Abstract
Purpose
To investigate threshold values for obstructive apnea–hypopnea index (OAHI) and nadir oxygen saturation (NspO2) in children with severe obstructive sleep apnea (OSA) to identify children most appropriate for preoperative echocardiography.
Methods
A multi-institutional retrospective chart review was performed on children who underwent echocardiography and polysomnogram within a year. Children with severe OSA as defined by OAHI > 10 or NspO2 < 80% were included. Receiver operator curves and Youden’s J index were used to assess the discriminatory ability and threshold values of OAHI and NspO2 for right heart strain (RHS) on echocardiography.
Results
A total of 173 prepubertal (< 10 years) children and 71 postpubertal (≥ 10 years) children of age were included. RHS was seen in 9 (5%) prepubertal children and 4 (6%) postpubertal children. In prepubertal children, OAHI and NspO2 were poor predictors of RHS (area under the curve [AUC] 0.53 [95%CI 0.45–0.61], p = 0.748; AUC 0.56 [95%CI 0.48–0.64], p = 0.609). In postpubertal children, threshold values of 55 events/hour and 69% were strong predictors for RHS (AUC 0.88 [95%CI 0.78–0.95], p < 0.001; AUC 0.92 [95%CI 0.83–0.97], p < 0.001).
Conclusion
In children with severe OSA, evidence of RHS is low. Postpubertal children with OAHI > 55 and NspO2 < 69% appear most appropriate for echocardiography. Clinicians should weigh the risks and benefits of preoperative echocardiography for each child with these threshold values in mind.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the pediatric population, obstructive sleep apnea (OSA) prevalence ranges from 1.2 to 5.7% [1]. Early detection and treatment of OSA are imperative, as untreated OSA can lead to systemic inflammation, cardiovascular disease, metabolic disorder, and neurocognitive and behavioral issues [2]. Polysomnography is currently the gold standard for diagnosing OSA because clinical history and physical examination alone are neither sensitive nor specific enough to differentiate OSA from primary snoring [1]. On polysomnogram, the obstructive apnea–hypopnea index (OAHI) and nadir oxygen saturation (NspO2) are the most commonly used parameters in assessing the severity of OSA [2].
Adenotonsillectomy is one effective treatment for OSA in children [1]. Although commonly performed, adenotonsillectomy is associated with certain risks from general anesthesia, pain, dehydration, bleeding, and acute airway and respiratory compromise during the perioperative period [1]. An increased risk of postoperative post-obstructive pulmonary edema, arrhythmia, and other cardiac events is seen in patients younger than 3 years of age or those with comorbidities, such as severe OSA, failure-to-thrive, obesity, craniofacial abnormality, neuromuscular disorder, or current respiratory infection [1,2,3]. Moreover, it has been highlighted that cardiac abnormalities and pulmonary hypertension are significant factors for developing postoperative pulmonary edema and respiratory compromise [4]. Because of these risks, it is best practice for surgeons to attempt to identify which children are at the highest risk of perioperative cardiopulmonary complications. Using polysomnogram and echocardiogram findings, multiple studies have investigated the relationship between OSA and perioperative cardiopulmonary complications [3, 5,6,7,8,9,10,11,12,13]. Some of these studies suggest obtaining an echocardiogram as part of the routine preoperative evaluation for some children. Since findings on echocardiograms such as pulmonary hypertension and right heart strain (RHS) are associated with OSA and are known risk factors for perioperative complications in children, preoperative echocardiography is often performed to identify patients with these conditions [7, 8]. The American Heart Association and American Thoracic Society have advocated for preoperative echocardiograms in children with severe OSA or cardiometabolic risk factors to identify cardiac abnormalities that would increase the risk of perioperative complications [14].
However, more recent retrospective reviews have highlighted that polysomnogram variables are inconsistent predictors of cardiac abnormalities on preoperative echocardiography [9,10,11,12,13]. Additionally, some studies have suggested that abnormal echocardiography findings might not adequately predict perioperative cardiovascular and respiratory complications after adenotonsillectomy [3, 9, 13]. A recent systematic review analyzing all studies on preoperative echocardiography could not draw any conclusions regarding their utility secondary to the heterogeneous published data and inconsistent results [15]. It is challenging for practitioners to determine which children with OSA should undergo a preoperative echocardiogram, potentially resulting in unnecessary imaging studies and delays in treatment.
