Abstract
Background
Studies about antioxidant supplementation and exercises combined, especially at hepatic liver tissue, are rare and still controversial. In this study, we aimed to evaluate if the association between a recognized antioxidant compound—Diphenyl Diselenide ([(PhSe)2])—and training can reduce homogenate liver and liver mitochondria oxidative stress in old rats.
Methods
Old male Wistar rats were divided into four groups (six animals per group): old-sedentary, old-sedentary [(PhSe)2] supplemented, old-trained, and old-trained [(PhSe)2] supplemented. Trained groups were submitted to swimming training sessions (3% of body weight, 20 min/day during 4 weeks); animals were fed daily with standard feed or standard feed supplemented with 1 ppm of [(PhSe)2] during 4 weeks.
Results
Trained and trained + [(PhSe)2] groups decreased reactive oxygen species (ROS) generation, while only the trained group reduces GSSG production and increased GSH/GSSG ratio when compared to trained + [(PhSe)2]. Mitochondrial ROS production was elevated in control sedentary group, but only swimming training prevented its elevation. However, MnSOD activity was found elevated at trained + [(PhSe)2] rats when compared to the trained and [(PhSe)2] supplementation groups. Mitochondrial Δψm in trained + [(PhSe)2] was decreased compared to trained group, while ratio (III/IV states) was increased when compared to control sedentary.
Conclusions
We conclude that the combination of [(PhSe)2] and swimming training did not manifest synergic effect since it does not prevent the aging-induced hepatic oxidative stress generation, but blunted the induced-exercise adaptations, including at mitochondrial mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regular exercise is recognized by health quality improvements, evidenced at all age and gender [1], contributing to the prevention of chronic diseases [2]. Studies indicate that the adaptations associated with exercising can be caused by continuous and moderate reactive oxygen species (ROS) generation, improving cellular defense systems [3], by raising the expression and activity of antioxidants enzymes [4, 5]. Training also modulates mitochondria bioenergetics’ metabolism [6], biogenesis and redox status, leading to a cell increased resistance and better energy synthesis.
Aging process results from continuous bouts of oxygen-free radical attacks at cell components throughout the lifespan [7]. Aging affects liver function through alterations in multiple metabolic pathways. These changes include a reduction in the antioxidant defense [8], decrease of antioxidant protein levels [9], and a chronic inflammatory status, contributing age-related diseases development [10].
Selenium is an essential trace element that is present in several enzymes, such as glutathione peroxidase and thioredoxin reductase [11, 12]; selenium deficiency has been implicated in oxidative stress, inflammatory, and diseases [13, 14]. Most selenium biological functions are attributed to the selenoproteins [15], reducing intracellular ROS content due to its antioxidant property. These proteins can trigger molecular mechanisms related to the anti-inflammatory process [16, 17]. Especially, the diphenyl diselenide [(PhSe)2], is an organoselenium molecule, which is recognized to their low toxicity, high antioxidant, and anti-inflammatory properties [18]. Besides, compound is known for its double face due to a contrasting behavior in biological systems that is dose dependent, whereas low doses have beneficial effects, high doses lead to toxicity. On the other hand, the therapeutic potential of [(PhSe)2] and related compounds seems to be superior to their toxic effects [19, 20]. It has been demonstrated that combined therapy with swimming exercise and diet supplemented with [(PhSe)2] have a favorable effects on levels of pro-inflammatory cytokines [21] and contributed to the hepatic glucose homeostasis in old rats [22].
Over the last years, exercise and dietary supplementation have been longed used as beneficial and not aggressive strategies to prevent aging-related symptoms and disease development. Therefore, this study aims to evaluate if [(PhSe)2] and swimming combined to have greater beneficial against the damages caused by age-related at rat’s liver and isolated liver mitochondria than their isolated effects.
Materials and methods supplemented in diet
Animals
Old (27 months y.o.) male Wistar rats were obtained from a local breeding colony and were housed in cages, with free access to food and water. Rats were placed in controlled environment conditions (12:12 h light–dark cycle, with an onset of light phase at 7:00 am, 25 ± 1 °C, 55% relative humidity) with food (Guaiba, RS, Brazil) and water at libitum. The experiment was conducted according to the guidelines of the Committee on Care and Use of Experimental Animal Resources 5394050115, the Federal University of Santa Maria, Brazil.
Drugs and (PhSe)2 supplementation in the diet
[(PhSe)2] was prepared according to the method described by Paulmier [23]. The animals were fed daily with a standard feed or standard feed supplemented with 1 ppm of [(PhSe)2] for 4 weeks. The preparation and concentration of supplemented standard chow were based on a previous study [24]. The standard diet was sprinkled with [(PhSe)2] [1 mg of [(PhSe)2]/100 g standard chow] dissolved in ethyl alcohol (1 mg/100 ml) using a spray bottle. The concentration of [(PhSe)2] found in the diet did not cause overt signals of toxicity [25, 26]. The diet was changed daily with 100 g of chows new and the diet was maintained in cages (two animals per cage) for 1 day. The standard and supplemented diets were kept at 60° for 3 h to evaporate ethyl alcohol and then kept at 4° by not more than 1 week.
