Abstract
Study objectives
Although insomnia is common among cancer patients, its prevalence remains variable, and its risk factors and correlation with other cancer-related symptoms are not fully explored in the literature. This study aims to determine the prevalence and severity of insomnia as well as risk factors and sleep-related symptom clusters in a sample of cancer patients.
Methods
A cross-sectional survey was conducted collecting data from 213 cancer patients undergoing chemotherapy (age = 53.1 ± 11.3 years, 60% female). Insomnia was measured using the Insomnia Severity Index, a sleep log, and Actigraph, while symptoms were assessed using the Memorial Symptom Assessment Scale and the Hospital Anxiety and Depression Scale. Quality of life was measured with the Functional Assessment of Cancer Therapy—General.
Results
Of the participants, 42.8% reported insomnia, with 31.9% of those with insomnia reporting severe insomnia. Insomnia occurrence and severity were not correlated with the participants’ characteristics, cancer-related or treatment-related factors, only with the participants’ anxiety/depression scores. Principal component analysis showed that insomnia, depression, and anxiety formed a symptom cluster (p < 0.001). There was no difference between sleep parameters measured by Actigraphy in insomnia and non-insomnia participants.
Conclusion
This study demonstrated that the prevalence of insomnia was high and indicated a symptom cluster of insomnia, depression, and anxiety. Therefore, interventions to reduce this symptom cluster may benefit cancer patients who are trying to manage these symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insomnia is common among patients undergoing chemotherapy treatment [1, 2]. Previous studies have revealed a wide range of insomnia prevalence in cancer patients from 8.95 to 93.3% [3,4,5,6]. Insomnia can be categorized as either a symptom or a disease [7]. When being categorized as a symptom, insomnia can be detected by using questionnaires such as the Insomnia Severity Index and Pittsburgh Sleep Quality Index. In this study, insomnia is categorized as a symptom based on an Insomnia Severity Index (ISI) score of 15 or above.
Insomnia generates adverse health problems. Short-term consequences include somatic problems and psychological and social issues, while the long-term consequences are more severe and include cardiovascular disease, obesity, and cancer [8]. Studies indicated that people with insomnia have a high risk of developing a wide range of cancers [8,9,10,11]. Results of a recent study stated that insomnia could damage DNA and lower the body’s ability to repair this, possibly increasing the risk of developing cancer [12]. Insomnia is a major problem and significantly associated with poorer quality of life among cancer patients [13,14,15]. Furthermore, short sleep duration is associated with mortality in cancer patients [16]. However, this condition often goes unrecognized because patients do not report their sleep difficulties [17]. Also, this condition has received little attention by clinicians and researchers [13, 18,19,20,21].
The lack of using standardized questionnaires has resulted in a wide range of insomnia prevalence in the literature. In addition, most studies are conducted on small sample sizes with diverse participant characteristics, which can lead to the inability to conduct subgroup analysis and identify potential risk factors of insomnia [17]. Moreover, few studies have included an objective measurement to explore specific impaired sleep patterns or quantify insomnia among participants [13, 17]. Although a variety of factors are associated with the development of insomnia in cancer patients (e.g., gender, age, cancer treatments, unpleasant symptoms), mixed results were shown across studies regarding risk factors and only a limited number of studies have been conducted to determine if insomnia affects individuals undergoing chemotherapy [22]. Therefore, more studies are needed to clarify these gaps in the literature.
Recent studies indicated that insomnia correlates with other symptoms to form a “symptom cluster” (SC). A symptom cluster has been defined as at least two symptoms co-occurring, being clinically meaningful together, and relating to each other [23, 24]. Different sleep-related symptom clusters (SCs) in cancer patients have been reported in the literature, including fatigue, depression, and insomnia [4, 13, 25]; pain, fatigue, and insomnia [26,27,28]; pain, depression, and insomnia in prostate cancer patients [29]; and depression, anxiety, pain, and insomnia in breast cancer patients receiving aromatase inhibitors [30]. However, the correlation between insomnia and other symptoms is not fully investigated due to limitations of previous studies, including not using validated questionnaires to measure insomnia [4, 13, 25, 26, 28, 31] and measuring a limited number of symptoms [4, 13, 25, 26, 28, 31]. Moreover, the use of small sample sizes [25, 26, 28, 31] and investigating insomnia on specific cancer diagnoses limits our ability to generalize sleep-related SCs in cancer patients [26, 28,29,30,31].