Therefore, the objective of this study aimed to compare polysomnogram variables between patients who showed evidence of RHS to patients who did not have RHS on echocardiogram. The primary aim of this study was to establish threshold values for OAHI and NspO2 that could help clinicians decide when to obtain a preoperative echocardiogram for children with severe OSA. We hypothesized that children with increasing severity of severe OSA were more likely to have RHS, meaning threshold values could be identified to aid clinicians on the proper time to order a preoperative echocardiogram. Furthermore, we hypothesized that OAHI and NspO2 would be more robust predictors in older children as they have had more time to develop RHS compared to younger children.
Methods
Patient selection
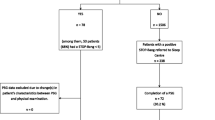
The Institutional Review Boards at Augusta University and Medical University of South Carolina (MUSC) approved this retrospective chart review. From 2001 to 2021, all pediatric patients from 0 to 18 years old who underwent echocardiogram and polysomnography studies within a year of each other were reviewed. There were no criteria describing which study had to be performed first chronologically. The two chronologically closest studies were recorded if a patient had more than one echocardiogram or polysomnogram within a year. Patients with OAHI > 10 or NspO2 < 80% were included in this study. Patients with congenital heart disease, ventilator dependence, and/or tracheostomy were excluded from the study. Patients were divided into the subgroups of prepuberal (< 10 years of age) and postpubertal (≥ 10 years of age). As a large study found that the mean age of 10 years was when children begin puberty [16], we felt this was an appropriate division to reduce heterogeneity among our cohort.
Polysomnogram
Polysomnogram data were obtained from the patient’s medical records. Demographic variables were extracted from the polysomnogram report, including age, gender, and body mass index (BMI). All polysomnograms were performed overnight. Continuous nocturnal audio-visual recording was performed using frontal, central, and occipital electroencephalograms (EEG), bilateral electrooculograms (EOG), submentalis and anterior tibialis electromyography (EMG), nasal and oral pressure flow transducer, electrocardiography (ECG), pulse oximeter, end-tidal carbon dioxide, and inductance plethysmography for thoracic and abdominal movements. The polysomnogram-specific variables extracted included OAHI, central apnea index (CAI), NspO2, mean SpO2, and total sleep time. Diagnostic and split study polysomnogram data were included in this study. For split study polysomnograms, only data from the diagnostic portion of the study was included. A polysomnogram was excluded if the polysomnogram was only a titration study without a diagnostic portion.
Furthermore, a polysomnogram was also excluded if a patient discontinued the study early or did not have enough total sleep time for the sleep medicine physician to define any variables. For this study, polysomnogram respiratory events were defined using the AASM Manual for the Scoring of Sleep and Associated Events [17]. An obstructive apnea event was defined as at least a 90% reduction of airflow in the presence of continued respiratory effort lasting at least two respiratory cycles. An obstructive hypopnea event was defined as at least a 30% airflow decrease lasting at least two respiratory cycles and was associated with a 3% oxygen desaturation or arousal [17]. OAHI was calculated as the sum of the number of obstructive apnea and obstructive hypopnea events per hour [17]. NspO2 was defined as the lower oxygen saturation percent across total sleep time [18].
Echocardiogram
Echocardiogram variables were also collected from the patient’s medical records. Evidence of RHS included direct documentation of pulmonary hypertension, right ventricular hypertension, right ventricular hypertrophy, right ventricular dilation, or right ventricular systolic pressure (RVSP) greater than 40 mmHg [19]. Only transthoracic echocardiograms were included. As transesophageal echocardiograms are typically reserved for children with significant cardiac disease rather than routine preoperative screening, transesophageal echocardiograms were excluded [20]. In addition, if the interpreting physician documented that the patient did not cooperate enough to determine the cardiac function, specifically the right ventricle, echocardiogram was excluded. Most patients received echocardiograms to “assess cardiac form and function” after being diagnosed with severe OSA, but other reasons included hearing a murmur on physical exam, changes on electrocardiogram (ECG), shortness of breath, and exercise intolerance. There were no standard criteria for when an echocardiogram should be ordered in this patient population, but it was based on clinical decision-making by each provider.