Experimental procedure and swimming exercise
The animals were divided into five groups (six animals per group): I—old-control (old animals did not perform exercise); II—[(PhSe)2] (old animals supplemented with 1 ppm of [(PhSe)2]; III—trained (old animals performed swimming training); and IV—trained + [(PhSe)2]-supplementation (old animals supplemented with 1 ppm of [(PhSe)2] and performed the swimming training) (Fig. 1). Thirty-month-old rats were submitted to the pre-training session 20 min/day for 1 week (III and IV groups). After this adaptation period, the rats performed the swimming training protocol with a workload (3% of body weight) for 20 min/day for 4 weeks [27]. The workload was placed in the back of animal, attached around their chest by an elastic band so that their movements were not restricted. The body weights of animals were measured once a week, for determining the workload. The swimming training occurred at a water temperature of 32 ± 1 °C, the tank used in this study was 80 cm in length, 50 cm in width, 90 cm in-depth and the swimming training was performed in water temperature of 31 ± 1 °C (70 cm depth). Sedentary rats (I and II groups) were placed in the bottom of a separate tank with shallow water (5 cm) at 32 ± 1 °C, without the workload (consists in adapting to water). At the end of the swimming exercise-training period, all animals were towel dried before being returned to their cages.
Liver homogenate preparation
At the end of the protocol, the liver was removed and quickly dissected, placed on ice and immediately homogenized in cold Tris–HCl 10 mM (pH 7.4). Homogenates were centrifuged at 2000×g for 10 min to yield the low-speed supernatant fractions that were used for different biochemical assays in all trials. Liver aliquots preparations were frozen (− 80 °C) for later analysis.
Hepatic mitochondria isolation
Rat liver mitochondria were isolated at 4 °C by differential centrifugation [28]. Livers were rapidly removed after sacrificed and immersed in ice-cold ‘Ionic Medium’ containing 100 mM sucrose, 10 mM EDTA, 46 mM KCl, and 100 mM Tris–HCl (pH 7.4). The tissue was minced using surgical scissors and then extensively washed, then homogenized in power-driven, tight-fitting Potter–Elvehjem homogenizer with Teflon Pestle. The resulting suspension was centrifuged for 10 min at 2500×g in a Hitachi CR 21E centrifuge. After centrifugation, the supernatant was centrifuged for 10 min at 10,000×g. The pellet was resuspended in ‘Ionic Medium + BSA’ containing 100 mM sucrose, 10 mM EDTA, 46 mM KCl, 0.1% bovine serum albumin free fatty acid, 100 mM Tris–HCl (pH 7.4), and centrifuged at 10,000×g for 10 min. The supernatant was decanted, and the final pellet was gently washed and resuspended in ‘Suspension Medium’ containing 230 mM mannitol, 70 mM sucrose, and 20 mM Tris–HCl (pH 7.4) [29]. An aliquot of the resulting mitochondrial suspension was separated and rapidly frozen at − 80 °C for following GSH content and mitochondrial enzyme.
Reactive oxygen species (ROS) production
ROS production in liver homogenates was determined by oxidation of reduced dichlorofluorescein diacetate (H2DCF-DA) [30]. Briefly, homogenates were added to the standard medium containing Tris–HCl as a buffer (10 mM, pH 7.4) and 2′, 7′—dichlorofluorescein diacetate (Sigma-Aldrich, catalogue 35845) (1 mM) for 60 min in a condition without light. Fluorescence quantification was determined at 488 nm for excitation and 525 nm for emission, with slit widths of 3 nm, in a spectrofluorimeter, using oxidized 2′, 7′—dichlorofluorescein (DCF) (Sigma-Aldrich, catalogue 35848) as a standard. Mitochondrial samples (150 µg of protein/ml) were incubated with buffer, and the respiratory substrates glutamate/malate (5 mM) and succinate (5 mM). The reaction was started using H2DCF-DA, and the medium was kept at constant stirring during the assay period [31].
Reduced glutathione (GSH) and oxidized glutathione (GSSG)
GSH levels were determined in liver homogenate with fluorescence detection after reaction of the supernatants from deproteinized containing H3PO4/NaH2PO4−EDTA, with O-Phthalaldehyde (OPT) [32]. In brief, 250 mg of liver were homogenized in 3.75 mL phosphate–EDTA buffer (100 mM NaH2PO4, 5 mM EDTA, pH 8.0) plus 1 mL H3PO4 (25%), resuspended in 1.5 mL phosphate–EDTA buffer and 500 ml H3PO4 (4.5%) were rapidly centrifuged at 13000 rpm (Hitachi,) for 10 min. For GSH determination, the supernatant was incubated in phosphate buffer and in the presence of OPT at room temperature for 15 min. GSSG determination, the supernatant was added with N-Ethylmaleimide incubation at room temperature for 30 min. After this was incubated in sodium hydroxide buffer and in the presence of OPT at room temperature for 15 min, the fluorescence of both was measured at 420 and 350 nm emission and excitation wavelengths, respectively.
