Abstract
Background
Natural ingredients have great demand to formulate the health supplements to enhance the physical endurance and stamina of any active persons. Fitnox™ is a natural ingredient formulation which contains the extracts of Kaempferia parviflora root, Punica granatum peel and Moringa oleifera leaves.
Aim
The study was designed to investigate the efficacy of Fitnox™ for physical endurance in healthy male adults before and after exercise for a period of 22 days.
Methods
Twenty four healthy male participants consumed 250 mg of either Fitnox™ or placebo (1:1 ratio) daily and they visited the clinic on day 0 and day 22 for physical examination. Serum chemistry, hematology and the levels of lactate dehydrogenase (LDH), malondialdehyde (MDA), heart rate, red blood cell (RBC) count, dopamine, nitrate and nitrite were analyzed before and after exercise on screening and final visit.
Results
There was a 31% increase in plasma nitrate before exercise and 45% after exercise for the test groups on day 22. Similarly, the nitrite content increased 49% and 47% for pre- and post-exercises, respectively, in plasma. In saliva, the concentrations of the nitrate increased 24% and 18% before and after exercise, respectively. The saliva nitrite also increased 18% and 11% in pre- and post-exercise. Moreover, the dopamine concentration is considerably increased (36%) after the administration of the Fitnox™ (P < 0.05). Serum inflammatory biomarker LDH decreased from 635 to 582 U/L (P < 0.05) in post-exercise and MDA decreased 47% in the Fitnox™ group (P < 0.01), but no considerable change in the placebo group.
Conclusions
It was concluded from the study that Fitnox™ not only increases the performance but it also reduces the oxidative stress to the muscles and tissues during exercise. There were no study-related adverse events reported between both the treatment groups and the results indicated that the Fitnox™ which enhances the physical endurance considerably.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The need of vitamins, minerals, increased levels of nitric oxide (NO), dopamine and red blood cells (RBC) before and during exercise are important biological parameters to determine the efficiency of the exercise. NO-mediated vasodilation and high RBC count can increase the oxygen supply to the cells and, hence, increase the physical endurance [1]. During exercise, the oxidative stress in the muscle tissue is increased due to the free radical production and thereby it would damage the tissues in the body [2]. The biomarkers such as LDH and MDA levels [3, 4] can be increased in any physical strain due to this oxidative damage [5]. Antioxidants play a vital role in protecting tissues from excessive tissue damage during exercise by scavenging the reactive oxygen species (ROS). Consumption of various sports supplements which contain antioxidants would increase the acute strenuous exercise and chronic exercise training; it is conceivable that dietary supplementation of specific antioxidants would be beneficial [6]. These sports supplements are the functional foods to provide health benefits for the athletes and to improve performance in sports [7, 8]. This can be achieved by minimizing the impact of the factors that cause fatigue and impair the performance of skilled tasks [9].
Some of the natural products fulfill all these requirements for intense trainings and are a better and more sorted option over the chemical supplements widely publicized in the world markets nowadays. Among the widely used natural products, black ginger root, pomegranate peel and moringa leaves are utilized to increase the energy levels in the body and thus prove their benefits as a physical fitness supplement. The mode of actions of these three is as described below.
Black ginger (Kaempferia parviflora) extract
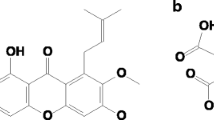
Black ginger or Thai Ginseng (Kaempferia parviflora) is used as an energy enhancer and a large number of studies have demonstrated the polymethoxyflavones present in it showing the antioxidative activity effectively [10, 11]. Among the methoxy flavones, the major constituents are 5,7-dimethoxyflavone, 5,7,4′-trimethoxyflavone, 5-hydroxy-3,7,4′-trimethoxyflavone and 5-hydroxy-3,7-dimethoxyflavone [12]. The general structure of these is shown in Fig. 1. UPLC chromatogram of methoxy flavones in black ginger extract has shown in Fig. S1a (Supplementary material): (1) 5,7,3′4′-tetramethoxy flavone, (2) 3,5,7,3′4′-pentamethoxy flavone, (3) 5,7-diemthoxy flavone, (4) 5,7,4′-trimethoxy flavone, (5) 3,5,7 trimethoxy flavone, (6) 3,5,7,4′ tetramethoxy flavone, (7) 5-hydroxy-3,7,3′4′ tetramethoxy flavone, (8) 5-hydroxy-7-methoxy flavone, (9) 5-hydroxy-3,7 dimethoxy flavone and (10) 5-hydroxy-3,7,4′ trimethoxy flavones.