Statistical analysis
Data were analyzed using SPSS 27.0 (IBM Corporation, Armonk, NY, USA) and MedCalc 20.011 (MedCalc Software, Belgium). Categorical variables were presented as frequency (N) and percentage (%), and continuous variables were presented as mean ± standard deviation or as median and interquartile range (IQR 25th–75th) where appropriate. All continuous variables were assessed for normality by the Kolmogorov–Smirnov test. Comparisons of nominal variables were performed using chi-square or Fisher’s exact test. As appropriate, comparisons of continuous variables between two groups were analyzed using an independent t-test or a Mann–Whitney U test. For the polysomnographic variables of OAHI and NspO2, the area under the receiver operator curve (AUC) was calculated to assess their discriminatory ability for RHS on echocardiogram. The max Youden J index (Sensitivity + Specificity − 1) was used to determine the threshold that would be the most optimal in predicting RHS [21]. A p-value of less than 0.05 was considered statistically significant for all statistical tests.
Results
Patient characteristics
A total of 244 patients were included in the analysis, with 67 from Augusta University and 177 from MUSC. Included patients had a median age of 4.8 years (2.0–11.7) and a BMI of 16.2 kg/m2 (13.9–22.8) (Table 1). More patients were male (N = 145, 59%). On polysomnogram, OAHI was 25.0 events/hour (14.3–50.0), and NspO2 was 74.0% (66.0–81.8). A total of 13 patients (5%) exhibited RHS on echocardiogram. The most common causes of RHS were right ventricular dilation (46%), right ventricular hypertrophy (39%), and pulmonary hypertension (30.8%). Most patients underwent adenotonsillectomy (N = 152, 62%), and a small minority underwent other sleep surgeries (N = 15, 6%). The other sleep surgeries were either performed without adenotonsillectomy or either before, during, or after adenotonsillectomy. In 51 (21%) patients, chronic lung disease was seen, with asthma most common (N = 34, 14%).
Right heart strain in prepubertal children
A total of 173 patients were less than 10 years of age at their polysomnogram, with 164 children (95%) showing no evidence of RHS and 9 children (5%) having RHS on echocardiography (Table 2). No statistical differences were seen in age, sex, and BMI between these two groups. Comparing children with RHS to children without RHS, no statistical significance was seen for OAHI (27.0 events/hour [13.3–35.3] vs. 24.0 events/hour [14.0–45.0], p = 0.753) nor NspO2 (81.0% [57.0–84.0] vs. 73.0% [64.0–81.0], p = 0.540). No differences were appreciated in total sleep time or mean SpO2.
Right heart strain in postpubertal children
Of 71 patients who were 10 years of age or older at their polysomnogram, 67 children (94%) did not have RHS, and 4 children (6%) did have RHS on echocardiography (Table 2). When comparing these two groups, no statistical differences were seen in age, sex, and BMI. Children with RHS had an elevated OAHI (88.3 events/hour [62.9–146.0] vs. 30.0 events/hour [17.0–60.0], p = 0.007) and lower NspO2 (45.0% [27.8–66.8] vs. 77.0% [69.0–83.0], p = 0.002) when compared to children without RHS. Mean SpO2 was also lower in children with RHS (77.0% [54.3–90.0] vs. 95.0% [91.8–97.0], p = 0.002), but no statistical differences were seen in total sleep time.
Threshold values for right heart strain in prepubertal children
The AUC for OAHI in predicting RHS was poor and nonsignificant (0.53 95%CI 0.45–0.61, p = 0.748) (Table 3). The criterion threshold that optimized sensitivity and specificity was 41 events/hour; however, the Youden J index was low at 0.18. At this threshold, sensitivity was 88.9%, and specificity was 29.3%. Figure 1 depicts the receiver operator curve (ROC) for OAHI in prepubertal children. For NspO2, the AUC was also poor and nonsignificant in discriminating RHS (0.56 95%CI 0.48–0.64, p = 0.609) (Fig. 2). The criterion threshold was 80%, corresponding to a Youden J index of 0.29. Sensitivity and specificity at this threshold were 55.6% and 73.8%, respectively.
