Abstract
Purpose
Obstructive sleep apnoea (OSA) involves recurrent obstructive apnoeas and hypopnoeas which cause cyclic hypoxia, reoxygenation and formation of reactive oxygen species (ROS). We aimed to investigate a member of the nicotinamide adenine dinucleotide phosphate oxidase (NOX) family of enzymes, specifically (NOX4), not previously studied in humans, as well as 8-OHdG/106dG, MDA and IMA, which are known to be associated with oxidative stress. We also evaluated these parameters in predicting the presence and severity of OSA.
Methods
All 120 subjects (90 with OSA, 30 healthy controls) underwent polysomnography and had blood serum samples taken at the same time of day. Subjects were grouped by presence and severity of OSA, and serum markers were compared among groups.
Results
Age and body mass index were not significantly different among groups. In the OSA group, the levels of NOX4, IMA, MDA and 8-OHdG/106dG were significantly higher than in the healthy control group. NOX4 and other parameters were positively correlated with the severity of OSA. For all parameters, the highest levels were detected in patients with severe OSA.
Conclusions
The repeated hypoxia of OSA is associated with increases in the serum levels of inflammatory mediators such as MDA, IMA and 8-OHdG/106dG and the ROS NOX4. In this study, NOX4 and other markers were associated with the presence and severity of OSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnoea (OSA) is a disease that is characterized by repetitive obstructive apnoea and hypopnoea caused by recurrent collapse of the upper respiratory tract during sleep. In the clinical course of OSA, there is a significant reduction or pause in airflow despite the respiratory effort during sleep. Additionally, snoring, oxyhemoglobin desaturation and recurrent arousal and fragmented sleep are also often seen in the course of OSA [1]. Excessive daytime sleepiness is responsible for impaired quality of life and neurocognitive performance in patients. It is also a chronic disease associated with significant morbidity, especially in the cardiometabolic functional area, and mortality [2]. OSA affects at least 2–4% of the adult population, mainly older adults, and its prevalence has been reported to exceed 30% in patients aged 65 years and older [3].
OSA tends to cause episodic hypoxia and reoxygenation that can lead to ischemia/reperfusion injury (I/R) [4]. Reoxygenation after hypoxia leads to the formation of the reactive oxygen species (ROS). Therefore, many studies investigated the relationship between OSA and oxidative stress (OS) [5]. Several apnoea markers were investigated in these studies, including malondialdehyde (MDA, the end product of lipid peroxidation), 8-hydroxy 2-deoxyguanosine (8-OHdG, an indicator of oxidative damage of deoxyribonucleic acid (DNA)), as well as activities of the antioxidant enzymes (e.g., superoxide dismutase and glutathione peroxidase). It was found that the markers of oxidative damage were significantly higher in patients with OSA [6]. Ischemia-modified albumin (IMA) is a modified form of human serum albumin (HSA) formed under the OS with a diminished capacity to bind metal ions compared to the normal HSA [7]. In recent studies, a close relationship between increased IMA levels and OSA was reported. However, the clinical use of IMA in OSA has not been established yet [8].
Nicotinamide adenine dinucleotide phosphate oxidase (NOX) enzyme family catalyses the oxidation of NADPH and has seven members (NOX1–5 and DUOX1 and 2). The increase in the concentration of these enzymes is thought to be responsible for the production of endogenous ROS [9]. NOX1, and especially NOX4, are the primary source of ROS productions in the kidney-mediating redox signalling in the presence of chronic intermittent hypoxia (CIH) [10]. In a rat model, NOX4 activity was reported to play an important role in the pathogenesis of CIH-induced hypertension; thus, it has been claimed that the inhibition of this enzyme can be used clinically [11]. However, we could not find a published study evaluating NOX4 activity in OSA patients.
