Abstract
Purpose
It may be considered that the duration of intervals of high-intensity interval exercise (HIIE) can affect skeletal muscle biochemical and physiological responses. Also, sex-based differences in muscle biochemistry and physiology play a role in different responses to exercise. The aim of this study was to investigate the effects of sex differences on biochemical parameters involved in muscle glycolysis in response to HIIE protocols with short and long-term intervals.
Methods
Forty male and female Wistar rats (aged 8 weeks, mean weighting 270 g and 225 g, respectively) were divided into HIIE with short-term interval (HIIESh), HIIE with long-term interval (HIIEL), and control groups. The phosphofructokinase (PFK), glycogen synthase 1 (GYS1), monocarboxylate transporter 4 (MCT4), and lactate dehydrogenase (LDH) levels were evaluated in gastrocnemius muscle after a single bout of exercise.
Results
The PFK, GYS1, and MCT4 levels were lower in HIIESh and HIIEL groups compared to the control group. However, there was no significant difference between sexes (P < 0.05). Moreover, the LDH activity was higher in both male and female HIIE rats compared to the control group.
Conclusion
It is likely that HIIE with short- and long-term intervals represents similar effects on the glycolytic pathway in both male and female rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, High-intensity interval training (HIIT) is being considered a time-efficient training method to improving cardiorespiratory and metabolic functions [1], providing a unique physiological stimulus compared to traditional exercise [2]. Furthermore, HIIT is now considered to improve exercise performance in both aerobic and anaerobic exercises [3,4,5]. High-intensity interval exercise (HIIE) is strongly associated with anaerobic metabolism. The glycolytic capacity has been introduced as the maximum rate of conversion of glucose to pyruvate or lactate, which can be clearly obtained by a cell [6]. The enzymes involved in glycolysis and muscle glycogenosis such as phosphofructokinase (PFK), lactate dehydrogenase (LDH), glycogen synthase 1 (GYS1) and protein levels of monocarboxylate transporter 4 (MCT4), represent the glycolytic capacity of the skeletal muscle which could be altered with HIIE [7]. A limited number of studies have examined the changes in glycolytic enzyme in response to exercise [8, 9]. There are many variables for designing this popular exercise modality that focused primarily on duration of the active and recovery intervals, which seem to have an effect on acute physiological response [1, 10]. The Norwegian (4 × 4) and standard (10 × 1) protocols were used in the various studies and the effects of each on improving oxidative capacity have been reported [11,12,13,14,15,16] but less attention has been paid to improving the glycolytic capacity.
There are differences in the physiological responses to acute exercise between men and women [17]. It has been suggested that men are more likely than women to have a greater type II muscle-fiber area, glycolytic enzyme activity, and greater reliance on glycolytic metabolism [18].
It is imperative that the physiological responses to various HIIE protocols be characterized and understood so that coaches can prescribe a safe, accurate, and effective HIIE protocol. Thus, the purpose of this study was to compare sex differences in response to two commonly used protocols (16 × 1 min and 4 × 4 min) with the same intensity on biochemical parameters involved in glycolysis and glycogenesis pathways in rat gastrocnemius muscle.
Materials and methods
Animals and exercise protocols
Forty male and female Wistar rats (aged 8 weeks, mean weighting 270 g and 225 g, respectively) were obtained from Birjand University of Medical Sciences (Birjand, South Khorasan, Iran). Rats were under a controlled environment (12 h light–dark cycle and room temperature of 25 ± 2 °C) with food and water ad libitum. All procedures were approved by the Ethics Committee of the Birjand University of Medical Sciences (IR.BUMS.REC.1396.55).