The objective of this study was to provide evidence of the prevalence of insomnia with its severity in cancer patients undergoing chemotherapy, objectively quantify insomnia among participants, clarify possible risk factors for insomnia, determine how insomnia impacts the participants' quality of life, and identify sleep-related symptom clusters.
Methods
Participants
This cross-sectional survey included cancer patients from three large cancer hospitals in Hanoi, Vietnam, including Vietnam National Cancer Institution, Bachmai Hospital, and Hanoi Oncology Hospital. Patients with any cancer diagnosis were invited if they were undergoing chemotherapy in the participating hospitals between May and July 2017 using convenience sampling method. The inclusion criteria were age 20–84 years old and having a Karnofsky Performance Index ≥ 80. Patients were excluded if they had cognitive impairments or psychiatric disorder (based on clinical records or clinician opinion) as these conditions would prohibit completion of the questionnaires or adhere with Actigraph use requirements, history of insomnia before the diagnosis of cancer (based on clinical records or as reported by patient) as the study aimed to capture new-onset insomnia related to cancer and its treatment time, and presence of a sleep disorder other than insomnia and receiving insomnia treatment for at least 1 month prior to the study.
Procedures
Data were collected by the principal researcher and two research assistants. Potential participants were approached and invited to the study on the first day they were admitted to the hospital. The purpose of the study was explained to them. Participants who agreed to participate were informed of the procedures in detail and were provided with the study information sheet. Each participant signed the consent form. Afterwards, they were given the questionnaires to complete. Before they were discharged from the hospital, the principal researcher gave them the actigraphy and explained how to use it. The study obtained ethical approval from the Human Subject Ethics Board, The Hong Kong Polytechnic University (Hong Kong SAR), and Hanoi School of Public Health (Vietnam).
Measurements
Insomnia
The Insomnia Severity Index (ISI), a sleep log, and Actigraph were used to assess insomnia. The ISI is a self-reported questionnaire consisting of seven items. A score of 15 and above indicated the patient had insomnia; the insomnia severity is interpreted as follows: moderate insomnia (ISI score of 15 to 21) and severe insomnia (ISI score of 22 to 28) [32]. This instrument has been validated and translated into Vietnamese [33]. In this study, the ISI had high reliability, with Cronbach’s α = 0.92.
Actigraph
The wGT3X-BT was used to collect the following data:
Total sleep time (TST): the period measured from sleep onset during nighttime to the final awakening.
True sleep time (TuST): the period that patient actually sleeps.
Wake after sleep onset (WASO): a period of time spent awake after the sleep onset, calculated by TST minus TuST.
Mid-sleep awakening: the number of awakenings during a sleep period.
Sleep efficiency: calculated by dividing TuST (at night) by TST (at night) multiplied by 100%.
The data were collected in 1-min epochs and scored with the ActiLife6 version 6.13.3 software using the Cole-Kripker algorithm, and the sampling rate was 30 Hz. Patients were asked to wear the Actigraph for three consecutive nights [34]. This device has been validated in measuring sleep parameters with Actiwatch 2 (a device that has been validated previously) among 49 adults in Hong Kong; results from the study indicated that Actigraph wGT3X-BT could measure sleep parameters similarly to Actiwatch 2 (Spearman correlations ranging from 0.74 to 0.90) [35].
A sleep log was used to calculate sleep onset and sleep efficiency in the study participants. The components of the sleep logs were:
The time that individuals went to bed
The time that individuals got out of bed
Time the Actigraph was removed from the participants’ wrist
Number of night awakenings
The sleep log helped in identifying misclassified sleep or wake during the recordings (e.g., motionless activities such as watching a movie, sitting in a car, which are scored as sleep). Therefore, participants were asked to complete a 3-day sleep log during the time they wore the Actigraph.
Other symptom assessments
The validated Vietnamese version of The Memorial Symptom Assessment Scale (MSAS) (α = 0.79) was used to measure 32 symptoms (Long NH, 2010, Factors related to postoperative symptoms among patients undergoing abdominal surgery. Burapha University, unpublished MSc thesis). In this study, the internal consistency was reported to be high, with the overall scale having α = 0.92, the psychological subscale α = 0.80, and the physical subscale α = 0.83.
