Abstract
Purpose
Sleep-disordered breathing (SDB) is associated with hypertension, poor glycemic control and dyslipidemia. Usually, apnoea events tend to be more prominent during rapid eye movement (REM) sleep than non-REM (NREM) sleep. We examined which SDB parameters are associated with blood pressure (BP), HbA1c and lipid profile in patients with type 2 diabetes (T2D).
Methods
A total of 185 patients with T2D who underwent polysomnography were analysed. Exclusion criteria were: the presence of pulmonary diseases, central sleep apnoea, treated SDB, or REM sleep < 30 min. To predict BP, HbA1c, and lipid profiles, we performed multiple linear regression analyses adjusted for known risk factors. Subsequently, we performed multivariable logistic regression analyses.
Results
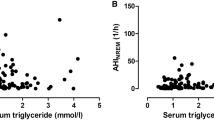
Patient characteristics (mean ± standard deviation/median) were as follows: age 58.0 ± 11.8 years, body mass index 26.0 kg/m2 (24.1–28.9 kg/m2 ), systolic BP 134 ± 19 mmHg, mean BP 98 ± 14 mmHg, HbA1c 7.4% (6.8–8.4%), triglyceride 143 mg/dL (97–195 mg/dL), non-high density lipoprotein (non-HDL) cholesterol 143 mg/dL (120–163 mg/dL), REM-apnoea–hypopnea index (AHI) 35.1/h (21.1–53.1/h). The analyses revealed that REM-AHI was independently associated with systolic and mean BP, whereas NREM-AHI was not. A statistically significant association was not observed between REM-AHI and HbA1c or lipid profile.
Conclusion
In patients with T2D, REM-AHI was associated with systolic and mean BP. The alteration of BP, associated with SDB during REM sleep, may be an important pathophysiological link between SDB and cardiovascular diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous studies reported that about 58 to 86% of type 2 diabetes mellitus (T2D) patients have obstructive sleep apnoea (OSA) [1]. The severity of sleep-disordered breathing (SDB) is related to metabolic syndrome parameters in diabetic patients, such as blood pressure (BP), diabetes mellitus and dyslipidemia [2,3,4]. Additionally, SDB is associated with cardiovascular events [5].
It has been shown that sympathetic activity is more aroused during rapid eye movement (REM) sleep than non-REM (NREM) sleep [6]. Furthermore, apnoea events during REM sleep are more severe than during NREM sleep [7]. However, currently, it is impossible to distinguish REM from NREM sleep with portable sleep tests. Previous studies assessing Caucasian patients with and without diabetes mellitus have associated REM-apnoea–hypopnea index (AHI) with hypertension [8, 9].
However, to date, there are limited studies assessing the relationship between SDB during REM sleep and metabolic syndrome parameters in T2D patients. Furthermore, to the best of our knowledge, there has been no study on the comprehensive assessment of metabolic syndrome parameters in diabetic patients. The present study aimed to reveal which SDB parameters had a higher correlation with metabolic syndrome parameters in Japanese T2D patients.
Methods
Patients
The present report was a single centre, cross-sectional study. We enrolled 528 T2D patients who underwent polysomnography in our hospital between May 2005 and June 2013. The diagnosis of diabetes was made according to the criteria released by the Japan Diabetes Society [10]. Patients treated with hypoglycaemic agents, with no history of type 1 or other types of diabetes, were also regarded as T2D patients. Exclusion criteria were as follows: patients missing essential data of polysomnography for analysis, insufficient information on T2D due to follow-up by other clinic, patients with diabetes other than T2D, pulmonary diseases and treated SDB, patients with REM sleeping hours shorter than 30 min when undergoing polysomnography, cases who underwent diagnostic polysomnography and CPAP titration in one night, a total sleep time < 60 min, presence of hypothyroidism, diagnosis of chronic kidney diseases (eGFR < 30 or on haemodialysis), past medical history of heart failure and atrial fibrillation, and recent history of cerebral vascular accidents.
Due to the unacknowledged nature of the data, the informed consent was obtained through an opt-out policy. The institutional review board of Toranomon Hospital approved the study protocol.