Currently, polysomnography (PSG) is the gold standard for OSA classification, used to diagnose and determine its severity. However, an assessment based solely on apnoea-hypopnoea index (AHI) may not be sufficient to determine I/R injury that may occur in patients with OSA as a result of repeated hypoxia/reoxygenation. Therefore, there is a need for biomarkers to assist in determining I/R injury in patients with OSA. We aim to evaluate the levels of NOX4, MDA, IMA and 8-OHdG/106dG in individuals with OSA classified by the AHI index, to examine the possible relationship of these parameters with OSA severity and to compare these results with healthy control individuals. These results may give information about the extent of I/R injury that may occur as a result of recurrent hypoxia/reoxygenation in these patients.
Material and methods
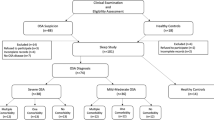
The study was carried out on 90 patients admitted to the sleep unit of Van Yuzuncu Yil University Faculty of Medicine, Chest Diseases Department, accepted to participate in the study and diagnosed with OSA. Thirty healthy volunteers participated in the study; they have no chronic inflammatory disease, no hospitalizations within the last 6 months and were not on any continuous medical or herbal medication. The volunteers had no OSA disease as determined by PSG.
Sleep study
The scoring and definition of OSA were performed according to the 2018 AASM manual using the following parameters, detected with a digital 16-channel Embla (Medicare, Reykjavik, Iceland) device. Sleep monitoring with airflow through a nasal pressure transducer associated with thermistor signals, pulse oximetry, arterial oxygen saturation, electrocardiography and abdominal and thoracic bands’ breathing efforts. Sleep staging was done with an electroencephalogram (EEG), electrooculogram and submental and pretibial electromyography (EMG) signals. Apnoea was defined by a > 90% reduction in the thermistor signal of the airflow for ≥ 10 s. Hypopnoea was defined as a reduction of sat 3% in oxygen saturation or > 50% reduction in the nasal pressure signal lasting ≥ 10 s with an associated decrease in EEG stimulation. An event was defined as obstructive in the presence of respiratory effort and centrally if such effort was absent. The AHI was defined as the number of obstructive and central apnoea and hypopnoea in 1 h of sleep and was calculated by dividing the total number of events by the total sleep time. The patients were divided into three groups according to the apnoea-hypopnoea index: severe (AHI > 30), moderate (15 < AHI < 30) and mild (5 < AHI < 15).
Analysis of study parameters
Blood samples (5 ml) from the patients and healthy volunteers were divided into serum (3 ml of blood per dry biochemistry tube with the gel) and whole blood (2 ml of blood per tube with ethylenediaminetetraacetic acid as the anticoagulant). The serum samples were obtained by centrifugation at 3500×g for 10 min. Serum and whole blood samples were kept at − 80 °C.
NOX4 and IMA analyses were performed by commercially available kits using enzyme-linked immunosorbent assay (YLbiont ELISA kits from Shanghai, Chine). DNA from leukocytes in the whole blood sample was isolated by a DNA isolation kit (Invitrogen Purelink Genomic DNA Mini kit, ThermoFisher Scientific, CA, USA). The isolated DNA was hydrolysed with formic acid [12] and analysed by high-performance liquid chromatography (HPLC Agilent 1200 Series system, Agilent Technologies, Waldbronn, Germany) with an electrochemical detector to determine 8-OHdG and dG concentrations as previously described [13].
MDA was determined by the HPLC system as described by Khoschsor et al. [14]. The 50-μl serum sample, H3PO4, (0.44 M, 0.75 mL) and a certain concentration of thiobarbituric acid (42 mM, 0.25 mL) were combined and incubated in a boiling water bath for 60 min. Then, 1500 μl of alkaline methanol (50 mL MeOH+4.5 mL 1 M NaOH) was added, and the tubes were centrifuged at 3000×g for 3 min. After adding the supernatant colour complex to the HPLC system, MDA concentrations were determined by an ultraviolet detector (532 nm). Analysis of MDA and oxidative DNA damage was performed by HPLC method. The HPLC system consists of the autosampler, column flame, gradient pump, fluorescent detector and electrochemical detector (Agilent 1200 series from USA).