Vallace et al. showed that vascular tone is controlled not only by nervous and hormonal influences, but also by local active factors produced by the endothelium by activation of the various nitric oxide synthase (NOS) [13]. Nitric oxide (NO) is also known as endothelium-derived relaxing factor (EDRF) functions as a cell signaling factor in physiological and pathological processes and NO contributes to the control of basal and stimulated regional blood flow in man [14]. Another study investigated the effect of Kaempferia parviflora extract (KPE) in human umbilical vein endothelial cells (HUVEC) showing that KPE dose-dependently increased eNOS mRNA, protein expression and nitrite concentrations in culture media [15].
Phosphodiesterases (PDEs) are a group of enzymes that have powerful effects on cellular signaling because they regulate the second messenger, cyclic adenosine monophosphate (cAMP) or cyclic guanosine monophosphate (cGMP) [16]. One study investigated the effect of KPE on the inhibitory activity against both PDE5 and PDE6 and the results showed that 7-methoxyflavones are the major reason for the inhibition towards the enzymes in vitro [17]. KPE administration increased oxygen consumption in mice fed on a high-fat diet [18] and ingestion of KPE was found to enhance physical fitness state in subjects without an exercise habit [19].
Pomegranate (Punica granatum) peel extract
The pomegranate husk or peels are comprised almost 26–30% of the total fruit weight and constitute a higher amount of phenolic compounds than in the fruit pulp [20]. The compounds in pomegranate peel include flavonoids (anthocyanins, catechins and other complex flavonoids) and hydrolysable tannins (punicalin, pedunculagin, punicalagin, gallic and ellagic acid), and these are responsible for its biological activity [21]. The methanol extract of pomegranate peel showed significantly high antioxidant activity and anti-mutagenic properties, especially effective on lipid peroxidation, hydroxyl radical scavenging activity and human low-density lipoprotein (LDL) oxidation [22,23,24,25]. Apart from the biological activity of the pomegranate peel, it could be used for the rapid synthesis of silver nanoparticles (AgNPs) in ambient conditions [26]. Chemical structures of some of the active components found in pomegranate peel are depicted below (Fig. 2). UPLC-chromatograms of pomegranate peel extract are shown in Fig. S1b (Supplementary material) Ellagic acid and Fig. S1c (Supplementary material) Punicalagin.
Moringa (Moringa oleifera) leaves extract
Moringa Oleifera leaves extract is rich in saponins [27] and compounds like Niaziminin A, Niazirin, Querecetin-3-glucoside, Chlorogenic acid and Kaempferol [28, 29] and the structures are shown in Fig. 3. UPLC-Q-TOF–MS chromatograms of Moringa leaf extract are shown in Fig. S(2) (Supplementary material)—(a) Niaziminin A, (b) Niazirin, (c) Querecetin-3-glucoside, (d) Chlorogenic acid and (e) Kaempferol. The consumption of the leaf extract increased the hemoglobin level in pregnant women and was able to retain ferritin serum level dismount up to 50% [30, 31]. Moreover, the aqueous extract of M. oleifera possesses anxiolytic and antiepileptic effects possibly mediated via GABA mimetic action and can be used in the treatment of epilepsy and anxiety [32]. The moringa leaf extract showed both antioxidant, monoamine oxidase type B (MAO-B) and PDE-5 suppression activities in rats [33] and, hence, could be substituted with expensive conventional protein sources without any deleterious effects on performance and blood parameters [34]. Thus, it could be utilized as a source of food supplement to improve the growth performance.
To reconstitute all the health benefits from these natural sources, we used a formulation (Fitnox™) which consists of the extracts of black ginger, pomegranate peel and moringa leaves. The purpose of the study is to investigate a randomized, double-blind, placebo-controlled, single center study to evaluate the efficacy of Fitnox™ for physical endurance in 24 healthy adults.
Methods
Product description
The composition of the sports supplement formulation contains the extracts of moringa leaf (50%), black ginger (15%) and pomegranate peel (35%). Each stage of the raw material extraction has been closely screened and controlled to ensure the quality of the product. The moringa oleifera leaves were extracted using food grade ethanol–water mixture and concentrated. The black ginger rhizomes were extracted using food grade ethanol and was further purified to enrich the methoxyflavones. Together with these two extracts, the aqueous extract of pomegranate was spray dried to obtain the product with uniform matrix and capsulated to 250 mg. Analysis carried out to determine total methoxy flavones (11.4%) by HPLC [35], total pholyphenols (15.8%) by Folin-Ciocalteau phenol reagent [36] and total saponins (23.7%) by gravimetry [37]. The chemical constituents of Fitnox™ are described in Table 1.