Threshold values for right heart strain in postpubertal children
The AUC for OAHI in predicting RHS was strong and significant (0.88 95%CI 0.78–0.95, p < 0.001) (Table 3). The criterion threshold that optimized sensitivity and specificity was 55 events/hour, with a Youden J index of 0.75 (Fig. 3). At this threshold, sensitivity was 100%, and specificity was 74.6%. For NspO2, the AUC was also strong and significant in discriminating RHS (0.93 95%CI 0.83–0.97, p < 0.001). The criterion threshold was 69%, corresponding to a Youden J index of 0.75. Sensitivity and specificity at this threshold were 100.0% and 74.6%, respectively. Figure 4 represents the ROC for NspO2 for children ≥ 10 years of age.
Discussion
The AHA and ATS currently recommend ordering preoperative echocardiograms to assess cardiac function in children with severe OSA undergoing adenotonsillectomy [14]. While some studies have supported this recommendation by finding evidence of pulmonary hypertension and RHS in these children [5, 8, 22], recent evidence has not established a connection between OSA severity and both echocardiography findings and postoperative complications [9,10,11,12,13]. Therefore, this study aimed to establish threshold values that clinicians could use when deciding to order an echocardiogram for a child with severe OSA. Our study found a low prevalence of RHS of 5%. When separating children by age, significant threshold values were not identified in prepubertal children. For postpubertal children, the OAHI threshold of 55 events/hour and NspO2 of 69% were strong discriminators of RHS.
The prevalence of RHS in children with OSA is low. Revenaugh et al. and Teplitzky et al. found no abnormal echocardiograms in their cohorts of 57 and 47 patients, respectively [6, 10]. Two other studies by Bitners et al. and Clements et al. found evidence of RHS and elevated RVSP to be 4.0% and 5.3%, but these findings were unrelated to the severity of OSA [11, 12]. Pettitt-Schieber et al. found that only 1.8% of their cohort had echocardiogram findings after excluding patients with congenital heart disease [9]. One of the largest cohorts of 163 patients by Burns et al. identified a similarly low rate of 1.8% [13]. Notably, both studies did not find a connection between echocardiogram findings and peri- and postoperative complications after adenotonsillectomy [9, 13]. The highest rate of RHS was seen in Larrier et al., with 11% of patients having right ventricular dilation or hypertrophy. However, these investigators included ECG in their evaluation of RHS [3]. Our study supports a low prevalence of RHS in children with severe OSA, even when separated by age (5% in prepubertal children and 6% in postpubertal children). These findings potentially question the utility of preoperative echocardiogram in identifying children with cardiac abnormalities before adenotonsillectomy.
Even though the prevalence is low, perioperative cardiac concerns for children with OSA are a vital consideration. An extensive review by Minai et al. concluded that pulmonary hypertension was an independent predictor for perioperative complications with increased risks of hypotension, prolonged extubation, respiratory compromise, and right heart failure [8]. Blum et al. described the cardiac function changes and subsequent anesthesia challenges for children with chronic upper airway obstruction [5]. A case-controlled study by Kalra et al. reported that children with significant postoperative respiratory complications had increased left ventricular mass and right ventricular dimensions compared to matched controls without postoperative complications [7]. However, recent evidence has shown that many of these cardiac changes are subclinical and resolve after adenotonsillectomy. There continues to be evidence that many small echocardiographic changes do not change the clinical course or outcome. One small study from Sofer et al. described children aged 1 to 3 and 1/2 with cor pulmonale and reduced right ventricular ejection fraction secondary to adenotonsillar hypertrophy that resolved after surgery [23]. Abd El-Moneim et al. and Chan et al. have shown lower right ventricular function in groups with moderate to severe OSA that resolved after adenotonsillectomy [22, 24]. Three independent systematic reviews on conventional and tissue Doppler echocardiography, a more sensitive form of echocardiogram, have shown improvements in subclinical cardiac changes after adenotonsillectomy [25,26,27]. While there certainly is evidence of cardiac changes in children with OSA, the current literature has become challenging for providers to interpret to know when an echocardiogram may provide meaningful results that would change management or risk stratify a patient for their safety.