The Vietnamese version of the Hospital Anxiety and Depression Scale (HADS) was used to measure depression and anxiety (Long NH, 2010, unpublished MSc thesis). This scale has seven items measuring anxiety and seven items measuring depression over the past week. In the current study, the Cronbach’s alpha for the scale was 0.87, for the anxiety subscale was 0.86, and for the depression subscale was 0.76.
Quality of life
The Vietnamese version of Functional Assessment of Cancer Therapy-General (FACT-G) was used in this study to assess the quality of life in participants [36]. The questionnaire consists of 27 items and has four subscales: The physical well-being (PWB), the social/family well-being (SWB), the emotional well-being (EWB), and the functional well-being (FWB). In this study, the Cronbach’s alpha for the scale was 0.89 and for the EBW subscales was 0.78, for the FWB was 0.82, for the PWB was 0.81, and for the SWB was 0.82.
Data analysis
Data were analyzed using SPSS 20.0. Between-group comparisons were performed using T tests and one-way ANOVA for continuous variables and χ2 test for categorical variables. Spearman’s correlations were used to assess the association between insomnia and other variables. Symptom clusters were identified by using principal component analysis (PCA). The statistical significance level was set at p < 0.05.
Results
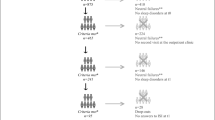
There were 224 patients included in the study; 11 patients withdrew from the study during the time they wore the Actigraph, leaving a final sample of 213 participants. Figure 1 depicts the flow of the study’s participant recruitment. The mean age of patients was 53.1 ± 11.3 (min 21, max 80), with 60% being female. Nearly one-third of the participants were diagnosed with breast cancer. The vast majority of the sample was in cancer stage 2 (n = 66, 31%) and stage 3 (n = 62, 29.1%). More details are shown in Table 1.
Prevalence and severity of insomnia
There were 42.8% of participants (n = 91) reporting insomnia. Among the participants reporting insomnia, 68.1% reported moderate insomnia and 31.9% reported severe insomnia.
Risk factors for insomnia
There was no difference between the average ISI score and insomnia occurrence regarding different gender, age groups, cancer diagnoses, cancer stages, and chemotherapy regimens (all p > 0.05). Patients with depression had a significantly higher ISI score and greater insomnia prevalence than patients with no depression (mean score = 15.4 vs. 11.36; 60.4% vs. 39.6%, respectively, both p < 0.01), and patients with anxiety had a higher ISI score and greater insomnia prevalence than those with no anxiety (mean score = 15.24 vs. 11.61; 63.7% vs. 36.3%, respectively, both p < 0.001) (Table 2). Insomnia occurrence was correlated with anxiety (r = 0.30; p < 0.01) and depression (r = 0.27; p < 0.01).
Symptom clusters analysis
Before performing PCA, we excluded the MSAS item of “Difficulty sleeping” and included the scores for ISI, anxiety, and depression. PCA was thus conducted on 34 items with varimax rotation. We determined SCs based on four dimensions: symptom frequency, symptom severity, symptom distress, and total symptom score. The results showed that across the insomnia symptom dimensions, anxiety, depression, and depressive symptoms (worrying, feeling sad) formed a clear SC (Table 3).
Among participants with insomnia, there were 44 participants (48.4%) reporting the sleep-related SC and 47 participants (51.6%) reporting insomnia only. Among participants reporting the sleep-related symptom cluster, 21 patients (47.7%) reported three symptoms (insomnia, depression, and anxiety) and 23 patients (52.7%) reported two symptoms (insomnia and depression/anxiety).
The impact of insomnia and sleep-related symptom cluster on participants' quality of life
Participants experiencing the sleep-related SC had significantly lower QOL scores in comparison with non-insomnia patients and participants reporting insomnia only (all p < 0.001). They also had worse scores than those without insomnia or those reporting insomnia only regarding emotional, functional, and physical well-being. Participants reporting insomnia only had significantly poorer scores in comparison with non-insomnia participants regarding functional well-being (p < 0.05) (Tables 4 and 5).