Ethics and informed consent
This trial was conducted in accordance with the clinical research guidelines established by the Drugs and Cosmetics Act, 1940 in India, Drugs and Cosmetics Rules, 1945 of India, Ethical Guidelines for Biomedical Research on Human Participants, 2006 by the Indian Council of Medical Research (ICMR) in India, the principles enunciated in the Declaration of Helsinki (Edinburgh, 2000) and the ICH-harmonized tripartite guideline regarding Good Clinical Practice (GCP). This study was registered at the Clinical Trials Registry-India under the identifier REF/2016/02/010815 on 27th February 2016. There were no changes to the methods or planned endpoints after study initiation.
Exclusion and inclusion criteria
Participants included in the study were male healthy adults ranging in age from 18 to 55 years (both inclusive) normotensive, physically active but not exercise trained. The willingness to follow the protocol requirements was evidenced by written informed consent. Participants agreed not to use any medication, including vitamins and minerals, during or before the course of this study. Participants whose blood chemistries are within a normal range were considered and excluded if they have any clinically significant medical history. All the participants were non-smokers and non-alcoholics. The maximum volume of oxygen (VO2) intake was tested for every individual during the screening test [38] and none of the participants with poor oxygen volume intake was enrolled into the study. Vital signs such as blood pressure, pulse rate, respiratory rate and oral temperature were measured before check-in, prior to oral dosing on dosing day and at different time intervals after the dose in each study group.
Study design
The disposition of the study participants is shown in Fig. 4. This randomized, mono-centric, double-blind, placebo-controlled, parallel-group, clinical trial was conducted in 24 healthy male volunteers. The sample size of the study was 24, with 12 participants randomized to each of the two study arms in a double-blinded manner at a 1:1 ratio and received dosing as per randomization code provided at site by an authorized person independently. The participants were followed up regularly for all concomitant dosing from the time of screening till the follow-up visit was captured and recorded.
The continuation of the study was for 3 weeks and the participants in both groups were given either 250 mg of sports supplement formulation or placebo (250 mg starch capsules) in capsules once daily. Participants were allowed to consume their regular diet and they visited the clinic on day 0 (In house) which is 2 days after the screening day. Subsequent visit was on day 22 (Visit 2). Physical examination, demographics, vitals recorded on all visits and blood samples have taken before and after exercise on the day 0 and 22. Adverse events and concomitant medication were recorded. Serum chemistry, hematology, lactate levels, heart rate, time to exhaustion, 12 lead-electrocardiogram (ECG) for arrhythmia patterns, ST and RR segments (efficacy assessments), exercise test on treadmill [39], serum and saliva NO3 and NO2 levels at pre- and post-exercise were conducted on screening and final visit.
Administration of the product
After overnight fasting for at least 10 h, sports supplement formulation and placebo were administered orally to each subject in sitting posture, with 240 mL of water at room temperature, as per the randomization schedule. Participants were provided with dinner on the pre-study day, thereafter fasted for 10 h before dosing and for 4 h after dosing. Water was not permitted 1 h before and after the administration, but was allowed at all other times ad libitum. The meal plan was identical for all the groups.
Vital signs (blood pressure, pulse rate, respiratory rate and oral temperature) were measured before check-in, prior to oral dosing on dosing day and at different time intervals after the dose in each study group.
Blood samples and saliva were collected after oral administration at different time intervals for a span of 24 h. Saliva samples directly freeze to −20 °C until they are analyzed. After collection of blood samples, all the blood samples were transferred in a box with pre-cooled gel refrigerant packs, and centrifuged at 3500 rpm under refrigeration at 4 °C for 10 min within 60 min of collection. After centrifugation, the separated plasmas were transferred into suitably labeled polypropylene tubes. The aliquot plasma was stored in an insulated box containing dry ice pending transfer into the deep freezer. The temperature inside the insulated box was monitored. All plasma samples were stored upright in a freezer set at −20 °C until they are analyzed.