Male and females rats were randomly divided into HIIE with short-term intervals (HIIESh, n = 14), HIIE with long-term intervals (HIIEL, n = 14), and control (n = 12) groups. Rats were weighed at the beginning of the experimental protocol. All animals in the HIIE groups were acclimatized to the treadmill running at 10 m/min for 10 min over 7 days. The control group was exposed to the same environment as both HIIE groups without running. The exercise protocols involved treadmill running of either 16 × 1 min work (HIIESh) or 4 × 4 min work (HIIEL) at 80% − 95% of VO2max (40 m/min), interspersed by 1 and 4 min active rest at 50% − 60% of VO2max (16 m/min), respectively [10, 19]. The treadmill speed changes during work and active rest intervals were taken in less than 10 s. The HIIE groups performed warm-up and cool-down periods for 5 min (40% VO2max, 15 m/min) at the beginning and the end of exercise (the treadmill slope was 0º at all intensities). The desired speed is selected based on previous studies [20, 21]. Figure 1 shows the two HIIE protocols used in this study.
Preparation and homogenization of tissue
The rats were anesthetized with a combination of ketamine/xylazine (100 mg and 10 mg/kg body weight, respectively) immediately after exercise and subsequently, samples from gastrocnemius muscle were removed in less than 5 min after the end of the exercise, and then the isolated tissue was immediately frozen in liquid nitrogen (− 196 °C) and stored at − 80 °C until the assay. For homogenization, the tissue was weighed, incised, and lysed in phosphate-buffered saline (PBS) (pH 7.4, 100 mM, 100 mg tissue/1 ml PBS) through a homogenizer. Anti-protease cocktail (Problock, Goldbio Inc., USA) for preventing protein degradation was added to the lysis buffer. Then, the homogenized samples were centrifuged (6000 rpm, + 4 °C for 10 min), then the supernatants were separated and stored at − 80 °C for subsequent assays.
Measurement of PFK, MCT4, GYS1 levels and LDH activity
At first, the total protein content of tissue samples was measured using a Bradford protein assay kit (intra-assay CV%:19, sensitivity: 5 µg/mL, Colorimetric, ZellBio GmbH, Ulm, Germany, prepared by Padginteb Co., Tehran, Iran), and then the biochemical variables were measured. The level of PFK (intra-assay: CV%:4.6; sensitivity: 0.2 ng/mL), MCT4 (intra-assay: CV%:4.9; sensitivity: 0.05 ng/mL), and GYS1 (intra-assay: CV%:5.1; sensitivity: 0.1 ng/mL) were determined using the specific-related enzyme-linked immunosorbent assay (ELISA) kits manufactured by ZellBio GmbH, Ulm, Germany, prepared by Padginteb Co., Tehran, Iran. Also, the LDH activity (intra-assay: CV%:2.1; sensitivity: 5 U/mL) was measured using an enzymatic assay kit (Photometric UV Test, manufactured by Parsazmoun, Tehran, Iran, prepared by Padginteb Co., Tehran, Iran).
Statistical analyses
The data were tested for normality using the Shapiro–Wilk test. Comparisons were made by two-way ANOVA (2[HIIE protocol] × 2[sex]) and Tukey’s post hoc test. Statistical analysis was performed using SPSS software version 20 and a P < 0.05 was considered statistically significant.
Results
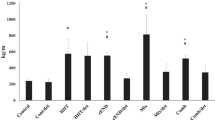
A two-way analysis of variance showed that there was a significant difference in the levels of PFK and GYS1 between the HIIE protocols (F (2, 34) = 8.48, P = 0.001 and F (2, 34) = 11.57, P = 0.0001; respectively), whereas there were no significant differences between the sexes (F (1, 34) = 0.01, P = 0.92 and F (1, 34) = 0.73, P = 39; respectively) and interaction effects (HIIE protocol × sex) (F (2, 34) = 0.75, P = 0.48 and F (2, 34) = 1.24, P = 0.30; respectively). The finding of the post hoc test showed a significant decrease in the level of PFK (P = 0.003 and P = 0.002; respectively) and GYS1 (P = 0.0001 and P = 0.001; respectively) in the HIIESh and HIIEL groups compared to controls (Fig. 2a and b). There were no significant difference between HIIESh and HIIEL for both PFK and GYS1 levels (P = 0.99 and P = 0.77; respectively).