This study’s strength is that we identified practical threshold values in children 10 years of age or older that may help clinicians order echocardiograms for suitable patients and avoid unnecessary testing in most patients. This finding could be used to develop further OAHI cut-off values to risk stratify children with OSA on potential cardiac damage. Unfortunately, significant thresholds were not identified for our larger cohort of children under 10 years of age. In the recent literature, there is increasing evidence that childhood OSA has a different pathophysiology compared to adolescent OSA [28, 29]. While childhood OSA is most likely due to adenotonsillar hypertrophy, adolescent OSA has been attributed more to combinations of obesity, reduced compensatory ventilatory response, and decreased upper airway neuromotor tone [28, 29]. These pathophysiology underpinnings have been proposed to explain why childhood OSA has a higher propensity to resolve spontaneously compared to OSA in adolescents, which is more likely to persist into adulthood [28, 29]. Interestingly, our findings follow this same age group division, suggesting that OAHI and NspO2 may be more decisive predictive factors of cardiac function changes in older children with OSA than in younger children with OSA. More research is needed to determine if other polysomnogram, demographic, or clinical factors may be more helpful in discriminating RHS in children under 10 years of age.
There are limitations to this study that need to be addressed. The first is the retrospective nature of the study. Aside from OSA, several other factors may lead to cardiac dysfunction, such as hypertension, diabetes, obesity, kidney disease, chronic lung diseases, and other pulmonary diseases. In addition, these conditions may confound each other and take several years to decades for a patient to develop any cardiac complication. Therefore, we could not definitively conclude that the right heart strain of these thirteen children was solely due to OSA rather than other medical comorbidities. While only including exams 1 year apart reduced some potential temporal confoundings, it is impossible to know how long a child met diagnostic criteria for OSA before receiving an echocardiogram. Therefore, we could not analyze if the amount of time before treatment of OSA influenced the changes in their echocardiogram. Furthermore, this study could not account for the potential differing interpretations of RHS on echocardiogram between readers. While we did include RVSP > 40 mmHg as an objective measurement, the other definitions of right ventricular hypertrophy, dilation, and hypertension were more subjective descriptions that relied on various measures from differing cardiac windows. Also, the analyses could not account for the variations in echocardiography protocols and procedures between the two institutions. We were unable to evaluate other important variables in our study, such as the central apneas and end-tidal carbon dioxide, which are undoubtedly essential variables to research in the future.
While this study was multi-institutional, our findings may be limited in application to the general population. While our cohort of 244 patients is one of the largest cohorts of patients with both polysomnograms and echocardiograms, it is difficult to make definitive conclusions with only thirteen patients exhibiting RHS. Furthermore, both institutions are in the southeastern United States, which has a different pediatric population than other regions [30]. A large, multicenter observational study with robust follow-up is necessary to define exact thresholds of polysomnogram variables that accurately predict echocardiographic abnormalities and to see if RHS does remit in patients after their sleep surgery.
This study did not address the postoperative outcomes and complications for the children who underwent adenotonsillectomy because not all of our children received this intervention. Only 62% of children had received adenotonsillectomy when their medical record was analyzed. Unfortunately, this study does not answer whether or not finding RHS on echocardiogram translates to an increased risk in peri-and postoperative complications after adenotonsillectomy. While findings of RHS are essential to identify and should be followed after the procedure, the clinical utility of these findings is unknown. Furthermore, whether or not RHS had any bearing on postoperative OAHI or cardiovascular improvements is unknown. More research is needed to identify if our threshold values correlate to complications which can further help protect the child's safety during adenotonsillectomy.
Conclusion
No significant threshold values for OAHI and NspO2 were found for prepubertal children; however, for postpubertal children, the thresholds of 55 events/hour for OAHI and 69% for NspO2 were strong discriminators in RHS. These thresholds provided a 100% sensitivity and 74.6% specificity for RHS. These values might help clinicians decide when a preoperative echocardiogram is appropriate for a particular child with severe OSA before adenotonsillectomy. A multicenter prospective trial is needed to understand further if identifying RHS on preoperative echocardiogram provides meaningful risk stratification for patients and if this RHS resolves or persists after adenotonsillectomy.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to concerns of compromise of individual privacy, but are available from the corresponding author on reasonable request.