Sleep parameters measured by Actigraph and sleep log
According to the results from Actigraphy, insomnia patients slept 363.75 min (≈ 6 h) per night on average, and their sleep efficiency was 89%. Furthermore, their average WASO was 44.2 min with 1.4 times on average being awakened. In comparison, the results measured by the participants’ sleep log reported that these same participants slept 354.82 min (≈ 6 h) and woke up 1.8 times on average per night.
The results also indicated that there was no significant difference in sleep parameters measured by Actigraph or sleep log in terms of TST, TuST, SE, and the number of night awakenings and WASO between insomnia and non-insomnia participants (Table 6).
Discussion
In the general population, insomnia is one of the most common sleep disorders with a prevalence that is approximately 10 to 20% [37, 38]. The findings of this study showed a high prevalence of new-onset complaints of insomnia among cancer patients undergoing chemotherapy which is double that of the general population. This prevalence in our study is higher than previous studies which also used ISI to measure insomnia in cancer patients [5, 29, 39]. The difference can be explained by the characteristics of the samples used. More specifically, Morris et al. [39] recruited participants who contacted a cancer care helpline and this could create a selection bias [3]. Davis et al. [5] recruited advanced cancer patients who participated in a palliative medicine program and did not report the type of cancer treatment the participants received [8]. However, two studies conducted in breast cancer patients and prostate cancer patients indicated that the chemotherapy period was associated with more insomnia severity than other times, which is in line with our findings [3, 40]. Furthermore, our sample was drawn from Vietnamese hospitals, where patients are often treated in overcrowded clinics, and patients often have to travel long distances from other regions to attend for treatment in Hanoi. Hence, we can conclude that the peri-chemotherapy time is a stressful time that is strongly linked with the development of insomnia complaints in cancer patients as a relatively high percentage of chemotherapy patients have moderate to severe insomnia complaints.
We did not find a significant difference in insomnia frequency and severity rates between male and female participants, participants in different age groups and different cancer diagnoses or cancer stages (although breast cancer patients and those receiving taxanes had numerically higher percentage of insomnia complaints in our sample). These findings are different from some of the existing literature. Gender and age were identified as predisposing factors for insomnia in cancer patients as well as in the general population [21, 41]. Nevertheless, these findings are in line with some recent studies in cancer patients, whereby there was no significant difference in insomnia rates between males and females and participants in different age groups [1, 15, 39, 42, 43]. Several large-scale studies, however, found significant differences in insomnia prevalence between lung/breast cancer patients compared to other cancer diagnoses [4, 44]. Some potential reasons for the differences may include the antiemetic drugs used (i.e., dexamethasone), tumor biology, and side effects from chemotherapy (i.e., menopause) [4, 44]. In addition, we found participants reporting depression and/or anxiety had significantly higher insomnia rates than those with no depression and anxiety. Studies in the literature also showed consistently that patients with depression experience insomnia, and insomnia is also a risk factor for developing depression and anxiety [45]. Anxiety brings more difficulty in falling asleep resulting in less restorative sleep and nightmares, while depression has been linked to difficulties maintaining sleep and unwanted early waking up in the morning as well as nightmares [46, 47]. Therefore, the rate and severity of insomnia may be related to the participants’ distressing symptoms and not only to the participant’s characteristics and cancer diagnosis or treatment variables.
In our study, participants with the sleep-related SC had a significantly worse global QOL score as well as other subscale scores (except for the SWB) than those without insomnia, or those reporting insomnia only. The sleep-related SC seemed to contribute to lower physical and FWB and poorer EWB of the patients. The consistent results from previous studies also support our findings of how insomnia impacts on patients’ quality of life [13,14,15, 19, 48, 49]. Insomnia caused cancer patients to be less able to cope with stress and carry on with their daily activities and had greater difficulty dealing with emotional problems [14, 15], which impaired them physically and emotionally [48, 49].