Exercise procedure
All the participants were fasted for 10 h before the exercise session with the exception of their morning supplement. During this session, heart rate and ratings of perceived exertion were monitored at rest, every 5 min during exercise and 10 min after recovery. The participants were mounted a level motorized treadmill and warmed up for 5 min at a self-selected pace. After the 5-min warm-up, treadmill speed increased slowly until a heart rate of 80% of predicted maximal heart rate was achieved and the maximum heart rate (100%) was about 220 minus the age of the participant [40]. The participants maintained this pace for 5 min. At this time, the treadmill grade was adjusted to −10%. The participants maintained this workload for 30 min, after that they completed a 5 min active cool-down at a self-selected pace and a 5-min seated passive recovery period.
Blood and saliva samples extraction and analysis
The blood samples kept to attain room temperature, added 2 mL of HPLC acetonitrile to the tube containing serum, mixed well for 2 min, pipetted the clear acetonitrile portion and evaporated under nitrogen flow. To this, 2 mL of water was added, mixed well for 2 min and pipetted 0.5 mL solution each into two vials to check nitrate and nitrite. Similarly, 2 mL of water was added to the saliva containing tube, mixed well for 2 min and pipetted 0.5 mL of the solution each into two vials. The nitrate and nitrite in both serum and saliva were checked by HPLC [41]. The analytical standards of sodium nitrate and sodium nitrite were obtained from Merck India.
Statistical analysis
The data analyzed as per statistical analysis system (SAS), expressed as the mean ± standard deviation (SD) and ‘P’ value ≤0.05, was considered as a significant value. Paired t test used to measure the change from the baseline. ANOVA was used to observe the changes in the various parameters after the follow-up visits scheduled, followed by appropriate post hoc test.
Results
The study assessed the improvement in physical endurance, safety and tolerability of test product Fitnox™ with the placebo in 24 healthy adults. The primary outcome of this study was to determine the LDH levels in pre- and post-exercise, heart rate, time to exhaustion, 12-lead electrocardiogram for arrhythmia patterns, performance test at 40 and 80% of VO2 max, serum and saliva NO3 and NO2 levels, MDA and LDH levels for oxidative stress at pre- and post-exercise on day 0 and day 22. The changes in the laboratory investigations (RBC Count, dopamine levels in urine) were also analyzed. The secondary outcome of this study was the safety of the product assessed by adverse events against placebo. The concentration of nitrate and nitrite in both plasma and saliva on day 0 and 22 in pre- and post-exercise is given in Figs. 5, 6, 7 and 8. The plasma nitrate increased from 6.7 µmol/L (day 0) to 9.7 µmol/L (day 22) before exercise and from 8.8 µmol/L (day 0) to 15.9 µmol/L (day 22) after exercise for the test groups. Similarly, the plasma nitrite increased from 37.5 to 73.1 (µmol/L) and from 42.5 to 80.3 (µmol/L) for pre- and post-exercises, respectively. In saliva, the concentrations of the nitrate increased from 6.5 (day 0) to 8.5 µmol/L (day 22) and 8.9 (day 0) to 10.9 µmol/L (day 22) in before and after exercise, respectively. The saliva nitrite also increased in the same manner from 37.9 to 46.4 (µmol/L) and 57.1 to 64.5 (µmol/L) in pre- and post-exercise. The LDH and MDA levels on day 0 and day 22 are shown in the Tables 2 and 3, respectively. Serum inflammatory biomarkers LDH and MDA decreased from 673.7 to 578 U/L and from 14.63 to 9.91 nmol/mL, respectively, in Fitnox™ group but no change in the placebo group. The dopamine concentration has considerably increased from 225.1 ug/24 h (day 0) to 354.3 ug/24 h (day 22) after the administration of the sports nutrition Fitnox™ (Table 3). Heart rate and RBC of the participants after the administration of Fitnox™ or placebo are given in Table 4 which clearly indicated that there are no significant changes between the treatment groups. Efficacy analysis for time to exhaustion (between groups) is shown in the Table 5. In the Fitnox™ group, the time to exhaustion significantly increased at P < 0.05 with that of the placebo.
Discussions
There were no study-related adverse events in any of the groups. All the 24 randomized volunteers enrolled had completed the study procedures with no withdrawal or dropouts during the study procedures. There were no unusual physical changes and abnormal changes in the vital signs like blood pressure, pulse rate, respiratory rate and oral temperature in both the treatment groups, indicating that natural sports supplement formulation Fitnox™ would be safe.