References
Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, Schechter MS et al (2012) Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 130:576–584. https://doi.org/10.1542/peds.2012-1671
Mitchell RB, Archer SM, Ishman SL, Rosenfeld RM, Coles S, Finestone SA, Friedman NR et al (2019) Clinical practice guideline: tonsillectomy in children (update). Otolaryngol Head Neck Surg 160:S1-s42. https://doi.org/10.1177/0194599818801757
Larrier DR, Huang ZJ, Wei Z, McHugh CH, Brock L, Reddy SC-B, Zhang W (2015) Is routine pre-operative cardiac evaluation necessary in obese children undergoing adenotonsillectomy for OSA? Am J Otolaryngol 36:744–747. https://doi.org/10.1016/j.amjoto.2015.05.007
Katz SL, Monsour A, Barrowman N, Hoey L, Bromwich M, Momoli F, Chan T et al (2020) Predictors of postoperative respiratory complications in children undergoing adenotonsillectomy. J Clin Sleep Med 16:41–48. https://doi.org/10.5664/JCSM.8118
Blum RH, McGowan FX Jr (2004) Chronic upper airway obstruction and cardiac dysfunction: anatomy, pathophysiology and anesthetic implications. Paediatr Anaesth 14:75–83. https://doi.org/10.1046/j.1460-9592.2003.01193.x
Revenaugh PC, Chmielewski LJ, Edwards T, Krishna J, Krakovitz P, Anne S (2011) Utility of preoperative cardiac evaluation in pediatric patients undergoing surgery for obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 137:1269–1275. https://doi.org/10.1001/archoto.2011.208
Kalra M, Kimball TR, Daniels SR, LeMasters G, Willging PJ, Rutter M, Witt SA et al (2005) Structural cardiac changes as a predictor of respiratory complications after adenotonsillectomy for obstructive breathing during sleep in children. Sleep Med 6:241–245. https://doi.org/10.1016/j.sleep.2004.10.004
Minai OA, Yared JP, Kaw R, Subramaniam K, Hill NS (2013) Perioperative risk and management in patients with pulmonary hypertension. Chest 144:329–340. https://doi.org/10.1378/chest.12-1752
Pettitt-Schieber B, Tey CS, Hill R, Vaughn W, Pakanati V, Leu R, Raol N (2021) The utility of preoperative echocardiography in pediatric obstructive sleep apnea. Sleep and Breathing. https://doi.org/10.1007/s11325-021-02303-5
Teplitzky TB, Pereira KD, Isaiah A (2019) Echocardiographic screening in children with very severe obstructive sleep apnea. Int J Pediatr Otorhinolaryngol 126:109626. https://doi.org/10.1016/j.ijporl.2019.109626
Bitners AC, Arens R, Mahgerefteh J, Sutton NJ, Silver EJ, Sin S, Khan MA et al (2021) Prevalence of elevated right ventricular pressure in children with obstructive sleep apnea syndrome undergoing pulmonary hypertension screening. J Clin Sleep Med 17:2225–2232. https://doi.org/10.5664/jcsm.9412
Clements AC, Walsh JM, Dai X, Skinner ML, Sterni LM, Tunkel DE, Boss EF et al (2021) Cardiopulmonary testing before pediatric adenotonsillectomy for severe and very severe obstructive sleep apnea syndrome. Laryngoscope. https://doi.org/10.1002/lary.29480
Burns AT, Hansen SL, Turner ZS, Aden JK, Black AB, Hsu DP (2019) Prevalence of pulmonary hypertension in pediatric patients with obstructive sleep apnea and a cardiology evaluation: a retrospective analysis. J Clin Sleep Med 15:1081–1087. https://doi.org/10.5664/jcsm.7794
Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD et al (2015) Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 132:2037–2099. https://doi.org/10.1161/cir.0000000000000329
Pettitt-Schieber B, Tey CS, Nemeth J, Raol N (2021) Echocardiographic findings in children with obstructive sleep apnea: a systematic review. Int J Pediatr Otorhinolaryngol 145:110721. https://doi.org/10.1016/j.ijporl.2021.110721
Zhang J, Chan NY, Lam SP, Li SX, Liu Y, Chan JW, Kong AP et al (2016) Emergence of sex differences in insomnia symptoms in adolescents: a large-scale school-based study. Sleep 39:1563–1570. https://doi.org/10.5665/sleep.6022