Manganese superoxide dismutase (MnSOD) activity
Enzymatic activity in isolated mitochondria was measured after disruption of mitochondria by freeze-thawing (3×), following centrifugation at 2000×g for 1 min at 4 °C, and the mitochondrial supernatant (0.1 mg protein/mL) was add to the reaction medium. Manganese superoxide dismutase (MnSOD) activity was measured as described previously [33]. The isolated mitochondria were assayed after incubation with 1 mM potassium cyanide (KCN). At this concentration, cyanide inhibits the CuZnSOD isoform of the enzyme but does not affect the MnSOD isoform [34].
Mitochondrial transmembrane electrical potential (Δψm)
Mitochondrial Δψm was estimated by fluorescence changes in Safranine-O 10 mM recorded by RF-5301 Shimadzu spectrofluorometer (Kyoto, Japan) operating at excitation and emission wavelengths of 495 and 586 nm, with slit widths of 5 nm [35]. The mitochondria (0.5 mg protein) were added, and 30 s later, mitochondrial respiration was initiated by the addition of succinate and glutamate.
Oxygen consumption of liver mitochondria
Oxygen consumption was monitored polarographically with a Clark-type electrode (Hansatech, UK) at 30 °C. Liver mitochondria were incubated in 1 ml of the respiratory medium consisted of 10 mM Tris–HCl (pH 7.4), 320 mM mannitol, 8 mM K2HPO4, and 4 mM MgCl2, 0.08 mM EDTA, 1 mM EGTA, 0.2 mg/ml BSA described previously [36]. Respiration of mitochondria (1 mg of protein/ml) was initiated with substrates at 5 mM malate, 5 mM glutamate, and 5 mM Succinate. To induce the phosphorylating (state III) respiration, 200 nmol ADP was used. Next, the oligomycin (1 μg/ml) was used to induce the non-phosphorylating (state IV) respiration. Respiration rates are given in nmol oxygen/min/mg protein. Respiratory control ratio (RCR) was determined by the State III/State IV ratio.
Protein determination
The protein content was determined as described previously [37] using bovine serum albumin (BSA) as standard.
Statistical analysis
Statistical analysis was performed using GraphPad (version 8.0 for Macintosh OSX, GraphPad Software, San Diego, CA). The Shapiro–Wilk test was used to confirm the normality of quantitative variables. With normal data distribution, a two-way analysis of variance (ANOVA), followed by Tukey’s Test for post hoc comparison between old groups (sedentary/trained) × (without/with (PhSe)2). Significance was set at p < 0.05, and data were expressed as mean ± standard deviation of the mean (SD).
Results
Effect of association (PhSe)2 and swimming on hepatic oxidative damage in old rats
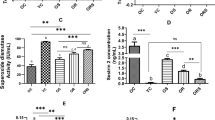
ROS levels showed a significant [(PhSe)2] × training interaction (F(1, 13) = 10.71; p = 0.0061). The control sedentary group showed an increase in the ROS levels when compared to control trained (p < 0.0001; 95% CI of diff. = 9.164–23.34) and [(PhSe)2] + trained (p = 0.0022; 95% CI of diff. = 4.159–18.33). Indeed, control trained demonstrated a reduction in ROS levels when compared to (PhSe)2 sedentary (p = 0.0028; 95% CI of diff. = − 17.09 to −3.640) (Fig. 2a).
Effect of association [(PhSe)2] and swimming training on hepatic oxidative damage markers in old rats. a reactive oxygen species (ROS) levels, b reduced glutathione (GSH), c oxidized glutathione (GSSG), and d GSH/GSSG ratio. Different letters indicate significant differences between groups (mean ± SD for n = 4–5 in each group, two-way test (ANOVA) followed by Tukey’s test, p < 0.05)
GSH contents revealed a significant main effect of training (F(1, 11) = 15.51; p = 0.0023). Training alone or association with [(PhSe)2] decreased the GSH levels in comparison to sedentary group ([p = 0.0265; 95% CI of diff. = 1.106–18.66], and [p = 0.0182; 95% CI of diff. = 1.761–19.32], respectively) (Fig. 2b). We observed also that GSSG contents (Fig. 2c) presented a significant interaction in [(PhSe)2] × training (F(1, 12) = 12.13; p = 0.0045). Training without association decrease the GSSG levels in comparison to control sedentary (p = 0.0104; 95% CI of diff. = 2.075–15.77), and training associated with [(PhSe)2] increased these levels (p = 0.0488; 95% CI of diff. = − 13.73 to − 0.03178) when compared to training.