The biomarkers LDH and MDA values were exhibited due to exercise bouts demonstrating a decreased trend in Fitnox™ group, but no considerable change in the placebo group. There was a significant decrease (P < 0.05) in the LDH on day 22 (608 for pre- and 583 U/L for post-exercise) in Fitnox™ group as compared with placebo (654 for pre- and 635 U/L for post-exercise) as in Table 2 which shows the values at baseline, end of the treatment. In the Fitnox™ group, there were significant differences compared with baseline values at P < 0.01 in pre-exercise and P < 0.05 in post-exercise. But in the placebo group, there were no significant differences compared with baseline in the LDH. The onset of acidosis during periods of intense exercise has commonly been attributed to accumulation of lactic acid by LDH [42]. From this, the lactate production is being a primary cause of muscle fatigue during exercise and while LDH activity is correlated to muscle fatigue, the production of lactate by means of the LDH complex works as a system to delay the onset of muscle fatigue [43].
Similarly, the MDA levels also decreased considerably in the Fitnox™ group from 15 to 10 (nmol/mL) as in the Table 3 and there were significant differences compared with baseline in the MDA (nmol/ml) (P < 0.01), whereas there was no difference in the placebo. The percentage of decrement in the MDA levels from day 0 to 22 was 47%. MDA is one of the most frequently used indicators of lipid preoxidation, which is the oxidative degradation of lipids [44]. It is the process in which free radicals absorb electrons from the lipids in cell membranes, resulting in cell damage and this process proceeds by a free radical chain reaction mechanism [45]. The antioxidants present in the sports supplement prevent the depletion of cells [46]. Thus, it is assumed that the post-exercise analysis of LDH and MDA values exhibiting a lower value than the placebo indicates the effectiveness of the ROS quenchers present in the Fitnox™.
Table 3 shows that the dopamine concentration is considerably increased (36%) from 225 µg/24 h to 354 µg/24 h after the administration of the sports nutrition Fitnox™ and there were significant differences compared with baseline in the dopamine (P < 0.05). On the other hand, in the placebo group, there was no difference compared with baseline in the dopamine levels. During and after exercise, the lower plasma levels of norepinephrine and epinephrine indicate a marked rise in conjugated dopamine, which suggests that norepinephrine and epinephrine metabolism is shifted towards conjugated dopamine by exercise training [47, 48]. Dopamine normally associated with neuromodulatory actions may directly affect local cortical blood flow [49]. Dopamine also serves to protect the gastrointestinal tract and improves immune function [50]. Thus, maintaining sufficient dopamine levels is beneficial for mental health and physical functioning [51].
Figures 5, 6, 7 and 8 show the concentration of nitrate and nitrite in both plasma and saliva on days 0 and 22 in pre- and post-exercise. There is an increase in nitrate and nitrite concentrations in blood of the Fitnox™ group for both pre- and post-exercises indicating that the sports supplement formulation enhances the production of these two in the blood. In contrast, there is no significant increase in the placebo. The plasma nitrate increased from 6.7 µmol/L (day 0) to 9.7 µmol/L (day 22) and the increment was 31% before exercise. Similarly, there was a 45% increase in plasma nitrate after exercise and it was elevated from 8.8 µmol/L to 15.9 µmol/L. The plasma nitrite increased from 37.5 to 73.1 (µmol/L) and from 42.5 to 80.3 (µmol/L) for pre- and post-exercises, respectively. There was a remarkable rise in plasma nitrite, and 49 and 47% for pre- and post-exercises, respectively. None of the placebo groups showed a considerable increase in nitrate and nitrite.
The same trend observed in saliva and the concentrations of the nitrate increased from 6.5 to 8.5 (µmol/L) and 8.9 to 10.9 (µmol/L) in before and after exercise, respectively. The percentage of increment was 24% before exercise and 18% after exercise. The saliva nitrite also increased in the same manner from 37.9 to 46.4 µmol/L (18% increase) and 57.1 to 64.5 µmol/L (11% increase) in pre- and post-exercise. KPE has been shown to improve physical fitness performance in clinical studies [52,53,54]. The most potent PDE5 inhibitor was 5,7-dimethoxyflavone [17] and the comparative effects of KPE with sildenafil citrate on cyclic guanosine monophosphate (cGMP) level and modulation of cardiac function were investigated in a rat model and the results showed that KPE has a positive effect on NO signaling in the heart, resulting in an increased cGMP level, similar to that of sildenafil citrate [55]. Polymethoxyflavones in black ginger extract increased energy production by activating AMP-activated protein kinase (AMPK) which improved the dysfunction of muscle metabolism that leads to metabolic syndrome and locomotive dysfunction in vitro [56].