This survey identified insomnia, anxiety, depression, and depressive symptoms (worrying and feeling sad) all forming a SC. Since worrying and feeling sad are symptoms of depression [50], we conclude that insomnia, anxiety, and depression form a clear and concrete SC. Previous studies in different cancer populations also reported similar findings [51, 52]. Results from a systematic review further suggested a bidirectional relationship between insomnia, depression, and anxiety [53]. However, previous studies used non-specific scales to measure symptoms [52] or identified symptom clusters on one dimension of the symptom experience (severity) [51, 53]. It is interesting to see that the correlations between the three symptoms were low in the correlational analysis, suggesting that other factors, not examined in this study, may be part of this relation. However, the strength of this relationship became moderate-to-strong in the SC analysis. Hence, anxiety and depression are clearly part of insomnia, but perhaps not the only parts. This supports the use of selective serotonin reuptake inhibitors (SSRIs) as an option to improve sleep in this population when anxiety and/or depression are present, balancing well the possible beneficial effect over side effects and polypharmacy. However, as SSRIs have multiple side effects, can be addictive, and there are other factors contributing to insomnia in cancer patients who often experience a high symptom burden, they have a place in the management of insomnia in select patients but should not be considered as the drug of choice in this population. To our knowledge, our study is the first study to date using standardized measurements to assess insomnia, depression, and anxiety and identify SCs based on different dimensions of the symptom experience that give more concrete evidence that insomnia, anxiety, and depression form a SC.
The results also demonstrated there was no difference between sleep parameters measured by Actigraph between insomnia and non-insomnia participants. The average true sleep time for both insomnia and non-insomnia participants was greater than 6 h per night, and the sleep efficiently was nearly 90%. Interestingly, we found that insomnia participants slept more than non-insomnia participants (although the difference was not significant) but they seemed to have lower sleep efficiency and tended to wake up more often. Using both objective and subjective measurements are being recommended to evaluate multiple sleep parameters of insomnia [34, 54]. However, our study indicates the sleep parameters between insomnia and non-insomnia participants are related to patients’ experience of insomnia, and how their perceived sleep quality impacts on their lives rather than objective quantifiable sleep parameters, which cannot incorporate patient perceptions. A study also found insomnia frequency is related to patients’ perception about sleep, but not the result from objective measurements [55].
Recent studies further reported a difference between the participants perception of sleep quality and objective measurements [56, 57]. A study by Siberfarb et al. indicated that perceptions of “poor” or “good” sleep were associated with the amount of delta sleep (the deepest form of sleep) but not with other objective sleep parameters in cancer patients [58]. This condition may also be explained by “the sleep discrepancy” referring to underestimation of the total sleep time and overestimation of the sleep latency, and wakening after sleep onset in insomnia patients [59]. Sleep experts define this sleep discrepancy as “paradoxical insomnia,” experienced by up to 50% of insomnia patients [60]. Considering the above evidence, Actigraphy can measure sleep parameters but it does not measure the sleep quality as perceived by the patients, and the latter informs the patient’s satisfaction with sleep, as part of the experience of insomnia. Furthermore, patients’ satisfaction with sleep may impact on their psychological states (anxiety/depression) and their quality of life, relating to the co-occurrence of sleep-related symptoms.
The use of a large sample size, multi-site data collection, sample diagnostic heterogeneity, and measuring insomnia both subjectively and objectively are strengths of this study. However, due to limitations inherent in cross-sectional surveys, the study cannot point out which symptom (anxiety, depression, and insomnia) occurred first nor how they influence each other or how these symptoms change over the trajectory of the illness. Our suggestion regarding the lack of difference between sleep parameters measured objectively between insomnia and non-insomnia participants should be interpreted with caution, as participants were required to wear Actigraph for three nights only, which is relatively short time and may easily be affected by intra-night variability among patients. A longer Actigraph measurement may potentially correlate better with subjective measurements.
In addition, not measuring day time napping is also a limitation of this study. Changes in sleep architecture and maladaptive sleep behavior include prolonged bedtime and daytime napping that are considered as perpetuating factors contributing to the maintenance of insomnia. Cancer patients, in an attempt to cope with sleeplessness, might try the above sleep behaviors, but as these become habits, insomnia persists for long term [21]. In addition, individuals with persistent insomnia, possibly including those with cancer, tend to engage in sleep-interfering activities in their bedroom for staying awake rather than inducing sleep (e.g., watching TV, listening to music, eating, working or reading in bed or the bedroom). These behaviors tend to weaken the association between certain normally sleep-inducing stimuli (bed, bedtime, and bedroom) and sleep [32]. Furthermore, sleep efficiency in our study may be misrepresented, as there are inherent weaknesses in its measurement when there is no standardized time in bed. Finally, as ISI measures sleep complaints in the past 2 weeks and diagnostic criteria for insomnia disorder require at least 3 months of sleep complaints [61], the insomnia rates reported in this study may not meet the full diagnostic criteria of chronic insomnia.