Statistical analysis
Statistical analyses were performed using SPSS 22.0 package program (IBM Corp., Armonk, N.Y., USA). Normality analysis of the data was performed by the Kolmogorov-Smirnov test. Independent sample t test was used to compare the normally distributed data. One-way ANOVA test was used to compare multiple numerical data. Mann-Whitney U and Kruskal-Wallis tests were used for the analysis of numerical data of multiple groups that did not conform to normal distribution. Additionally, the ROC curves determined with Medcalc (15.6.1) software program (MedCalc Software, Mariakerke, Belgium) were used to examine the ability of parameters to differentiate between OSA and healthy control groups. A p value less than 0.05 was considered statistically significant. The sample size was based upon the original observations of Sunnetcioglu et al. [15]. We recruited the 90 individuals for the OSA patient group and 30 individuals for healthy control group based on the power calculation of 95% power with a 5% type I error level to detect a minimal clinically significant difference.
Results
In our study, the mean of age in the OSA and healthy control groups was 50.9 ± 13.2 and 45.9 ± 11.3, respectively. There was no significant difference between the OSA and healthy control groups in terms of age (p = 0.129). When we compared the age of patients with mild, moderate and severe OSA and the healthy control group (48.4 ± 13.2, 49.5 ± 13.5, 54.2 ± 12.9 and 45.9 ± 11.3, respectively), there was no significant difference between groups (p = 0.191). In terms of gender, there was no statistically significant difference between patients with mild (10 females, 20 males), moderate (13 females, 17 males) and severe (12 females, 18 males) OSA, and the healthy volunteers (14 females, 16 males) (p = 0.751). The level of NOX4 in the OSA group was significantly higher compared to the healthy control group (p < 0.001). Also, the level of IMA in the OSA group was significantly higher than in the healthy control group (p < 0.001). When we investigated the lipid peroxidation and oxidative DNA damage levels, we found that the levels of MDA and 8-OHdG/106dG in OSA were significantly higher than in the healthy control group. There was no significant difference in BMI between the OSA and the healthy control groups (Table 1).
When the OSA group was classified as severe, moderate and mild, we found the highest level of NOX4 in the severe OSA group. The level of NOX4 in the mild OSA group was higher than that in the healthy control group, but not statistically significant. In addition, there was no significant difference in NOX4 levels between the mild and moderate groups. The level of NOX4 in the severe OSA group was significantly higher than in the other groups. Similarly, the level of MDA in the severe OSA group was significantly higher than in the other groups. The level of MDA in the moderate OSA group was significantly higher than the mild OSA group. There was no significant difference in MDA levels between the mild OSA and the healthy control groups. The highest and the lowest 8-OHdG/106dG levels were detected in the severe OSA and the healthy control groups, respectively. The 8-OHdG/106dG levels were significantly different in each group. The upward trend of IMA in the groups was similar to the MDA. The level of IMA in the severe OSA group was significantly higher than the other groups. The level of IMA in the moderate OSA group was significantly higher than in the mild OSA group. There was no significant difference in IMA levels between the mild OSA and healthy control groups (Table 2).
We performed ROC analysis in all study populations to determine OSA and healthy condition. The AUC values for 8-OHdG/106dG, IMA, MDA and NOX4 were 0.927, 0.914, 0.979 and 0.823, respectively, and no significant difference was observed (Fig. 1). In the ROC analysis, NOX4 was able to distinguish between the OSA and health status with AUC 0.823. We summarized sensitivity and specificity values in Table 3.
For the severe, moderate and mild OSA groups, the highest sensitivity and specificity values were (96%, 100%), (88%, 80%) and (60%, 70%), respectively (Fig. 2).
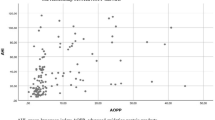
We found a statically significant correlation between the levels of AHI and NOX4 (r = 0.751, p < 0.001). The scatter plot of this correlation is shown in Fig. 3.