Furthermore, GSH/GSSG ratio revealed a significant [(PhSe)2] × training interaction (F(1, 11) = 18.19; p = 0.0013). Training alone increased the ratio when compared to control sedentary (p = 0.0033; 95% CI of diff. = − 3.211 to − 0.6903), [(PhSe)2] group (p = 0.0077; 95% CI of diff. = 0.4709–2.992) and training in association with [(PhSe)2] (p = 0.0012; 95% CI of diff. = 0.9545–3.475) (Fig. 2d).
Effect of association (PhSe)2 and swimming on hepatic mitochondrial oxidative damage and Δψm in old rats
Mitochondrial ROS levels revealed a significant [(PhSe)2] × training interaction (F(1, 12) = 6.173; p = 0.0225). The analysis demonstrated that sedentary old rats preset higher mitochondrial ROS levels without association (p = 0.0028; 95% CI of diff. = 2.185–11.39) or with [(PhSe)2] association (p = 0.0013; 95% CI of diff. = − 12.55 to − 2.894) when compared to the training control group. However, (PhSe)2 association with training was not effective against this increase (p = 0.0027; 95% CI of diff. = − 11.43 to − 2.225) (Fig. 3a).
Effect of association [(PhSe)2] and swimming on hepatic mitochondrial oxidative damage in old rats. a Mitochondrial reactive oxygen species (ROS), b manganese superoxide dismutase (MnSOD) activity, and c mitochondrial Δψm. Different letters indicate significant differences between groups (mean ± SD for n = 4–5 in each group, two-way test (ANOVA) followed by Tukey’s test, p < 0.05)
Nevertheless, mitochondrial MnSOD activity (Fig. 3b) showed a significant [(PhSe)2] × training interaction (F(1, 8) = 18.97; p = 0.0024). Analysis demonstrated that [(PhSe)2] associated with training was effective promoting increased MnSOD activity when compared to trained group (p < 0.0045; 95% CI of diff. = − 119.1 to − 26.37) and [(PhSe)2] sedentary (p = 0.0138; 95% CI of diff. = − 106.3 to − 13.61).
Mitochondrial Δψm revealed a significant [(PhSe)2] × training interaction (F(1, 12) = 5.685; p = 0.0318). The swimming training associated with [(PhSe)2] supplementation decreased Δψm when compared to control training (p = 0.0306; 95% CI of diff. = 6.651–152.2) (Fig. 3c).
(PhSe)2 effects on hepatic mitochondrial respiration rates and phosphorylation efficiency in old rats
Figure 4a shows that the training and treatment with [(PhSe)2] altered the state III of mitochondria respiration in the liver. State III of mitochondria respiration revealed a significant [(PhSe)2] × training interaction (F(1, 12) = 92.03; p < 0.0001). The training associated with [(PhSe)2] increased the oxygen consumption in the state III of mitochondria when compared to the other groups, control sedentary (p < 0.0001; 95% CI of diff. = − 131.0 to − 72.65), control trained (p < 0.0001; 95% CI of diff. = − 142.9 to − 84.47), and [(PhSe)2] sedentary (p < 0.0001; 95% CI of diff. = − 147.4 to − 91.99.
[(PhSe)2] effects on hepatic mitochondrial respiration rates and phosphorylation efficiency in old trained rats. Mitochondria were incubated in the respiration medium for a State III was added ADP (200 nmol) and b State IV oligomycin (1 µg/ml). Respiratory control ratio (RCR) (c) was determined by State III/State IV ratio. Different letters indicate significant differences between groups (mean ± SD for n = 4–5 in each group, two-way test (ANOVA) followed by Tukey’s test, p < 0.05)
In addition, state IV showed a main effect of [(PhSe)2] (F(1, 12) = 5.946; p = 0.0253) and training (F(1,12) = 8.410; p = 0.0095). Old rats trained associated with [(PhSe)2] showed increased state IV of mitochondria respiration when compared to control sedentary group (p = 0.0069; 95% CI of diff. = − 29.94 to − 4.302) (Fig. 4b).
RCR (Fig. 4c) indicated a significant main effect of [(PhSe)2] (F(1, 12) = 5.852; p = 0.0298) and training (F(1, 12) = 5.222; p = 0.0384).The supplementation with [(PhSe)2] decreased RCR in the liver of old trained rats when compared to the sedentary group (p = 0.0229; 95% CI of diff. = 0.2486–3.691).