The levels of a phosphofructokinase (PFK), b glycogen synthase1 (GYS1), c monocarboxylate transporter 4 (MCT4), and d activity of lactate dehydrogenase (LDH) of HIIESh and HIIEL groups. HIIESh, HIIE with short-term intervals; HIIEL, HIIE with long-term intervals; *P < 0.05 difference compared to the control group; †P < 0.05 difference between male and female rats
The results showed no significant difference between sexes and interaction effects of HIIE protocol × sex in the level of MCT4 (F (1, 34) = 0.46, P = 0.49 and F (2, 34) = 1.26, P = 0.29; respectively), while there was a significant difference between HIIE protocols (F (2, 34) = 10.52, P = 0.0001). The result demonstrated that MCT4 level in the HIIESh and HIIEL groups was lower than that of the control group (P = 0.001 and P = 0.002; respectively) (Fig. 2c).
The results of LDH activity showed a significant difference between HIIE protocols and sexes (F (2, 34) = 44.52, P = 0.0001 and F (1, 34) = 9.21, P = 0.005; respectively). However, no significant difference was found in the HIIE protocol × sex interaction (F (2, 34) = 0.44, P = 0.64). Following Tukey’s post hoc test, LDH activity in HIIESh and HIIEL groups was significantly higher than the control group (P = 0.0001), while this difference between the HIIESh and HIIEL was not significant (P = 0.95). Also, LDH activity was significantly higher in male rats than in females (P = 0.005) (Fig. 2d).
Discussion
The findings of the present study showed that in the gastrocnemius muscle the levels of PFK, GYS1, and MCT4 were lower in both male and female rats compared to control group after HIIESh and HIIEL protocols, while LDH activity was higher than the control group in both sexes after these two protocols and in male rats it was higher than in the females. The muscle glycogen storage and glycolytic capacity have a key role in performance during high-intensity exercise [7]. Glycogen depletion is recognized as an important molecular signal linked to the gene expression regulation [21]. Glycogen synthase1 plays a major role in glycogen synthesis for muscle contraction and glycogen depletion is a leading cause of impaired skeletal muscle contraction and development of fatigue during exercise [23]. We observed that GYS1 reduction immediately after HIIESh and HIIEL were similar between male and female rats in the gastrocnemius muscle. This reduced level of GYS1 has been reported previously as a common parameter used to confirm the exhaustive characteristic following exercise [24]. It has been demonstrated that glycogen depletion occurs in sprint activities and a short period of physical activity reduces the rate of glycogen utilization [25]. Glycogen synthase has been observed to diminish or remain unchanged during a single bout of exercise and rapidly increase after the cessation of exercise [26, 27]. The mechanisms responsible for inhibition and especially glycolytic enzyme activation have not been fully elucidated. Taken together, there seems to be a consensus that exercise and insulin regulate glycogen synthesis differentially [28].
Glycolytic metabolism is triggered by the initiation of sprint exercise [7]. In the present study, the level of PFK enzyme was significantly decreased following the short and long-term exercise intervals in gastrocnemius muscle in male and female rats. In particular, the marked reduction in the PFK level observed in the gastrocnemius muscle represents the important role of metabolic control of this enzyme. As PFK is the rate-limiting enzyme of glycolysis, then the maximal potential flux through glycolysis would drop considerably after 40 min exercise to 75% VO2max in skeletal muscles where large PFK down-regulation occurred. Therefore, down-regulation of PFK following exercise in the gastrocnemius muscle can be due to any of the following reasons: (1) oxidation of amino acid residues with possible proteolytic degradation; (2) reversible changes in the phosphorylation state of the enzyme; and (3) calcium-activated proteolysis [9]. Tremblay et al. demonstrated that the endurance training program decreased PFK enzyme activity in both men and women. This is consistent with the fact that athletes competing in endurance events generally exhibit muscle PFK enzyme activity within the range of sedentary subjects. They also indicated that the HIIE resulted in a substantial increase in the activity of the glycolytic enzymes [29]. This type of exercise needs a high energy level via glycolytic metabolic pathway; therefore high concentrations of lactate is produced after a repeated bout of maximal short duration exercises [29, 30]. However, although there are several studies about exercise-induced changes in aerobic metabolism-related signaling pathways, little is known about the molecular mechanisms involving changes in anaerobic metabolism during exercise.