Discussion
OSA is a disease that can cause metabolic abnormalities and is characterized by occlusion of the upper airways during the sleep breathing with repeated pauses and increased drowsiness during the day. It is often associated with a decrease in blood oxygen saturation. In experimental studies, intermittent hypoxia has been reported to cause cell damage [5, 9, 16]. Reoxygenation after hypoxia leads to the formation of the regenerative oxygen radicals. For this reason, many studies have been conducted on OSA patients and OS [17, 18]. OS, systemic inflammation and endothelial dysfunction have been reported to play a role in the pathogenesis of OSA [19]. Therefore, in this study, we analysed 8-OHdG/106dG, which is a marker of DNA damage, MDA and IMA, known to be associated with OS, and NOX4 that has not been studied in humans before.
The function of NOX4, which is a family member of NAD(P)H oxidases producing ROS, has not been fully understood and remains to be explained. NOX4 has been associated with an increase in endogenous ROS production [9, 20]. In the current study, NOX4 was significantly increased in the serum of OSA patients, and this increase was positively correlated with the AHI index. NOX4 levels were significantly higher in the patients with severe and moderate OSA compared to those with mild OSA and the healthy control group. This finding supports the results of Bedard et al. [9] regarding the increase in reactive oxygen radical production and NOX4 due to recurrent hypoxia. Previous studies have shown that night-time hypoxia causes endothelial damage, and this damage was frequently seen in severe OSA [16, 20, 21]. In experimental studies, OS conditions occur under artificial hypoxia and hyperglycaemia, contributing to the formation of NOX4 [20, 22, 23]. During OSA, recurrent hypoxia constitutes OS conditions in relation to the AHI index, which is known to correlate with the severity of OSA [3]. Patients with the high AHI index are expected to develop more frequent and recurrent severe hypoxic conditions. While an association between the NOX4 and OS conditions has been reported in experimental or animal studies [11], this association remains unexplored in patients with OSA. Therefore, ours is the first study examining the association between NOX4 and severity of OSA in humans. We found that NOX4 levels were significantly increased in serum of patients with severe and moderate OSA, supporting previous experimental studies reporting an increase of NOX4 under OS conditions. Also, the low levels of NOX4 in the mild OSA and healthy control groups compared to the moderate and severe OSA groups support our conclusion that NOX4 production is associated with the severity of recurrent hypoxia in OSA.
Many studies have explored MDA, another indicator of the cellular OS [6, 24, 25]. MDA is the last lipid oxidation product, caused by ROS, which attacks the cell membrane and the electron transport chain [24, 25]. However, there are also opposite results reported in the MDA studies. For example, Sunnetcioglu et al. reported a significant increase in serum MDA in OSA and lung cancer patients compared with healthy controls in a prospective and case-cross-sectional study [15]. Similarly, Tichanon P et al. showed that MDA serum levels in OSA patients were significantly higher than those of the control group, and this high MDA serum level decreased significantly after treatment [26]. Nevertheless, in a rat-based animal model, Kang et al. found no association of OSA with DNA damage measured by comet assays or OS according to serum MDA levels [6]. In their model, an OS condition is induced by artificial hypoxia, similar to the course of OSA, and a significant increase in serum MDA levels was observed in rats. In their study, the fact that MDA was significantly increased in all patient groups compared to the control group supports previous studies’ findings and our study’s findings that revealed the relationship between MDA and OSA. Therefore, we believe that measuring the serum level of MDA can be an important indicator of the OSA severity useful for monitoring response to treatment.
IMA is a modified form of HSA formed under OS conditions; it has a lower capacity to bind metal ions than HSA [8]. It is known that IMA levels in circulating serum increase within minutes after the onset of OS, and this increase lasts up to 24 h [27]. Although IMA has been shown to play a potential role in clinical diseases other than OSA, the effect of OSA and apnoea-induced hypoxia on IMA has not been fully established until recently. However, several studies have shown that OSA may have an impact on IMA [15]. In a meta-analysis, Varikasuvu SR et al. [8] showed that OSA was associated with a significant increase in IMA levels, and this increase was more prominent in patients with severe OSA compared to those with mild to moderate OSA. Similar to this result, the IMA was found to be significantly increased in our OSA patients. However, unlike the results of this meta-analysis, we found that the increase occurred not only in patients with severe OSA but also in patients with moderate OSA. Therefore, our study shows that IMA levels are related to OSA severity and may be an indicator of OS and ischaemic damage resulting from recurrent hypoxia.