Berry RB, Albertario CL, Harding SM, Al E (2018) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.5. Am Acad Sleep Med 1–32
Wali SO, Abaalkhail B, AlQassas I, Alhejaili F, Spence DW, Pandi-Perumal SR (2020) The correlation between oxygen saturation indices and the standard obstructive sleep apnea severity. Ann Thorac Med 15:70–75. https://doi.org/10.4103/atm.ATM_215_19
Hsu WC, Kang KT, Chiu SN, Weng WC, Lee PL, Lin CY (2018) 24-hour ambulatory blood pressure after adenotonsillectomy in childhood sleep apnea. J Pediatr 199:112-117.e6. https://doi.org/10.1016/j.jpeds.2018.03.072
Kavanaugh-McHugh A, Tobias JD, Doyle T, Heitmiller ES, Meagher C (2000) Transesophageal echocardiography in pediatric congenital heart disease. Cardiol Rev 8:288–306. https://doi.org/10.1097/00045415-200008050-00008
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35. https://doi.org/10.1002/1097-0142(1950)3:1%3c32::aid-cncr2820030106%3e3.0.co;2-3
Abd El-Moneim ES, Badawy BS, Atya M (2009) The effect of adenoidectomy on right ventricular performance in children. Int J Pediatr Otorhinolaryngol 73:1584–1588. https://doi.org/10.1016/j.ijporl.2009.08.013
Sofer S, Weinhouse E, Tal A, Wanderman KL, Margulis G, Leiberman A, Gueron M (1988) Cor pulmonale due to adenoidal or tonsillar hypertrophy or both in children. Noninvasive Diagnosis Follow-up Chest 93:119–122. https://doi.org/10.1378/chest.93.1.119
Chan JY, Li AM, Au CT, Lo AF, Ng SK, Abdullah VJ, Ho C et al (2009) Cardiac remodelling and dysfunction in children with obstructive sleep apnoea: a community based study. Thorax 64:233–239. https://doi.org/10.1136/thx.2007.094904
Poupore NS, Gudipudi R, Nguyen SA, Pecha PP, Pecha TJ, Carroll WW (2022) Tissue Doppler echocardiography in children with OSA before and after tonsillectomy and adenoidectomy: a systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol 152:111002. https://doi.org/10.1016/j.ijporl.2021.111002
Sun YL, Yuan B, Kong F, Li XM (2021) Effects of adenoidectomy or adenotonsillectomy on the cardiovascular system in children: a meta-analysis. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-021-06986-0
Teo DT, Mitchell RB (2013) Systematic review of effects of adenotonsillectomy on cardiovascular parameters in children with obstructive sleep apnea. Otolaryngol - Head Neck Surg (United States) 148:21–28. https://doi.org/10.1177/0194599812463193
Spilsbury JC, Storfer-Isser A, Rosen CL, Redline S (2015) Remission and incidence of obstructive sleep apnea from middle childhood to late adolescence. Sleep 38:23–29. https://doi.org/10.5665/sleep.4318
Chan KC, Au CT, Hui LL, Ng SK, Wing YK, Li AM (2019) How OSA evolves from childhood to young adulthood: natural history from a 10-year follow-up study. Chest 156:120–130. https://doi.org/10.1016/j.chest.2019.03.007
Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, Lee A et al (2018) The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA 319:1444–1472. https://doi.org/10.1001/jama.2018.0158
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Nicolas S Poupore, Hussein Smaily, James D Sullivan, and Calvin W Myint. The first draft of the manuscript was written by Nicolas S Poupore, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of the Medical University of South Carolina approved this study.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poupore, N.S., Smaily, H., Sullivan, J.D. et al. Is there an OAHI or O2 nadir that predicts the need for preoperative echocardiogram prior to adenotonsillectomy for children with severe obstructive sleep apnea?. Sleep Breath 28, 411–418 (2024). https://doi.org/10.1007/s11325-023-02910-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-023-02910-4