Monocarboxylate transporters (MCTs) play an important role in lactate uptake and release [31] and MCT4 is widely expressed especially in tissues that rely on anaerobic metabolism. It plays a key role in the export of lactic acid from skeletal muscle [32]. In the present study, the MCT4 was significantly decreased following the HIIESh and HIIEL in male and female rats but did not differ between the sexes. Despite limited research, it appears that MCT4 can be rapidly affected by an acute exercise. Tonouchi et al. reported a reduction in the plasma membrane content of MCT4 immediately following 10 min high-intensity electrical stimulation performed in rats [33]. Therefore, our findings are consistent with the previous studies and supported the hypothesis that HIIE can acutely decrease relative MCT4 abundance. Although a limited number of studies have examined MCT changes in response to HIIE, the molecular mechanisms involved in this regulation have not yet been fully understood. The literature has previously suggested that the decline in MCT4 content after acute high-intensity electrical stimulation contractions may have been due to translocation of this protein to an intracellular pool [33]. Alternatively, the fall in the membrane MCT content could be attributed to the fact that exhaustive exercise increases free-radical concentration, where lipid peroxidation induced by free radicals may also lead to alterations in membrane fluidity and permeability [31, 34], intracellular translocation, and protein carbonylation [34]. The decline in MCT4 in the present study may be expected to reduce the rate of lactate (and H) removal from the cell [35].
In the present study, the LDH activity was higher following HIIESh and HIIEL in male and female rats compared to the control group and the level of LDH activity in male rats was higher than in females. Our findings are in line with a previous study revealing rises in LDH activity after a single session of high-intensity exercise performed on the treadmill until exhaustion in rats [36]. Also, Takekura et al., [37] investigated the effect of acute exhaustive exercise on LDH activity and PFK level at different times after exercise (0, 1, 6, 24, 48, and 72 h post-exercise) in slow and fast muscles of rats. The activity of LDH increases immediately after exercise only in the fast muscle (extensor digitorum longus). Also, two peaks were showed in slow and fast muscles at 1 and 24 h after exercise. Our findings confirm previous results that suggested increasing LDH activity after a single session exercise. This indicates that the metabolic and mechanical stimuli in skeletal muscle in male rats are almost different from female rats.
This study has some limitations. The one limitation of this study that can be mentioned is the lack of the measurement of these variables in slow and fast muscles and red and white areas of the skeletal muscle because these measurements can obtain more accurate information on the possible differences in response to these protocols in the two sexes. Another limitation of this study is the lack of measurements of these variables at different times after exercise because it can provide more accurate information about changes in these variables following exercise.
Conclusion
In conclusion, the present study indicated that HIIE with short-term and long-term intervals has similar effects on the glycolytic pathway in both male and female rats. However, long-term studies are warranted to answer the possible differences between the two sexes after two HIIE protocols.