Red blood cell (RBC) counts and heart rate of the participants in both groups indicated that there were no significant changes which clearly indicated that there was no harmful effect after intake of both Fitnox™ and placebo as given in Table 4. The work rate during each exercise bout is presented in Table 5 and efficacy analysis between Fitnox™ and placebo groups for 40 and 80% of the distance covered and time to exhaustion (T40 & T80) demonstrates the statistical significance observed between the groups at the level of P < 0.05. Interestingly, other efficacy parameters like heart rate showed a high statistical significance between active and placebo groups (P < 0.05). Hemoglobin (Hb) is contained in the red blood cells, which, being circulated by the cardiovascular system, deliver O2 to the periphery where it is released from its Hb-bond (deoxygenation) and diffuses into the cells [1]. All these results have shown the good efficacy of the Fitnox™ for cardiovascular performance.
It is clear that contracting skeletal muscles generate free radicals and that intense exercise can result in oxidative damage to cellular constituents. During the past three decades, our knowledge about the biological implications of exercise-induced oxidative stress has expanded rapidly. Indeed, it is now appreciated that high levels of free radicals and ROS can damage cellular components. The strong antioxidants like methoxy flavones present in the black ginger and poly phenols in pomegranate such as ellagic acid exhibited potent free radical and ROS quenching during the post-exercise.
Conclusions
To conclude, the natural sports supplement formulation Fitnox™ was assisted to increase the nitrate, nitrite, RBC, and dopamine levels in blood before exercise period as well as in post exercise sessions during a period of 22 days. Similarly, the oxidative stress biomarkers such as MDA and LDH also gradually reduced during these periods in both pre- and post-exercises. The increased dopamine content helped the efficiency in exhaustion tests and these results suggest that Fitnox™ could enhance the physical fitness and stamina of any active persons. There were no unusual physical changes and abnormal changes noticed in the participants. Therefore, the consumption of Fitnox™ is very useful for sports persons who participate in acute, chronic and strenuous exercises that require physical endurance to perform effectively without tiredness.
References
Mairbäurl H (2013) Red blood cells in sports: effects of exercise and training on oxygen supply by red blood cells. Front Physiol 4(332):1–13
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88(4):1243–1276
Aziz IA, Yacoub M, Rashid L, Solieman A (2015) Malondialdehyde; lipid peroxidation plasma biomarker correlated with hepatic fibrosis in human Schistosoma mansoni infection. Acta Parasitol 60(4):735–742
O’Driscoll S, Height SE, Dick MC, Rees DC (2008) Serum lactate dehydrogenase activity as a biomarker in children with sickle cell disease. Br J Haematol 140(2):206–209
Deminice R, Rosa FT, Franco GS, Jordao AA, de Freitas EC (2013) Effects of creatine supplementation on oxidative stress and inflammatory markers after repeated-sprint exercise in humans. Nutrition 29(9):1127–1132
Ji LL (1995) Oxidative stress during exercise: implication of antioxidant nutrients. Free Radic Biol Med 18(6):1079–1086
Haff GG, Lehmkuhl MJ, McCoy LB, Stone MH (2003) Carbohydrate supplementation and resistance training. J Strength Cond Res 17(1):187–196
Urso ML, Clarkson PM (2003) Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189(1–2):41–54
Poortmans JR, Kumps A, Duez P, Fofonka A, Carpentier A, Francaux M (2005) Effect of oral creatine supplementation on urinary methylamine, formaldehyde and formate. Med Sci Sports Exerc 37(10):1717–1720
Rujjanawate C, Kanjanapothi D, Amornlerdpison D, Pojanagaroon S (2005) Anti-gastric ulcer effect of Kaempferia parviflora. J Ethnopharmacol 102:120–122
Sutthanut K, Sripanidkulchai B, Yenjai C, Jay M (2007) Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J Chromatogr A 1143(1–2):227–233
Chowdhary S, Townend JN (2001) Nitric oxide and hypertension: not just an endothelium derived relaxing factor! J Hum Hypertens 15(4):219–227