Previous studies reported that 8-OHdG could be used to evaluate the oxidative DNA damage [28, 29]. However, there are different insights about the use of 8-OHdG as a marker of OS. Sunnetcioglu et al. [30] compared serum levels of 8-OHdG in patients with OSA and COPD, in lung cancer patients and healthy individuals; they found significant increases only for cancer patients. In their study, no significant difference of 8-OHdG levels was found between COPD, OSA and lung cancer patients. Similar to our result, they found that serum 8-OHdG levels were elevated in OSA patients, although this increase was not significant compared with the control group. In our study, 8-OHdG/106dG showed oxidative damage in leukocyte DNA, but Sunneticoglu et al. analysed the 8-OHdG level in serum samples. This difference may be a reason for the differences between the results of the two studies. While Chen CY et al. [31] found no significant increase in 8-OHdG levels in OSA patients, the analysis was done in urine samples, potentially accounting for the observed difference. Wang et al. [29] reported significantly higher levels of 8-OHdG urine of inpatients with OSA compared to those without, and their results are similar to ours despite the sample difference. Dayyat EA et al. [32] observed significantly elevated 8-OHdG in rodents exposed to recurrent hypoxia and OS, as seen in patients with OSA in our study. Elevated 8-OHdG is associated with cardiovascular diseases, chronic inflammatory diseases and cancer risk [33, 34]. Increased 8-OHdG/106dG in OSA patients may play a role in the development of severe conditions, e.g., cardiovascular diseases and cancer, because modified bases in DNA induce alteration in transcription and translation of encoded enzymes and hormones. Therefore, our results regarding oxidative DNA damage are important. Additionally, 8-OHdG may be used as an indicator of the presence and severity of OSA.
Results of the study indicate that a relationship exists between OSA-related repetitive hypoxia/reoxygenation and biomarkers of oxidative stress. One limitation of this study is a significant difference between the sample size in the patient and control groups. Another limitation is a non-consecutive enrolment of participants, so selection bias might exist.
Conclusions
In conclusion, this study is the first report to suggest that NOX4 can be used as an indicator of OS in patients with OSA. Additionally, we think that the investigated parameters are associated with OSA severity. We believe that these parameters can be used as predictive markers for the severity of OSA. Further studies are needed to investigate the effects of NOX4 inhibition on OS parameters in experimental animal-based models.
References
Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL (2011) Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 306(6):613–619. https://doi.org/10.1001/jama.2011.1115
Bruyneel M (2019) Telemedicine in the diagnosis and treatment of sleep apnoea. Eur Respir Rev 28(151):180093. https://doi.org/10.1183/16000617.0093-2018
Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, Weinstein MD, Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep M (2009) Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 5(3):263–276
Jordan W, Cohrs S, Degner D, Meier A, Rodenbeck A, Mayer G, Pilz J, Ruther E, Kornhuber J, Bleich S (2006) Evaluation of oxidative stress measurements in obstructive sleep apnea syndrome. J Neural Transm 113(2):239–254. https://doi.org/10.1007/s00702-005-0316-2
Lavie L (2003) Obstructive sleep apnoea syndrome - an oxidative stress disorder. Sleep Med Rev 7(1):35–51. https://doi.org/10.1053/smrv.2002.0261
Kang IG, Jung JH, Kim ST (2013) The effect of obstructive sleep apnea on DNA damage and oxidative stress. Clin Exp Otorhinolar 6(2):68–72. https://doi.org/10.3342/ceo.2013.6.2.68