References
Buchheit M, Laursen PB (2013) High-intensity interval training, solutions to the programming puzzle. Sports Med 43(10):927–954
Gibala MJ, Little JP, MacDonald MJ, Hawley JA (2012) Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 590(5):1077–1084
García-Hermoso A, Cerrillo-Urbina A, Herrera-Valenzuela T, Cristi-Montero C, Saavedra J, Martínez-Vizcaíno V (2016) Is high-intensity interval training more effective on improving cardiometabolic risk and aerobic capacity than other forms of exercise in overweight and obese youth? A meta-analysis Obesity. Reviews 17(6):531–540
Horii N, Hasegawa N, Fujie S, Uchida M, Miyamoto-Mikami E, Hashimoto T, Tabata I, Iemitsu M (2017) High-intensity intermittent exercise training with chlorella intake accelerates exercise performance and muscle glycolytic and oxidative capacity in rats. Am J Physiol-Regul Integr Comp Physiol 312(4):R520–R528
Jacobs RA, Flück D, Bonne TC, Bürgi S, Christensen PM, Toigo M, Lundby C (2013) Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol 115(6):785–793
Mookerjee SA, Nicholls DG, Brand MD (2016) Determining maximum glycolytic capacity using extracellular flux measurements. PLoS ONE 11(3):e0152016
Abe T, Kitaoka Y, Kikuchi DM, Takeda K, Numata O, Takemasa T (2015) High-intensity interval training-induced metabolic adaptation coupled with an increase in Hif-1α and glycolytic protein expression. J Appl Physiol 119(11):1297–1302
Boström S, Fahlen M, Hjalmarson Å, Johansson R (1974) Activities of rat muscle enzymes after acute exercise. Acta Physiol Scand 90(3):544–554
Lawler JM, Powers SK, Visser T, Van Dijk H, Kordus MJ, Ji L (1993) Acute exercise and skeletal muscle antioxidant and metabolic enzymes: effects of fiber type and age. Am J Physiol-Regul Integr Comp Physiol 265(6):R1344–R1350
Tucker WJ, Sawyer BJ, Jarrett CL, Bhammar DM, Gaesser GA (2015) Physiological responses to high-intensity interval exercise differing in interval duration. J Strength Cond Res 29(12):3326–3335
Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA (2014) High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol 119(6):753–758
Gillen JB, Percival ME, Ludzki A, Tarnopolsky MA, Gibala MJ (2013) Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity 21(11):2249–2255
Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, Jung ME, Gibala MJ (2011) Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol 111(6):1554–1560
Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ (2010) A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588(6):1011–1022
Moholdt TT, Amundsen BH, Rustad LA, Wahba A, Løvø KT, Gullikstad LR, Bye A, Skogvoll E, Wisløff U, Slørdahl SA (2009) Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: a randomized study of cardiovascular effects and quality of life. Am Heart J 158(6):1031–1037
Sultana RN, Sabag A, Keating SE, Johnson NA (2019) The effect of low-volume high-intensity interval training on body composition and cardiorespiratory fitness: a systematic review and meta-analysis. Sports Med 49(11):1687–1721
Astorino TA, Allen RP, Roberson DW, Jurancich M, Lewis R, McCarthy K, Trost E (2011) Adaptations to high-intensity training are independent of gender. Eur J Appl Physiol 111(7):1279–1286
Russ DW, Lanza IR, Rothman D, Kent-Braun JA (2005) Sex differences in glycolysis during brief, intense isometric contractions. Muscle Nerve 32(5):647–655
Saghebjoo M, Sadeghi-Tabas S, Saffari I, Ghane A, Dimauro I (2019) Sex differences in antiaging response to short- and long-term high-intensity interval exercise in rat cardiac muscle: Telomerase activity, total antioxidant/oxidant status. Chin J Physiol 62(6):261–266
Akmali A Saghebjoo M (2019) High-intensity interval training with long duration intervals is more effective than short duration intervals for improving glycolytic capacity in the rats’ gastrocnemius muscle. Horm Mol Biol Clin Investig (In press)