Polysomnography
We performed sleep tests with a digital polygraph (SomnoStar α Sleep System; SensorMedics Corp., Yorba Linda, CA) monitored by sleep technicians. An AHI was determined based on the number of apnoea and hypopnea events per hour during sleeping time. Apnoea was defined as a > 90% reduction of airflow for ≥ 10 s. Hypopnea was described as a > 30% reduction of airflow for ≥ 10 s with desaturation or arousal [11,12,13]. A REM-AHI was defined as an AHI during REM sleep.
Definitions and data collection
The following information and laboratory data were collected from the medical records: HbA1c, fasting plasma glucose and lipid profile, BP (taken the morning after polysomnography was performed), T2D’s duration, height, weight, medications, past medical histories and so on. The glycated haemoglobin (HbA1c) value was assessed as a National Glycohemoglobin Standardisation Program equivalent value. To this end, the following formula was used: HbA1c (%) = 1.02 × HbA1c (Japan Diabetes Society) (%) + 0.25(%) [14]. Finally, lipid profiles were calculated with a commercially available measurement system.
Statistical analysis
Continuous variables were described as mean ± standard deviation or median plus interquartile ranges. The relationship between SDB and cardiovascular disease (CVD) risk factors, such as BP, was assessed by determining dependent and independent variables. Specifically, when assessing the association between SDB and hypertension, the systolic, diastolic and mean BPs were set as independent variables. In the latter analyses, dependent variables included the following: age, sex, body mass index (BMI), use of sleeping pills and smoking history. When assessing hyperglycaemia, HbA1c was set as an independent variable. The dependent variables in this analysis included the following: age, sex, BMI, use of sleeping pills, T2D’s duration and use of insulin. Lipid profile assessment, non-high density lipoprotein cholesterol (non-HDL-C), triglyceride (TG) and high density lipoprotein cholesterol (HDL-C) were described as independent variables. In these analyses, dependent variables included the following: age, sex, BMI and the use of sleeping pills and statin.
For dependent variables, we used multiple linear regression analysis. Of note, non-normal distributed numbers were logarithmically transformed. Specifically, T2D’s duration was logarithmically transformed after adding 0.01 to calculate the zero value. Finally, four multivariate models were used after adjusting for multiple dependent variables as follows: (1) model 1 included REM-AHI, (2) model 2 included NREM-AHI, (3) model 3 included both REM-AHI and NREM-AHI, (4) model 4 included AHI. Additionally, we conducted a multivariable logistic regression analyses with target values (systolic BP < 130 mmHg, HbA1c < 7%, non-HDL-C < 150 mg/dL, TG < 150 mg/dL and HDL-C > 40 mg/dL), which were recommended by the Japan Diabetes Society [15]. Furthermore, we examined REM-AHI as categories with stratification into quartiles, calculating odds ratio and 95% confidence intervals. All data were analysed with Dr. SPSS II for Windows (SPSS Japan Inc., Tokyo, Japan), and p values < 0.05 were considered statistically significant.
Results
Of the 528 patients who underwent polysomnography during the research period, 185 were included in analyses (Fig. 1). The clinical characteristics of our cases are described in Table 1. Table 2 shows the results of the multiple linear regression analyses. We observed that REM-AHI was associated with systolic and mean BP (β = 0.18; p = 0.18) while NREM-AHI or AHI was not. In contrast, REM-AHI was not associated with HbA1c or lipid profile. When BP, HbA1c or lipid profile were regarded as binary independent variables, logistic regression analyses revealed no statistically significant association between binary BP, HbA1c or lipid profile and REM-AHI, NREM-AHI or AHI.
Discussion
The current study demonstrated that, in multiple linear regression analyses, REM-AHI was independently associated with systolic and mean BP, while NREM-AHI or AHI was not. Additionally, REM-AHI was not associated with HbA1c, non-HDL-C, TG or HDL-C.
Previous studies reported the association between SDB and hypertension [2, 16]. For instance, Marin et al. showed that OSA’s presence was associated with an increased adjusted risk of incident hypertension vs. participants without OSA [2]. Two additional studies associated REM-AHI with hypertension’s presence or onset, while NREM-AHI was not [8, 9]. In multiple linear regression analyses, our study showed a statistically significant association between continuous systolic BP or mean BP and REM-AHI (Table 3). On the contrary, the logistic regression analyses indicated no association between the presence of hypertension and REM-AHI. Similarly, no association was revealed between continuous BP and AHI. Such findings may be explained by some factors such as the percentage of patients taking anti-hypertensive drugs, the definition of hypertension and patient characteristics limited to T2D patients.