Turedi S, Gunduz A, Mentese A, Karahan SC, Yilmaz SE, Eroglu O, Nuhoglu I, Turan I, Topbas M (2007) Value of ischemia-modified albumin in the diagnosis of pulmonary embolism. Am J Emerg Med 25(7):770–773. https://doi.org/10.1016/j.ajem.2006.12.013
Varikasuvu SR, Dutt N, Sahu D (2019) Obstructive sleep apnea and the effect of CPAP treatment on ischemia-modified albumin levels: a multi effect size meta-analysis with diagnostic test accuracy. Sleep Breath 23(1):179–191. https://doi.org/10.1007/s11325-018-1679-6
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313. https://doi.org/10.1152/physrev.00044.2005
Rubattu S, Pagliaro B, Pierelli G, Santolamazza C, Di Castro S, Mennuni S, Volpe M (2015) Pathogenesis of target organ damage in hypertension: role of mitochondrial oxidative stress. Int J Mol Sci 16(1):823–839. https://doi.org/10.3390/ijms16010823
Lu W, Kang J, Hu K, Tang S, Zhou XF, Xu LF, Li YY, Yu SH (2017) The role of the Nox4-derived ROS-mediated RhoA/Rho kinase pathway in rat hypertension induced by chronic intermittent hypoxia. Sleep Breath 21(3):667–677. https://doi.org/10.1007/s11325-016-1449-2
Kaur H, Halliwell B (1996) Measurement of oxidized and methylated DNA bases by HPLC with electrochemical detection. Biochem J 318(Pt 1):21–23. https://doi.org/10.1042/bj3180021
Taken K, Alp HH, Eryilmaz R, Donmez MI, Demir M, Gunes M, Aslan R, Sekeroglu MR (2016) Oxidative DNA damage to sperm cells and peripheral blood leukocytes in infertile men. Med Sci Monit 22:4289–4296. https://doi.org/10.12659/MSM.898631
Khoschsorur GA, Winklhofer-Roob BM, Rabl H, Auer T, Peng Z, Schaur RJ (2000) Evaluation of a sensitive HPLC method for the determination of malondialdehyde, and application of the method to different biological materials. Chromotographia 52(3):181–184
Sunnetcioglu A, Asker S, Alp HH, Gunbatar H (2016) Increased asymmetric dimethylarginine and ischemia-modified albumin levels in obstructive sleep apnea. Respir Care 61(8):1038–1043. https://doi.org/10.4187/respcare.04472
Zhang F, Wu Y, Feng G, Ni X, Xu Z, Gozal D (2018) Polysomnographic correlates of endothelial function in children with obstructive sleep apnea. Sleep Med 52:45–50. https://doi.org/10.1016/j.sleep.2018.07.023
Tamisier R, Pepin JL, Remy J, Baguet JP, Taylor JA, Weiss JW, Levy P (2011) 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J 37(1):119–128. https://doi.org/10.1183/09031936.00204209
Dewan NA, Nieto FJ, Somers VK (2015) Intermittent hypoxemia and OSA: implications for comorbidities. Chest 147(1):266–274. https://doi.org/10.1378/chest.14-0500
Drummond GR, Selemidis S, Griendling KK, Sobey CG (2011) Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov 10(6):453–471. https://doi.org/10.1038/nrd3403
Munoz M, Lopez-Oliva ME, Rodriguez C, Martinez MP, Saenz-Medina J, Sanchez A, Climent B, Benedito S, Garcia-Sacristan A, Rivera L, Hernandez M, Prieto D (2019) Differential contribution of Nox1, Nox2 and Nox4 to kidney vascular oxidative stress and endothelial dysfunction in obesity. Redox Biol 28:101330. https://doi.org/10.1016/j.redox.2019.101330
Lee EJ, Alonso LC, Stefanovski D, Strollo HC, Romano LC, Zou B, Singamsetty S, Yester KA, McGaffin KR, Garcia-Ocana A, O'Donnell CP (2013) Time-dependent changes in glucose and insulin regulation during intermittent hypoxia and continuous hypoxia. Eur J Appl Physiol 113(2):467–478. https://doi.org/10.1007/s00421-012-2452-3
Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hebert RL (2010) Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299(6):F1348–F1358. https://doi.org/10.1152/ajprenal.00028.2010
Wang F, Bao Y, Shen X, Zengin G, Lyu Y, Xiao J, Weng Z (2019) Niazirin from Moringa oleifera Lam. attenuates high glucose-induced oxidative stress through PKCzeta/Nox4 pathway. Phytomedicine:153066. https://doi.org/10.1016/j.phymed.2019.153066
