a reason for increased habitat heterogeneity such as bare ground, specific vegetation structure and thermoregulatory site by ecosystem engineering (Buyandelger et al. 2021a). Our results support the hypothesis that Mongolian marmot functions as a keystone species for certain species in the steppes of Mongolia because of their engineering activities (Murdoch et al. 2013).
The breeding habitats and nest sites with a favorable microclimate can be advantageous, especially for birds breeding in harsh environments (Rauter and Reyer 2000). The microclimate in marmot burrows is relatively stable, especially concerning to ground temperatures, not rising too high in hot weather nor too low in cold weather (Nikol’skii 2002). They may also provide decrease mortality in their brood life stage development (e.g. egg, nestling and fledgling). One of our camera trap surveys represented avian abundance was significantly higher on colony sites compared to off-colony sites in three different areas of Mongolia (Buyandelger et al. 2021a, b). Our results provide a measure of the effect of temperature on bird detection. Probability of detecting the species at a given site was greatest at 25 °C based on our model. Furthermore, our model estimated that detection probability is > 62.6% between 19 and 30 °C.
The concentration of prey (i.e. the higher density of other species using burrows) and favorable microhabitat of burrows create a rich niche that a variety of species exploited (Hansell 1993). As an insect eater, Isabelline wheatear likely consumes marmot burrow dwellers, which can accumulate in large volumes in and around individual burrows. For example, a previous study in Hustai National Park showed that marmot burrow mounds had more flowers and consequently higher visitation rates by insect pollinators than other non-mound sites, indicating that burrows not only impacted the distribution of some species (insects and vascular plants in this case) but also have the potential to influence important ecosystem services (the supporting service of pollination) (Yoshihara et al. 2010a). Isabelline wheatear comprises that characteristic of chalk outcrops with low vegetation cover (Banik 2017).
Do these species have symbiotic co-occurring for breeding shelter or food resources? Our ability to evaluate this idea is constrained by the scarcity of autecological data for the Isabelline wheatear occurrence, but our work has identified species that would be logical choices for mechanistic studies of their response to disturbance and their functional roles in the semi-arid region.
Mongolian marmots are well protected in Hustai National Park, but only 6% of the species’ range falls in protected areas in Mongolia (Clayton 2016). Marmots face clear threats from overharvesting, which represents the main driver of decline (Kolesnikov et al. 2009). Government bans on hunting, investment in reducing poaching, and reintroduction efforts have contributed to reducing declines and recovery of the species in some areas (Townsend 2009). Intensive livestock grazing, a ubiquitous practice, and means of subsistence in rural Mongolia represent another threat in the form of competition that can lower marmot fitness by reducing food availability just before hibernation (Buuveibaatar and Yoshihara 2012). Our results suggest that recovering marmots across much of their range through broad-scale actions related to minimizing harvest and livestock impacts will have positive benefits for birds, and potentially their effects on other species and ecosystem processes.
Our study examined the effects of marmot burrows and coarse-scale habitats on the Isabelline wheatear occupancy. We recommend future studies considering finer scale variables of bird distribution, such as the availability of food to gain further insight into the value of burrows as a resource. Impacts of changes in beetle distribution on other species and environmental conditions would also lead to a better understanding of their effects on ecosystem processes.
References
Adiya Y (2000) Mongolian marmots: biology, ecology, conservation and use. Mammalian Ecology Laboratory. Institute of Biology, Mongolian Academy of Sciences. Ulaanbaatar, Mongolia (in Mongolian)
Aspinal S (2009) Relationship between nesting Isabelline wheatears Oenanthe isabellina and a burrowing rodent (a jird Meriones sp). Sandgrouse 31(2):179–181
BirdLife International (2020) Species factsheet: Oenanthe isabellina. Downloaded from http://www.birdlife.org
Banik MV (2017) Breeding bird communities in hills with chalk outcrops in national nature park ‘Dvorichanskyi.’ J VN Karazin Kharkiv Natl Univ 28:101–110
Burnham K, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Buuveibaatar B, Yoshihara Y (2012) Effects of food availability on a time budget and home range of Siberian marmots in Mongolia. Mong J Biol Sci 10:25–31
Buyandelger S, Otgonbayar B, Reading PR (2017) Mongolian racerunners (Eremias argus) occupancy in active and inactive Siberian marmot (Marmota sibirica) colonies. J Biodivers Endanger Species 5:204. https://doi.org/10.4172/2332-2543.1000204
Buyandelger S, Otgonbayar B, Bayartogtokh B, Murdoch JD (2018) Siberian marmot (Marmota sibirca) ecosystem engineering supports darkling beetles (Blaps rugosa) in Hustai National Park, Mongolia. 7th International Conference on the Genus Marmota, Marmotas of the Old and New World, Ulaanbaatar, Mongolia
Buyandelger S, Otgonbayar B, Reading PR (2019) The strange case of the common shelduck Tadorna tadorna and the endangered Mongolian marmot Marmota sibirica. BirdingASIA 31:62–64
Buyandelger S, Enkhbayar T, Otgonbayar B, Zulbayar B, Bayartogtokh B (2021a) Ecosystem engineering effects of Mongolian marmots (Marmota sibirica) on terrestrial arthropod communities. Mong J Biol Sci 19:17–30
Buyandelger S, Otgonbayar B, Bayartogtokh B, Reading PR (2021b) Assessing wildlife biodiversity using camera trap data on Mongolian marmot (Marmota sibirca) colonies. J Arid Environ 188:104409. https://doi.org/10.1016/j.jaridenv.2020.104409
Clark EL, Munkhbat J, Dulamtseren S, Baillie JEM, Batsaikhan N, King SRB, Samiya R, Stubbe M (2006) Summary conservation action plans for Mongolian mammals. Regional Red List Series, Zoological Society of London, London
Clayton E (2016) Marmota sibirica. IUCN Red List of Threatened Species e.T12832A22258643.