Discussion
Here, we appraised the effect of the association between [(PhSe)2] compound and swimming exercise to reduce the oxidative stress in liver homogenate and liver mitochondria from old rats. We used well-known interventions that exert antioxidative properties (i.e. [(PhSe)2] supplementation and swimming exercise), as an associated treatment for better understating the exercise effects integrated with antioxidants and the aging process. It seems that individually, swimming training may have hepatoprotective effects against oxidative damage process (Fig. 2). Regular physical exercise is known to play an essential role at adaptations mechanisms, reaching cell homeostasis due to the activation of redox-sensitive signaling pathways [38]. However, our results demonstrated that [(PhSe)2] associated seems to blunt this adaptive effects to swimming training.
Briefly, aging is characterized by progressively morphological and biochemical changes, ROS overproduction and accumulation induce an imbalance of the antioxidant defense system, which causes injury in the cellular membrane components interfering on molecular functions [9, 39] and contribute to diseases development [40]. In this sense, strategies to prevent or slow down aging need to be investigated, and this way, it can be applied to improve the health quality of this elderly population.
Previously, [(PhSe)2] was seemed to mimic endogenous antioxidant enzymes, such as glutathione peroxidase (GPx), metabolized by thioredoxin reductase to form a selenol intermediate, performing the same function of the antioxidant seleno-enzymes [12]. Moreover, the decrease in oxidative damage markers has been attributed to exercise-training adaptations in rat liver [41]. In this sense, these antioxidant properties were not replicated here, when combined [(PhSe)2] compound and physical exercise. Despite the association (training + [(PhSe)2]) reduced ROS generation in old rats, the GSSG levels remain higher. On the other hand, in the liver of old trained rats, it was possible to observe a lower ROS generation and an increase at GSH/GSSG ratio, reinforcing the effectiveness of the physical training as an efficient antioxidant strategy. During physical exercise, the liver is a detoxification organ because, when stimulated, induces oxidative stress, and inflammatory responses [42]. Under other conditions, the lack of [(PhSe)2] redox protection may be related to the fact that our dose remains high to produce a chronic protective effect, and this way, more studies are needed to investigate the threshold dose of [(PhSe)2] with the positive effects expected against age-related damage.
In mitochondria, our results demonstrated that just the old trained rats reduce the ROS levels and, despite no increases in MnSOD activity, it may be linked to antioxidant response modulation against the oxidative alterations caused by physical exercise [41, 43]. Besides, regular exercise down-regulates the rate of mitochondrial ROS generation in the liver [44] and also improves the mitochondrial antioxidant system, resulting in lower oxidative damage markers in several tissues [45]. Besides, old trained rats associated with [(PhSe)2] treatment showed increased activity of MnSOD. However, this does not lead to changes in mitochondrial ROS production, corroborating with studies that have evidenced that previous antioxidant supplementation attenuates the physical training adaptive responses, such as mitochondrial biogenesis and cellular defense mechanisms [44, 46, 47].
To support this, we found that [(PhSe)2] supplementation plus exercise presents a lower liver mitochondrion potential index (Δψm) than those from old trained. In the same way, according to Puntel et al. (2010), the [(PhSe)2] responds negatively to Δψm because of a partial depolarization of mitochondria in a concentration-dependent manner [48]. It is known that maintenance of the proton gradient is of vital importance for cell health, bioenergetics, and alterations in mitochondrial functionality compromise the energy balance [49]. Here, our results demonstrated that the old trained plus [(PhSe)2] supplementation rats aggravated the hepatic mitochondrial dysfunction, considering the Δψm decreased, associated to increased mitochondrial ROS generation and MnSOD activity, proving that the combination of antioxidant and exercise training might not be the best strategy. In contrast, our results demonstrated that the training increased Δψm making the mitochondrial membrane more negative and reducing ROS production, thus, once more, the training shows potential benefits to old rats, reducing the production of ROS.
Finally, increases of oxidative stress markers paralleled respiration unbalance, with increases on O2 consumption (State III) and non-ADP-stimulated respiration (state IV) associated with Δψm decrease may be related to mitochondrial dysfunction. We suggest that the exercise plus [(PhSe)2] supplementation may produce a higher O2 consumption at state III, which is not transmitted to ATP production, and consequently, increase the formation of reactive species (i.e., mitochondrial ROS levels). In this regard, it is known that [(PhSe)2] can inhibit the mitochondrial complex II activity by decreasing the mitochondrial respiration supported by complex I or complex II substrates [50]. Previous studies observed that exposure to acute or chronic toxic doses of [(PhSe)2] can increase selenium deposition in liver [51]. Furthermore, selenium compounds can be toxic to naive animals and cultured cells [52, 53] attributed to the oxidation of thiol groups present in the mitochondrial membrane [54], leading to several alterations in the mitochondrial functions, and this way, interfering on possible mitochondrial adaptation improvements.