Several mechanisms may link REM-AHI and BP. However, we believe that the critical mechanism is the difference in sympathetic activity between REM and non-REM sleep. SDB plays a role as a cardiovascular risk factor by boosting sympathetic activity through its effect on BP and heart rate [17, 18]. Of note, sympathetic activity is more aroused during REM than NREM sleep [6]. Additionally, the hypoglossal nerve’s inhibition during REM sleep exacerbates the upper airways’ obstruction [19]. Therefore, SDB events during REM sleep may be more critical than those during NREM sleep as a risk factor related to hypertension.
A large body of literature suggests an independent association between SDB and glycemic control. Specifically, the previous meta-analysis associated SDB with the development of diabetes [20]. In T2D patients, there is a robust, graded relationship between OSA’s severity and HbA1c [21]. Furthermore, in their report, Grimaldi et al. showed an independent association between REM-AHI and increasing levels of HbA1c, contrary to NREM-AHI [22]. Our study, however, did not show a statistically significant association between AHI, REM-AHI or NREM-AHI and HbA1c. We believe that these results may be because, as opposed to Grimaldi et al.’s study, where all patients were under stable glycemic control in the previous 3 months [22], individuals in the present study were not. As a consequence, patients may have been analysed under fluctuating glycemic control. Another study showed a significant association between NREM-AHI and HbA1c level in total and non-diabetic individuals, but not T2D patients [23]. This finding is in agreement with our study, showing no independent association between HbA1c and REM or NREM-AHI in patients with T2D. Patients’ characteristics (whether non-diabetic patients were included or not) and SDB’s severity might affect the results.
A previous report demonstrated that AHI was associated with HDL-C but not with total cholesterol, TG or LDL-C. Sympathetic activities are thought to be a mechanism linking OSA and lipid profile through some studies focusing on α- and β-adrenoceptor blockers [4]. Another previous article showed the association between NREM-AHI and lipid profile in patients with and without T2D [24]. Importantly, to date, there have been few reports assessing the relationship between REM-AHI and lipid profile. We did not observe an association between AHI, REM-AHI, NREM-AHI and lipid profile. Of note, we believe that our results may have been affected by the fact we included more patients being treated for dyslipidemia than the previous study and by the fact patients with T2D were more likely to suffer from hypertriglyceridemia.
The present study has several limitations. First, we were unable to remove the selection bias thoroughly because this was a single centre, cross-sectional study. Future cohort or intervention studies are needed. Second, the small sample size of our study may limit the associations between SDB and CVD risk factors. Therefore, additional investigations with a larger sample size are required. Third, since only T2D patients were selected for the analysis, we are unsure whether or not the results of our study can be applied to the general population. Lastly, we used BP data measured at one time point. Considering BP’s fluctuation, using BP data taken at several time points or ambulatory blood pressure monitoring may need to be investigated.
In conclusion, the present study has three strengths. First, we used polysomnography rather than home sleep apnoea testing. Second, it shows an independent association between continuous BP value and REM-AHI, adjusted by multiple risk factors. Third, we demonstrated comprehensive analyses for assessing association between REM-AHI and several cardiovascular risk factors in the same sample. We believe that this study provides further evidence that REM-AHI is associated with systolic and mean BP in T2D patients.
Data availability
The data were collected by the authors in Toranomon Hospital.