Vallance P, Collier J, Moncada S (1989) Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 2(8670):997–1000
Chaturapanich G, Chaiyakul S, Verawatnapakul V, Yimlamai T, Pholpramool C (2012) Enhancement of aphrodisiac activity in male rats by ethanol extract of Kaempferia parviflora and exercise training. Andrologia 44:323–328
Wattanapitayakul SK, Suwatronnakorn M, Chularojmontri L, Herunsalee A, Niumsakul S, Charuchongkolwongse S, Chansuvanich N (2007) Kaempferia parviflora ethanolic extract promoted nitric oxide production in human umbilical vein endothelial cells. J Ethnopharmacol 110(3):559–562
Temkitthawon P, Viyoch J, Limpeanchob N, Pongamornkul W, Sirikul C, Kumpila A, Suwanborirux K, Ingkaninan K (2008) Screening for phosphodiesterase inhibitory activity of Thai medicinal plants. J Ethnopharmacol 119(2):214–217
Temkitthawon P, Hinds TR, Beavo JA, Viyoch J, Suwanborirux K, Pongamornkul W, Sawasdee P, Ingkaninan K (2011) Kaempferia parviflora, a plant used in traditional medicine to enhance sexual performance contains large amounts of low affinity PDE5 inhibitors. J Ethnopharmacol 137(3):1437–1441
Yoshino S, Kim M, Awa R, Kuwahara H, Kano Y, Kawada T (2014) Kaempferia parviflora extract increases energy consumption through activation of BAT in mice. Food Sci Nutr 2(6):634–637
Toda K, Kohatsu M, Takeda S, Hitoe S, Shimizu N, Shimoda H (2016) Enhancement of physical fitness by black ginger extract rich in polymethoxyflavones: a double-blind randomized crossover trial. Integr Mol Med 3(2):628–634
Li Y, Guo C, Yang J, Cheng S (2006) Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem 96(2):254–260
Ismail T, Sestili P, Akhtar S (2012) Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J Ethnopharmacol 143(2):397–405
Singh RP, Murthy CKN, Jayaprakasha GK (2002) Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem 50:81–86
Negi PS, Jayaprakasha GK, Jena BS (2003) Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem 80(3):393–397
Murthy CKN, Jayaprakasha GK, Singh RP (2002) Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem 50:4791–4795
Aviram M, Volkova N, Coleman R, Dreher M, Reddy MK, Ferreira D, Rosenblat M (2008) Pomegranate phenolics from the peels, arils, and flowers are antiatherogenic: studies in vivo in atherosclerotic apolipoprotein e-deficient (E 0) mice and in vitro in cultured macrophages and lipoproteins. J Agric Food Chem 56(3):1148–1157
Edison TJ, Sethuraman MG (2013) Biogenic robust synthesis of silver nanoparticles using Punica granatum peel and its application as a green catalyst for the reduction of an anthropogenic pollutant 4-nitrophenol. Spectrochim Acta A Mol Biomol Spectrosc 104:262–264
Makkar HPS, Becker K (1997) Nutrients and antiquality factors in different morphological parts of the Moringa oleifera tree. J Agric Sci Camb 128:311–322
Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani A (1992) Isolation and structure elucidation of novel hypotensive agents, niazinin A, niazinin B, niazimicin and niaziminin A+B from Moringa oleifera: the first naturally occurring thiocarbamates. J Chem Soc Perkin Trans 1:3237–3241
Vongsak B, Sithisarn P, Gritsanapan W (2014) Simultaneous HPLC quantitative analysis of active compounds in leaves of Moringa oleifera Lam. J Chromatogr Sci 52(7):641–645
Iskandar I, Hadju V, As’ad S, Natsir R (2015) Effect of Moringa oleifera leaf extracts supplementation in preventing maternal anemia and low-birth-weight. IJSRP 5(2):1–3
Nadimin Hadjub V, As’ad S, Bucharid A (2015) The extract of moringa leaf has an equivalent effect to iron folic acid in increasing hemoglobin levels of pregnant women: a randomized control study in the coastal area of Makassar. IJSBAR 22(1):287–294
Ingale SP, Gandhi FP (2016) Effect of aqueous extract of Moringa oleifera leaves on pharmacological models of epilepsy and anxiety in mice. Int J Epilepsy 3(1):12–19
Prabsattroo T, Wattanathorn J, Iamsaard S, Somsapt P, Sritragool O, Thukhummee W, Muchimapura S (2015) Moringa oleifera extract enhances sexual performance in stressed rats. J Zhejiang Univ Sci B 16(3):179–190
Oghenebrorhie O, Oghenesuvwe O (2016) Performance and haematological characteristics of broiler finisher fed Moringa oleifera leaf meal diets. J Northeast Agric Univ 23(1):28–34