Conclusions
In summary, our study is the first to test if [(PhSe)2] supplementation, when associated with swimming training, would interfere in the development of liver oxidative damage during the aging process. Despite [(PhSe)2] and regular physical exercises present individually antioxidant properties, as well as produce tissue adaptations and improve redox mechanisms, in this study, the combination of these two strategies did not manifest the expected benefits. This way, our hypothesis was not confirmed since the association did not present synergic effect, but, surprisingly, blunted the induced-exercise adaptations, including at mitochondrial mechanisms. In future, more researches are needed to investigate the effective dose and possible mechanisms of action, especially at hepatic liver tissue.
Abbreviations
- [(PhSe)2]:
-
Diphenyl diselenide
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- MnSOD:
-
Manganese superoxide dismutase
- Δψm :
-
Mitochondrial transmembrane electrical potential
- H2DCF-DA:
-
Reduced dichlorofluorescein diacetate
- DCF:
-
Oxidized dichlorofluorescein
- OPT:
-
O-Phthalaldehyde
- KCN:
-
Potassium cyanide
References
Alberti KGMM, Zimmet P, Shaw J (2007) International diabetes federation: a consensus on Type 2 diabetes prevention. Diabet Med 24:451–463. https://doi.org/10.1111/j.1464-5491.2007.02157.x
Warburton DER, Nicol CW, Bredin SSD (2006) Health benefits of physical activity: the evidence. CMAJ 174:801–809. https://doi.org/10.1503/cmaj.051351
Viña J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC (2012) Exercise acts as a drug; The pharmacological benefits of exercise. Br J Pharmacol 167:1–12
Steinbacher P, Eckl P (2015) Impact of oxidative stress on exercising skeletal muscle. Biomolecules 5:356–377
Gomez-Cabrera MC, Domenech E, Viña J (2008) Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44:126–131. https://doi.org/10.1016/j.freeradbiomed.2007.02.001
Rasmussen UF, Krustrup P, Kjær M, Rasmussen HN (2003) Experimental evidence against the mitochondrial theory of aging A study of isolated human skeletal muscle mitochondria. Exp Gerontol 38:877–886. https://doi.org/10.1016/S0531-5565(03)00092-5
Ji LL (1993) Antioxidant enzyme response to exercise and aging. Med Sci Sports Exerc 25:225–231
Navarro A, Boveris A (2007) The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292:C670–C686. https://doi.org/10.1152/ajpcell.00213.2006
Houtkooper RH, Argmann C, Houten SM, Cantó C, Jeninga EH, Andreux PA, Thomas C, Doenlen R, Schoonjans K, Auwerx J (2011) The metabolic footprint of aging in mice. Sci Rep. https://doi.org/10.1038/srep00134
Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C (2009) Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 8:18–30
Arnér ESJ (2009) Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim Biophys Acta Gen Subj 1790:495–526
Nogueira CW, Rocha JBT (2011) Toxicology and pharmacology of selenium: emphasis on synthetic organoselenium compounds. Arch Toxicol 85:1313–1359
Brenneisen P, Steinbrenner H, Sies H (2005) Selenium, oxidative stress, and health aspects. Mol Aspects Med 26:256–267
Parnham M, Sies H (2000) Ebselen: prospective therapy for cerebral ischaemia. Expert Opin Investig Drugs 9:607–619. https://doi.org/10.1517/13543784.9.3.607
Ryan-Harshman M, Aldoori W (2005) The relevance of selenium to immunity, cancer, and infectious/inflammatory diseases. Can J Diet Pract Res 66:98–102
Luchese C, Pinton S, Nogueira CW (2009) Brain and lungs of rats are differently affected by cigarette smoke exposure: antioxidant effect of an organoselenium compound. Pharmacol Res 59:194–201. https://doi.org/10.1016/j.phrs.2008.11.006
Prigol M, Schumacher RF, WayneNogueira C, Zeni G (2009) Convulsant effect of diphenyl diselenide in rats and mice and its relationship to plasma levels. Toxicol Lett 189:35–39. https://doi.org/10.1016/j.toxlet.2009.04.026
Carvalho NR, Da Rosa EF, Da Silva MH, Tassi CC, Corte CLD, Carbajo-Pescador S, Mauriz JL, González-Gallego J, Soares FA (2013) New therapeutic approach: diphenyl diselenide reduces mitochondrial dysfunction in acetaminophen-induced acute liver failure. PLoS One. https://doi.org/10.1371/journal.pone.0081961
Nogueira CW, Rocha JBT (2010) Diphenyl diselenide a janus-faced molecule. J Braz Chem, Soc
Rosa RM, Roesler R, Braga AL, Saffi J, Henriques JAP (2007) Pharmacology and toxicology of diphenyl diselenide in several biological models. Braz J Med Biol Res 40:1287–1304