The results of the study confirm the high prevalence of insomnia among cancer patients undergoing chemotherapy. In addition, insomnia and sleep-related SCs significantly reduced the patient’s quality of life. Approximately half of the insomnia burden in the sample was accounted for by the sleep-related SC while the other half was experienced only insomnia. This finding suggests that insomnia is driven both by shared and unique mechanisms. The results from this study also indicate that insomnia is a subjective symptom, and the patients’ perception of insomnia or quality of sleep may differ from the actual objectively measured insomnia severity they experience. The results highlight a high unmet need in this population requiring interventions to help patients manage these symptoms.
References
Romito F, Cormio C, De Padova S, Lorusso V, Berio MA, Fimiani F, Piattelli A, Palazzo S, Abram G, Dudine L, Guglielmi A, Galise I, Romito S, Mattioli V (2014) Patients attitudes towards sleep disturbances during chemotherapy. European Journal of Cancer Care 23(3):385–393. https://doi.org/10.1111/ecc.12106
Tian J, Chen GL, Zhang HR (2015) Sleep status of cervical cancer patients and predictors of poor sleep quality during adjuvant therapy. Support Care Cancer 23(5):1401–1408. https://doi.org/10.1007/s00520-014-2493-8
Savard J, Ivers H, Savard MH, Morin CM (2015) Cancer treatments and their side effects are associated with aggravation of insomnia: results of a longitudinal study. Cancer 121(10):1703–1711. https://doi.org/10.1002/cncr.29244
Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, Heckler C, Purnell JQ, Janelsins MC, Morrow GR (2010) Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol 28(2):292–298. https://doi.org/10.1200/jco.2009.22.5011
Davis MP, Khoshknabi D, Walsh D, Lagman R, Platt A (2014) Insomnia in patients with advanced cancer. Am J Hosp Palliat Care 31(4):365–373. https://doi.org/10.1177/1049909113485804
Shao Y-J, Ji K, Hao J-L, Cheng X-J, Guan B-Q, Liu W-S, Wang K (2016) Nonpain symptom prevalence and intensity of inpatients with moderate to severe cancer pain in China. Am J Hosp Palliat Med 33(5):448–455. https://doi.org/10.1177/1049909114565109
Billiard M, Bentley A (2004) Is insomnia best categorized as a symptom or a disease? Sleep Med 5(Suppl 1):S35–S40
Medic G, Wille M, Hemels ME (2017) Short- and long-term health consequences of sleep disruption. Nat Sci Sleep 9:151–161. https://doi.org/10.2147/NSS.S134864
Zhang X, Giovannucci EL, Wu K, Gao X, Hu F, Ogino S, Schernhammer ES, Fuchs CS, Redline S, Willett WC, Ma J (2013) Associations of self-reported sleep duration and snoring with colorectal cancer risk in men and women. Sleep 36(5):681–688. https://doi.org/10.5665/sleep.2626
Luo J, Sands M, Wactawski-Wende J, Song Y, Margolis KL (2013) Sleep disturbance and incidence of thyroid cancer in postmenopausal women the Women’s Health Initiative. Am J Epidemiol 177(1):42–49. https://doi.org/10.1093/aje/kws193
Fang H-F, Miao N-F, Chen C-D, Sithole T, Chung M-H (2015) Risk of cancer in patients with insomnia, parasomnia, and obstructive sleep apnea: a nationwide nested case-control study. J Cancer 6(11):1140–1147. https://doi.org/10.7150/jca.12490
Cheung V, Yuen VM, Wong GTC, Choi SW (2019) The effect of sleep deprivation and disruption on DNA damage and health of doctors. Anaesthesia 74:434–440. https://doi.org/10.1111/anae.14533
Davidson JR, MacLean AW, Brundage MD, Schulze K (2002) Sleep disturbance in cancer patients. Soc Sci Med 54(9):1309–1321