Wang Y, Chai Y, He X, Ai L, Sun X, Huang Y, Li Y (2017) Intermittent hypoxia simulating obstructive sleep apnea causes pulmonary inflammation and activates the Nrf2/HO-1 pathway. Exp Ther Med 14(4):3463–3470. https://doi.org/10.3892/etm.2017.4971
Tian XH, Liu CL, Jiang HL, Zhang Y, Han JC, Liu J, Chen M (2016) Cardioprotection provided by Echinatin against ischemia/reperfusion in isolated rat hearts. BMC Cardiovasc Disord 16:119. https://doi.org/10.1186/s12872-016-0294-3
Tichanon P, Wilaiwan K, Sopida S, Orapin P, Watchara B, Banjamas I (2016) Effect of continuous positive airway pressure on airway inflammation and oxidative stress in patients with obstructive sleep apnea. Can Respir J 2016:3107324–3107327. https://doi.org/10.1155/2016/3107324
Zhong Y, Wang N, Xu H (2013) Is there a dynamic change in ischemia-modified albumin in patients with obstructive sleep apnea, which often leads to ischemic diseases? Clinics 68(11):1474. https://doi.org/10.6061/clinics/2013(11)15
Cooke MS, Evans MD, Dizdaroglu M, Lunec J (2003) Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17(10):1195–1214. https://doi.org/10.1096/fj.02-0752rev
Wang SY, Luo J, Dong YF, Liu XY, Fan YL, Deng M, Chen DW, Li P, Cheng XS (2017) Risk of glomerular filtration rate decline in patients with hypertrophic cardiomyopathy and obstructive sleep apnoea. Sci Rep 7(1):17399. https://doi.org/10.1038/s41598-017-17818-9
Sunnetcioglu A, Alp HH, Sertogullarindan B, Balaharoglu R, Gunbatar H (2016) Evaluation of oxidative damage and antioxidant mechanisms in COPD, lung cancer, and obstructive sleep apnea syndrome. Respir Care 61(2):205–211. https://doi.org/10.4187/respcare.04209
Chen CY, Chen CL, Yu CC, Chen TT, Tseng ST, Ho CH (2015) Association of inflammation and oxidative stress with obstructive sleep apnea in ischemic stroke patients. Sleep Med 16(1):113–118. https://doi.org/10.1016/j.sleep.2014.07.027
Dayyat EA, Zhang SX, Wang Y, Cheng ZJ, Gozal D (2012) Exogenous erythropoietin administration attenuates intermittent hypoxia-induced cognitive deficits in a murine model of sleep apnea. BMC Neurosci 13:77. https://doi.org/10.1186/1471-2202-13-77
He F, Zuo L (2015) Redox roles of reactive oxygen species in cardiovascular diseases. Int J Mol Sci 16(11):27770–27780. https://doi.org/10.3390/ijms161126059
Pereira C, Gracio D, Teixeira JP, Magro F (2015) Oxidative stress and DNA damage: implications in inflammatory bowel disease. Inflamm Bowel Dis 21(10):2403–2417. https://doi.org/10.1097/MIB.0000000000000506
Author information
Authors and Affiliations
Contributions
SE contributed to the hypothesis, design, statistical analysis and drafting. HY and HHA contributed to data collection, design, statistical analysis and drafting. HY and HHA contributed to statistical analysis in the revision version of the manuscript and prepared the final version. SE and HHA managed the ethical approval process of the study. All the authors approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Informed consent
Informed consent was obtained from all participants included in the study.
Ethical approval
Prior to the initiation of the study, the non-invasive clinical ethics committee of Van Yuzuncu Yıl University was consulted, and approval (Approbation date: 31 December 2018; No: B.30.2.YYU.0.01.00.00/163) was obtained according to the ethical standards and 1964 Helsinki declaration.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ekin, S., Yildiz, H. & Alp, H.H. NOX4, MDA, IMA and oxidative DNA damage: can these parameters be used to estimate the presence and severity of OSA?. Sleep Breath 25, 529–536 (2021). https://doi.org/10.1007/s11325-020-02093-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-020-02093-2