Tuntiyasawasdikul S, Limpongsa E, Jaipakdee N, Sripanidkulchai B (2014) Transdermal permeation of Kaempferia parviflora methoxyflavones from isopropyl myristate-based vehicles. AAPS PharmSciTech 15(4):947–955
Box JD (1983) Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res 17(5):511–525
Ezeabar CA, Okeke CU, Aziagba BO, Ilodibia CV, Emeka AN (2014) Determination of saponin content of various parts of six citrus species. Int Res J Pure Appl Chem 4(1):137–143
Cooper KH (1968) A means of assessing maximal oxygen intake. J Am Med Assoc 203:135–138
Bruce RA, Lovejoy FW Jr, Pearson R, Yu PNG, Brothers GB, Velasquez T (1949) Normal respiratory and circulatory pathways of adaptation in exercise. J Clin Invest 28(6):1423–1430
Nes BM, Janszky I, Wisløff U, Støylen A, Karlsen T (2013) Age-predicted maximal heart rate in healthy subjects: the HUNT fitness study. Scand J Med Sci Sports 23(6):697–704
Croitoru MD (2012) Nitrite and nitrate can be accurately measured in samples of vegetal and animal origin using an HPLC-UV/VIS technique. J Chromatogr B 911:154–161
Sahlin K, Harris RC, Nylind B, Hultman E (1976) Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch 367(2):143–149
Facey A, Irving R, Dilworth L (2013) Overview of lactate metabolism and the implications for athletes. Am J Sports Med 1(3):42–46
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11(1):81–128
Halliwell B, Gutteridge JM (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219(1):1–14
Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2(2):219–236
Ransford CP (1982) A role for amines in the antidepressant 383 effect of exercise: a review. Med Sci Sports Exerc 14(1):1–10
Bove AA, Dewey JD, Tyce GM (1984) Increased conjugated dopamine in plasma after exercise training. J Lab Clin Med 104(1):77–85
Krimer LS, Muly EC, Williams GV, Goldman-Rakic PS (1998) Dopaminergic regulation of cerebral cortical microcirculation. Nat Neurosci 1(4):286–289
Sikirić P, Rotkvić I, Mise S, Krizanac S, Gjuris V, Jukić J, Suchanek E, Petek M, Udovicić I, Kalogjera L (1988) The influence of dopamine agonists and antagonists on indomethacin lesions in stomach and small intestine in rats. Eur J Pharmacol 158(1–2):61–67
Deslandes A, Moraes H, Ferreira C, Veiga H, Silveira H, Mouta R, Pompeu FA, Coutinho ES, Laks J (2009) Exercise and mental health: many reasons to move. Neuropsychobiology 59(4):191–198
Promthep K, Eungpinichpong W, Sripanidkulchai B, Chatchawan U (2015) Effect of Kaempferia parviflora extract on physical fitness of soccer players: a randomized double-blind placebo-controlled trial. Med Sci Monit Basic Res 21:100–108
Wattanathorn J, Muchimapura S, Tong-Un T, Saenghong N, Thukhum-Mee W, Sripanidkulchai B (2012) Positive modulation effect of 8-week consumption of Kaempferia parviflora on health-related physical fitness and oxidative status in healthy elderly volunteers. Evid Based Complement Alternat Med:1–7
Toda K, Hitoe S, Takeda S, Shimoda H (2016) Black ginger extract increases physical fitness performance and muscular endurance by improving inflammation and energy metabolism. Heliyon 2:e00115
Weerateerangkul P, Palee S, Chinda K, Chattipakorn SC, Chattipakorn N (2012) Effects of Kaempferia parviflora wall. ex. baker and sildenafil citrate on cGMP level, cardiac function, and intracellular Ca2+ regulation in rat hearts. J Cardiovasc Pharmacol 60(3):299–309
Toda K, Takeda S, Hitoe S, Nakamura S, Matsuda H, Shimoda H (2016) Enhancement of energy production by black ginger extract containing polymethoxy flavonoids in myocytes through improving glucose, lactic acid and lipid metabolism. J Nat Med 70(2):163–172
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Ethical approval
All procedures were approved by the Clinical Trials Registry-India the identifier REF/2016/02/010815 on 27th February 2016 and were carried out in accordance with the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gopi, S., Jacob, J., Varma, K. et al. Natural sports supplement formulation for physical endurance: a randomized, double-blind, placebo-controlled study. Sport Sci Health 13, 183–194 (2017). https://doi.org/10.1007/s11332-017-0352-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-017-0352-y