Collar NJ (2005) Family Turdidae (thrushes and chats). In: del Hoyo J, Elliott A, Christie DA (eds) Handbook of the birds of the world, vol 10. Lynx Edicions, Barcelona, pp 514–807
Gharajehdaghipour T, Roth JD, Fafard PM, Markham JH (2016) Arctic foxes as ecosystem engineers: increased soil nutrients lead to increased plant productivity on fox dens. Sci Rep 6:24020
Gombobaatar S, Monks EM (2011) Regional red list series, Vol 7. In: Seidler R, Sumiya D, Tseveenmyadag N, Bayarkhuu S, Baillie JEM, Boldbaatar S, Uuganbayar Ch (eds) Birds. Zoological Society of London, National University of Mongolia and Mongolian Ornithological Society, London
Hansell MH (1993) The ecological impact of animal nest and burrows. Funct Ecol 7:5–12
Hastings A, Byers JE, Crooks JA, Cuddington K, Jones CG, Lambrinos JG, Talley TS, Wilson WG (2007) Ecosystem engineering in space and time. Ecol Lett 10:153–164
Hustai National Park Trust (2009) Assessment of Mongolian marmot density and distribution of Hustai National Park. Mammal Ecology Laboratory of the Institute of Biology, Mongolian Academy of Science and Hustai National Park, Mongolia
Hustai National Park Trust (2016) The management plan of the Hustai National Park and its buffer zone (2016–2020). Hustai National Park, Mongolia
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Jones CG, Lawton JH, Shachak M (1997) Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78:1946–1957
Khanal B (2007) New report on the symbiotic relation of Ochotona roylei (Lagomorpha: Ochotonidae) and scaly breasted Wren-Babbler (Pnoepyge albiventer) at Ganesh Himalaya area of Central Nepal. Our Nat 5:37–40
Kolesnikov V, Brandler O, Badmaev B, Zoje D, Adiya Y (2009) Factors that lead to a decline in numbers of Mongolian marmot populations. Ethol Ecol Evol 21:371–379
Kucheruk VV (1983) Fauna and ecology of rodents. Proceeding on the study of the fauna and flora of the USSR. Section of Zoology. #52. Moscow State University, pp 5–39. (in Russian)
Li S, Xin L (2012) Reproductive ecology of isabelline wheatears at the extreme of their altitude distribution. Ardeola 59(2):301–307
MacKenzie DI, Bailey LL (2004) Assessing the fit of site-occupancy models. J Agric Biol Environ Stat 9:300–318
MacKenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255
Murdoch JD, Munkhzul T, Buyandelger S, Reading RP, Sillero-Zubiri C (2009) The endangered Siberian marmot Marmota sibirica as a keystone species? Observations and implications of burrow use by corsac foxes Vulpes corsac in Mongolia. Oryx 43:431–434
Murdoch JD, Davie H, Munkhchuluun G, Donovan T, Reading RP (2013) Do Siberian marmots influence toad-headed agama occupancy? Examining the influence of marmot colonies and three steppe habitats in Mongolia. J Arid Environ 92:76–80
Nikol’skii AA (2002) Relative effects of soil and surface air on marmot burrow temperature: a study of the bobac as an example. Dokl Biol Sci 328:25–27 (in Russian)
Purewsuren T, Jargalsaikhan L (2019) Birds of Mongolia: a photographic guide. Munkhiin Useg Printing, Ulaanbaatar (in Mongolian)
Reichman OJ, Seabloom EW (2002) The role of pocket gophers as subterranean ecosystem engineers. Trends Ecol Evol 17:44–49
Rauter C, Reyer HU (2000) Thermal and energetic consequences of nest location and breeding times in water pipits (Anthus spinoletta). J Ornithol 141:391–407
Roman J, Estes JA, Morissette L, Smith C, Costa D, McCarthy J, Nation JB, Nicol S, Pershing A, Smetacek V (2014) Whales as marine ecosystem engineers. Front Ecol Environ 12:377–385
Ross S, Kamnitzer R, Munkhtsog B, Harris S (2010) Den-site selection is critical for Pallas’s cats (Otocolobus manul). Can J Zool 88:905–913
Sasaki T, Kakinuma K, Yoshihara Y (2013) Marmot disturbance drives trait variations among five dominant grasses in a Mongolian grassland. Rangel Ecol Manage 66:487–491