Sadeghi-Tabas S Saghebjoo M Sarir H Hedayati M (2019) Effects of work/rest interval manipulation of high-intensity interval training and detraining on telomerase activity and p53 levels in cardiac muscle. Sci & Sports (In press)
Philp A, Hargreaves M, Baar K (2012) More than a store: regulatory roles for glycogen in skeletal muscle adaptation to exercise. American Journal of Physiology-Endocrinology And Metabolism 302(11):E1343–E1351
Xirouchaki CE, Mangiafico SP, Bate K, Ruan Z, Huang AM, Tedjosiswoyo BW, Lamont B, Pong W, Favaloro J, Blair AR (2016) Impaired glucose metabolism and exercise capacity with muscle-specific glycogen synthase 1 (gys1) deletion in adult mice. Mol Metab 5(3):221–232
Dubouchaud H, Eydoux N, Granier P, Prefaut C, Mercier J (1999) Lactate transport activity in rat skeletal muscle sarcolemmal vesicles after acute exhaustive exercise. J Appl Physiol 87(3):955–961
Abernethy PJ, Thayer R, Taylor AW (1990) Acute and chronic responses of skeletal muscle to endurance and sprint exercise. Sports Med 10(6):365–389
Katz A, Raz I (1995) Rapid activation of glycogen synthase and protein phosphatase in human skeletal muscle after isometric contraction requires an intact circulation. Pflügers Archiv 431(2):259–265
Yan Z, Spencer M, Katz A (1992) Effect of low glycogen on glycogen synthase in human muscle during and after exercise. Acta Physiol Scand 145(4):345–352
Nielsen JN, Richter EA (2003) Regulation of glycogen synthase in skeletal muscle during exercise. Acta Physiol Scand 178(4):309–319
Tremblay A, Simoneau J-A, Bouchard C (1994) Impact of exercise intensity on body fatness and skeletal muscle metabolism. Metabolism 43(7):814–818
Hermansen L (1981) Muscular fatigue during maximal exercise of short duration. Physiological Chemistry of Exercise and Training vol 13 Karger Publishers 45 52
Bishop D, Edge J, Thomas C, Mercier J (2007) High-intensity exercise acutely decreases the membrane content of MCT1 and MCT4 and buffer capacity in human skeletal muscle. J Appl Physiol 102(2):616–621
Halestrap AP, Wilson MC (2012) The monocarboxylate transporter family—role and regulation. IUBMB Life 64(2):109–119
Hamada T, Takimoto M (2013) Regulation of the exercise-induced expression of the monocarboxylate transporters MCT1 and MCT4 in skeletal muscle. J Phys Fit Sports Med 2(1):85–92
Thomas C, Bishop DJ, Lambert K, Mercier J, Brooks GA (2011) Effects of acute and chronic exercise on sarcolemmal MCT1 and MCT4 contents in human skeletal muscles: current status. Am J Physiol-Regul Integr Comp Physiol 302(1):R1–R14
Green H, Fraser I, Ranney D (1984) Male and female differences in enzyme activities of energy metabolism in vastus lateralis muscle. J Neurol Sci 65(3):323–331
Malaguti M, Angeloni C, Garatachea N, Baldini M, Leoncini E, Collado PS, Teti G, Falconi M, Gonzalez-Gallego J, Hrelia S (2009) Sulforaphane treatment protects skeletal muscle against damage induced by exhaustive exercise in rats. J Appl Physiol 107(4):1028–1036
Takekura H, Yoshioka T (1988) Acute exhaustive exercise changes the metabolic profiles in slow and fast muscles of rat. Jpn J Physiol 38(5):689–697
Acknowledgments
We would like to thank Dr. Mehdi Hedayati, the Head of Cellular and Molecular Endocrine Research Center at Shahid Beheshti University of Medical Sciences for his sincere cooperation in conducting laboratory tests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have financial or other conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures were approved by the Ethics Committee of the Birjand University of Medical Sciences (IR.BUMS.REC.1396.55).
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saghebjoo, M., Saffari, I., Sadeghi-Tabas, S. et al. Do sex-related differences and time of intervals affect the skeletal muscle glycolytic response to high-intensity interval exercise?. Sport Sci Health 16, 473–478 (2020). https://doi.org/10.1007/s11332-020-00627-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-020-00627-5