References
Pamidi S, Tasali E (2012) Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol 3:126. https://doi.org/10.3389/fneur.2012.00126
Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbé F, Vicente E, Wei Y, Nieto FJ, Jelic S (2012) Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 307:2169–2176. https://doi.org/10.1001/jama.2012.3418
Punjabi NM, Beamer BA (2009) Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med 179:235–240. https://doi.org/10.1164/rccm.200809-1392OC
Börgel J, Sanner BM, Bittlinsky A et al (2006) Obstructive sleep apnoea and its therapy influence high-density lipoprotein cholesterol serum levels. Eur Respir J Off J Eur Soc Clin Respir Physiol 27:121–127. https://doi.org/10.1183/09031936.06.00131304
Marin JM, Carrizo SJ, Vicente EAA (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365:1046–1053. https://doi.org/10.1016/S0140-6736(05)71141-7
Somers VK, Dyken ME, Mark AL, Abboud FM (1993) Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328:303–307. https://doi.org/10.1056/NEJM199302043280502
Findley LJ, Wilhoit SC, Suratt PM (1985) Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest 87:432–436
Appleton SL, Vakulin A, Martin SA, Lang CJ, Wittert GA, Taylor AW, McEvoy RD, Antic NA, Catcheside PG, Adams RJ (2016) Hypertension is associated with undiagnosed OSA during rapid eye movement sleep. Chest 150:495–505. https://doi.org/10.1016/j.chest.2016.03.010
Mokhlesi B, Finn LA, Hagen EW, Young T, Hla KM, van Cauter E, Peppard PE (2014) Obstructive sleep apnea during REM sleep and hypertension: results of the Wisconsin sleep cohort. Am J Respir Crit Care Med 190:1158–1167. https://doi.org/10.1164/rccm.201406-1136OC
Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus, Seino Y, Nanjo K et al (2010) Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 1:212–228. https://doi.org/10.1111/j.2040-1124.2010.00074.x
Ikeda Y, Kasai T, Kawana F, Kasagi S, Takaya H, Ishiwata S, Narui K (2012) Comparison between the apnea-hypopnea indices determined by the REMstar Auto M series and those determined by standard in-laboratory polysomnography in patients with obstructive sleep apnea. Intern Med 51:2877–2885. https://doi.org/10.2169/internalmedicine.51.8249
Ueno K, Kasai T, Brewer G, Takaya H, Maeno KI, Kasagi S, Kawana F, Ishiwata S, Narui K (2010) Evaluation of the apnea-hypopnea index determined by the S8 auto-CPAP, a continuous positive airway pressure device, in patients with obstructive sleep apnea-hypopnea syndrome. J Clin Sleep Med 6:146–151
Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep Medicine (2012) Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 8:597–619. https://doi.org/10.5664/jcsm.2172
Kashiwagi A, Kasuga M, Araki E et al (2012) International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. Diabetol Int 3:8–10. https://doi.org/10.1007/s13340-012-0069-8
Tajima N, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, Goto A, Fujimoto K, Sakamoto M, Haneda M (2015) Evidence-based practice guideline for the treatment for diabetes in Japan 2013. Diabetol Int 6:151–187. https://doi.org/10.1007/s13340-015-0206-2
Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered. N Engl J Med 342:1378–1384
Palatini P, Julius S (2009) The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr Hypertens Rep 11:199–205
Kohler M, Stradling JR (2012) CrossTalk proposal: most of the cardiovascular consequences of OSA are due to increased sympathetic activity. J Physiol 590:2813–2815. https://doi.org/10.1113/jphysiol.2012.229633
Grace KP, Hughes SW, Horner RL (2013) Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med 187:311–319. https://doi.org/10.1164/rccm.201209-1654OC
Wang X, Bi Y, Zhang Q, Pan F (2013) Obstructive sleep apnoea and the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Respirology 18:140–146. https://doi.org/10.1111/j.1440-1843.2012.02267.x
Aronsohn RS, Whitmore H, Van Cauter E, Tasali E (2010) Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med 181:507–513. https://doi.org/10.1164/rccm.200909-1423OC
Grimaldi D, Beccuti G, Touma C, van Cauter E, Mokhlesi B (2014) Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care 37:355–363. https://doi.org/10.2337/dc13-0933
Kurosawa H, Saisho Y, Fukunaga K et al (2018) Association between severity of obstructive sleep apnea and glycated hemoglobin level in Japanese individuals with and without diabetes. Japan Endocr Soc 65:121–127
Xu H, Xia Y, Li X, Qian Y, Zou J, Fang F, Yi H, Wu H, Guan J, Yin S (2020) Association between obstructive sleep apnea and lipid metabolism during REM and NREM sleep. J Clin Sleep Med 16:475–482. https://doi.org/10.5664/jcsm.8242
Acknowledgements
We thank Fumie Takano for her work of data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The institutional review board of Toranomon Hospital approved the study protocol.
Consent to participate
The informed consent was obtained through an opt-out policy.
Consent to publication
The informed consent was obtained through an opt-out policy.
Code availability
Dr. SPSS II for Windows (SPSS Japan Inc., Tokyo, Japan).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uchida, T., Nishimura, A., Kasai, T. et al. Relationship between obstructive sleep apnoea during rapid eye movement sleep and metabolic syndrome parameters in patients with type 2 diabetes mellitus. Sleep Breath 25, 309–314 (2021). https://doi.org/10.1007/s11325-020-02129-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-020-02129-7