Smith AT, Foggin JM (1999) The plateau pika (Ochotona curzoniae) is a keystone species for biodiversity on the Tibetan plateau. Anim Conserv 2:235–240
Todgerel T (1998) Assessment of Mongolian marmot distribution and density of Hustai Naitonal Park. Hustai National Park, Mongolia. Annual Report of Hustai National Park. (In Mongolian)
Todgerel T (2020) Assessment of Mongolian marmot distribution and density of Hustai National Park. Hustai National Park, Mongolia (in Mongolian)
Todgerel T, Dorzhiev TSZ (2018) Vegetation on marmot mounds in the steppes of central Mongolia. Proceeding of the 7th International Conference on the Genus Marmota “Marmots of the Old and New World” 13–17 August 2018. Ulaanbaatar, Mongolia. Narud Design LLC. 285–296
Townsend SE (2006) Burrow cluster as a sampling unit: an approach to estimate marmot activity in the eastern steppe of Mongolia. Mong J Biol Sci 4:31–36
Townsend SE (2009) Estimating Siberian marmot (Marmota sibirica) densities in the Eastern Steppe of Mongolia. Ethol Ecol Evol 21:325–338
Tserendulam T, Bayarsaikhan U, Oyuntsetseg B, Wesche K (2018) The vascular plant flora of Hustai National Park, Mongolia: composition, life forms, ecological groups and geographical elements. Feddes Repertorium 129:137–160
Van Staalduinen MA, Werger MJA (2007) Marmot disturbances in Mongolia steppe vegetation. J Arid Environ 69:344–351
VanNimwegen RE, Kretzer J, Cully JF (2008) Ecosystem engineering by a colonial mammal: how prairie dogs structure rodent communities. Ecology 89:3298–3305
Wright JP, Jones CG (2006) The concept of organisms as ecosystem engineers ten years on progress, limitations, and challenges. Bioscience 56:203–209
Yoshihara Y, Ohkuro T, Buuveibaatar B, Takeuchi K (2009) Effects of disturbance by Siberian marmots (Marmota sibirica) on spatial heterogeneity of vegetation at multiple spatial scales. Grassland Sci 55:89–95
Yoshihara Y, Ohkuro T, Buuveibaatar B, Undarmaa J, Takeuchi K (2010a) Pollinators are attracted to mounds created by burrowing animals (marmots) ina Mongolian grassland. J Arid Environ 74:159–163
Yoshihara Y, Okuro T, Buuveibaatar B, Undarmaa J, Takeuchi K (2010b) Responses of vegetation to soil disturbance by Siberian marmots within a landscape and between landscape positions in Hustai National Park, Mongolia. Grassland Sci 56:42–50
Zahler P, Lkhagvasuren B, Reading RP, Wingard GJ, Amgalanbaatar S, Gombobaatar S, Barton N, Onon Y (2004) Illegal and unsustainable wildlife hunting and trade in Mongolia. Mong J Biol Sci 2:23–31
Zhang Y, Zhang Z, Liu J (2003) Burrowing rodents as ecosystem engineers: the ecology and management of plateau zokors Myospalax fontanierii in alpine meadow ecosystems on the Tibetan Plateau. Mammal Rev 33:284–294
Acknowledgements
We thank the Mohammed bin Zayed Species Conservation Fund (13257538) and International Foundation for Science (D/5836-1) for funding the project. We also thank D. Usukhjargal and G. Uuganbayar and the staff of Hustai National Park for logistical and administrative support, M. Zulbayar and S. Batdorj for assistance in data collection, Dr. Prof. James D, Murdoch for guiding us through the challenging process of choosing a problem and developing methods to tackle it and Bayartogtokh Badamdorj provided helpful comments on a previous version of this manuscript. The project received administrative support from the National University of Mongolia and the Institute of Biology, Mongolian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buyandelger, S., Otgonbayar, B. Mongolian marmot burrow influences an occupancy of Isabelline wheatear. Landscape Ecol Eng 18, 239–245 (2022). https://doi.org/10.1007/s11355-022-00494-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11355-022-00494-x