Leite MR, Cechella JL, Mantovani AC, Duarte MMMF, Nogueira CW, Zeni G (2015) Swimming exercise and diphenyl diselenide-supplemented diet affect the serum levels of pro- and anti-inflammatory cytokines differently depending on the age of rats. Cytokine. https://doi.org/10.1016/j.cyto.2014.09.006
Heck SO, Fulco BCW, Quines CB, Oliveira CES, Leite MR, Cechella JL, Nogueira CW (2017) Combined therapy with swimming exercise and a diet supplemented with diphenyl diselenide is effective against. J Cell Biochem. https://doi.org/10.1002/jcb.25819
Paulmier C (1986) Selenium reagents and intermediates in organic synthesis. Pergamon Press. https://doi.org/10.1002/ange.19881000236
de Bem AF, Portella RDL, Colpo E, Duarte MMMF, Frediane A, Taube PS, Nogueira CW, Farina M, da Silva EL, Teixeira Rocha JB (2009) Diphenyl diselenide decreases serum levels of total cholesterol and tissue oxidative stress in cholesterol-fed rabbits. Basic Clin Pharmacol Toxicol 105:17–23. https://doi.org/10.1111/j.1742-7843.2009.00414.x
de Bem AF, de Lima Portella R, Perottoni J, Becker E, Bohrer D, Paixão MW, Nogueira CW, Zeni G, Rocha JBT (2006) Changes in biochemical parameters in rabbits blood after oral exposure to diphenyl diselenide for long periods. Chem Biol Interact. https://doi.org/10.1016/j.cbi.2006.04.005
De Bem AF, Portella RDL, Farina M, Perottoni J, Paixão MW, Nogueira CW, Rocha JBT (2007) Low toxicity of diphenyl diselenide in rabbits: a long-term study. Basic Clin Pharmacol Toxicol. https://doi.org/10.1111/j.1742-7843.2007.00073.x
Ravi Kiran T, Subramanyam MVV, Asha Devi S (2004) Swim exercise training and adaptations in the antioxidant defense system of myocardium of old rats: relationship to swim intensity and duration. Comp Biochem Physiol B Biochem Mol Biol 137:187–196. https://doi.org/10.1016/j.cbpc.2003.11.002
Bhattacharya SK, Thakar JH, Johnson PL, Shanklin DR (1991) Isolation of skeletal muscle mitochondria from hamsters using an lonic medium containing ethylenediarninetetraacetic acid and nagarse. Anal Biochem 192:344–349. https://doi.org/10.1016/0003-2697(91)90546-6
Kruglov AG, Teplova VV, Saris NEL (2007) The effect of the lipophilic cation lucigenin on mitochondria depends on the site of its reduction. Biochem Pharmacol 74:545–556. https://doi.org/10.1016/j.bcp.2007.05.012
Myhre O, Andersen JM, Aarnes H, Fonnum F (2003) Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 65:1575–1582
García-Ruiz C, Colell A, Marí M, Morales A, Fernández-Checa JC (1997) Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species: role of mitochondrial glutathione. J Biol Chem. https://doi.org/10.1074/jbc.272.17.11369
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226. https://doi.org/10.1016/0003-2697(76)90326-2
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(3170–3175):4623845
Geller BL, Winge DR (1984) Subcellular distribution of superoxide dismutases in rat liver. Methods Enzymol 105:105–114. https://doi.org/10.1016/S0076-6879(84)05014-X
Åkerman KEO, Wikström MKF (1976) Safranine as a probe of the mitochondrial membrane potential. FEBS Lett 68:191–197. https://doi.org/10.1016/0014-5793(76)80434-6
Da-Silva WS, Gómez-Puyou A, De Gómez-Puyou MT, Moreno-Sanchez R, De Felice FG, De Meis L, Oliveira MF, Galina A (2004) Mitochondrial bound hexokinase activity as a preventive antioxidant defense. Steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J Biol Chem 279:39846–39855. https://doi.org/10.1074/jbc.M403835200
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ji LL (2015) Redox signaling in skeletal muscle: role of aging and exercise. Adv Physiol Educ 39:352–359. https://doi.org/10.1152/advan.00106.2014
Safwat MH, El-Sawalhi MM, Mausouf MN, Shaheen AA (2014) Ozone ameliorates age-related oxidative stress changes in rat liver and kidney: effects of pre- and post-ageing administration. Biochem 79:450–458. https://doi.org/10.1134/S0006297914050095
Kan H, Hu W, Wang Y, Wu W, Yin Y, Liang Y, Wang C, Huang D, Li W (2015) NADPH oxidase-derived production of reactive oxygen species is involved in learning and memory impairments in 16-month-old female rats. Mol Med Rep 12:4546–4553. https://doi.org/10.3892/mmr.2015.3894
Lima FD, Stamm DN, Della-Pace ID, Dobrachinski F, de Carvalho NR, Royes LFF, Soares FA, Rocha JB, González-Gallego J, Bresciani G (2013) Swimming training induces liver mitochondrial adaptations to oxidative stress in rats submitted to repeated exhaustive swimming bouts. PLoS One. https://doi.org/10.1371/journal.pone.0055668
Santos-Alves E, Marques-Aleixo I, Coxito P, Balça MM, Rizo-Roca D, Rocha-Rodrigues S, Martins S, Torrella JR, Oliveira PJ, Moreno AJ, Magalhães J, Ascensão A (2014) Exercise mitigates diclofenac-induced liver mitochondrial dysfunction. Eur J Clin Invest 44:668–677. https://doi.org/10.1111/eci.12285
Barcelos RP, Souza MA, Amaral GP, Stefanello ST, Bresciani G, Fighera MR, Soares FAA, Barbosa NV (2014) Caffeine supplementation modulates oxidative stress markers in the liver of trained rats. Life Sci 96:40–45. https://doi.org/10.1016/j.lfs.2013.12.002
Radák Z, Chung HY, Naito H, Takahashi R, Jung KJ, Kim HJ, Goto S (2004) Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J 18:749–750. https://doi.org/10.1096/fj.03-0509fje
Radak Z, Taylor AW, Ohno H, Goto S (2001) Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev 7:90–107
Merry TL, Ristow M (2016) Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol. https://doi.org/10.1113/JP270654
Barcelos RP, Bresciani G, Rodriguez-Miguelez P, Cuevas MJ, Soares FAA, Barbosa NV, González-Gallego J (2016) Diclofenac pretreatment effects on the toll-like receptor 4/nuclear factor kappa B-mediated inflammatory response to eccentric exercise in rat liver. Life Sci. https://doi.org/10.1016/j.lfs.2016.02.006
Puntel RL, Roos DH, Folmer V, Nogueira CW, Galina A, Aschner M, Rocha JBT (2010) Mitochondrial dysfunction induced by different organochalchogens is mediated by thiol oxidation and is not dependent of the classical mitochondrial permeability transition pore opening. Toxicol Sci 117:133–143. https://doi.org/10.1093/toxsci/kfq185
Bhatti JS, Bhatti GK, Reddy PH (2017) Mitochondrial dysfunction and oxidative stress in metabolic disorders—a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis 1863:1066–1077
Puntel RL, Roos DH, Seeger RL, Rocha JBT (2013) Mitochondrial electron transfer chain complexes inhibition by different organochalcogens. Toxicol Vitr 27:59–70. https://doi.org/10.1016/j.tiv.2012.10.011
Nogueira CW, Zeni G, Rocha JBT (2004) Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev. https://doi.org/10.1021/cr0406559
de Jong N, Gibson RS, Thomson CD, Ferguson EL, McKenzie JE, Green TJ, Horwath CC (2001) Selenium and zinc status are suboptimal in a sample of older New Zealand women in a community-based study. J Nutr 131:2677–2684
Shen CL, Song W, Pence BC (2001) Interactions of selenium compounds with other antioxidants in DNA damage and apoptosis in human normal keratinocytes. Cancer Epidemiol Biomarkers Prev 10:385–390
Morin D, Zini R, Ligeret H, Neckameyer W, Labidalle S, Tillement JP (2003) Dual effect of ebselen on mitochondrial permeability transition. Biochem Pharmacol 65:1643–1651. https://doi.org/10.1016/S0006-2952(03)00114-X
Funding
This work was supported by Brazilian National Council of Technological and Scientific Development (CNPq), “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES), “Programa de Apoio a Núcleos Emergentes” (PRONEM) MCTI/CNPq [Grant number 472669/2011-7, 475896/2012-2], and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior CAPES/PROEX [process number: 23038.005848/2018-31]. FAAS received a fellowship from CNPq. PCR, DDH, STS, JLC, MRL and NRC received a fellowship from CAPES.
Author information
Authors and Affiliations
Contributions
All authors were involved in the development of this manuscript. As the corresponding author, Rômulo P. Barcelos oversaw the complete manuscript development. The study was designed by José L. Cechella; the training was designed and performed by Marlon R. Leite; data were collected and analyzed by Martin T. B. Leite, Micaela B. Souza, Thayanara C. da Silva and Nelson R. De Carvalho; data interpretation and article preparation were undertaken by Pamela C. Da Rosa, Diane D. Hartmann, and Sílvio T. Stefanello; the study conceived and supervisioned, and review of final version by Félix A. A. Soares, Gustavo O. Puntel. All authors have approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The author declares that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Da Rosa, P.C., Hartmann, D.D., Stefanello, S.T. et al. Diphenyl diselenide blunts swimming training on mitochondrial liver redox adaptation mechanisms of aged animals. Sport Sci Health 16, 281–290 (2020). https://doi.org/10.1007/s11332-019-00603-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-019-00603-8