Vargas S, Wohlgemuth WK, Antoni MH, Lechner SC, Holley HA, Carver CS (2010) Sleep dysfunction and psychosocial adaptation among women undergoing treatment for non-metastatic breast cancer. Psycho-Oncology 19(6):669–673. https://doi.org/10.1002/pon.1603
Phillips KM, Jim HS, Donovan KA, Pinder-Schenck MC, Jacobsen PB (2012) Characteristics and correlates of sleep disturbances in cancer patients. Support Care Cancer 20(2):357–365. https://doi.org/10.1007/s00520-011-1106-z
Collins KP, Geller DA, Antoni M, Donnell DM, Tsung A, Marsh JW, Burke L, Penedo F, Terhorst L, Kamarck TW, Greene A, Buysse DJ, Steel JL (2017) Sleep duration is associated with survival in advanced cancer patients. Sleep Med 32:208–212. https://doi.org/10.1016/j.sleep.2016.06.041
Dahiya S, Ahluwalia MS, Walia HK (2013) Sleep disturbances in cancer patients: underrecognized and undertreated. Cleve Clin J Med 80(11):722–732. https://doi.org/10.3949/ccjm.80a.12170
Savard J, Morin CM (2001) Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol 19(3):895–908
O’Donnell JF (2004) Insomnia in cancer patients. Clin Cornerstone 6(1):S6–S14. https://doi.org/10.1016/S1098-3597(05)80002-X
Raghava R, Induru DW (2014) Cancer-related insomnia. Am J Hosp Palliat Med 3(7):777–785
Lowery AE (2015) Sleep and cancer. In: Psycho-oncology. Oxford University Press, Oxford, pp 231–238. https://doi.org/10.1093/med/9780199363315.003.0030
Otte JL, Carpenter JS, Manchanda S, Rand KL, Skaar TC, Weaver M, Chernyak Y, Zhong X, Igega C, Landis CA (2015) Systematic review of sleep disorders in cancer patients: can the prevalence of sleep disorders be ascertained? Cancer Med 4(2):183–200. https://doi.org/10.1002/cam4.356
Dodd MJ, Miaskowski C, Paul SM (2001) Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum 28(3):465–470
Molassiotis A, Wengström Y, Kearney N (2010) Symptom cluster patterns during the first year after diagnosis with cancer. J Pain Symptom Manag 39(5):847–858. https://doi.org/10.1016/j.jpainsymman.2009.09.012
Okuyama T, Akechi T, Kugaya A, Okamura H, Imoto S, Nakano T, Mikami I, Hosaka T, Uchitomi Y (2000) Factors correlated with fatigue in disease-free breast cancer patients: application of the Cancer Fatigue Scale. Support Care Cancer 8(3):215–222
Chen ML, Tseng HC (2006) Symptom clusters in cancer patients. Support Care Cancer 14(8):825–830. https://doi.org/10.1007/s00520-006-0019-8
Ivanova MO, Ionova TI, Kalyadina SA, Uspenskaya OS, Kishtovich AV, Guo H, Mendoza TR, Novik A, Cleeland CS, Wang XS (2005) Cancer-related symptom assessment in Russia: validation and utility of the Russian M. D. Anderson Symptom Inventory. J Pain Symptom Manag 30(5):443–453. https://doi.org/10.1016/j.jpainsymman.2005.04.015
Hoffman AJ, Given BA, von Eye A, Gift AG, Given CW (2007) Relationships among pain, fatigue, insomnia, and gender in persons with lung cancer. Oncol Nurs Forum 34(4):785–792. https://doi.org/10.1188/07.onf.785-792
Savard J, Simard S, Hervouet S, Ivers H, Lacombe L, Fradet Y (2005) Insomnia in men treated with radical prostatectomy for prostate cancer. Psycho-Oncology 14(2):147–156. https://doi.org/10.1002/pon.830
Gehrman PR, Garland SN, Matura LA, Mao J (2016) Insomnia in breast cancer: independent symptom or symptom cluster? Palliat Support Care:1–7
Ho SY, Rohan KJ, Parent J, Tager FA, McKinley PS (2015) A longitudinal study of depression, fatigue, and sleep disturbances as a symptom cluster in women with breast cancer. J Pain Symptom Manag 49(4):707–715. https://doi.org/10.1016/j.jpainsymman.2014.09.009
Morin CM, Belleville G, Bélanger L, Ivers H (2011) The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34(5):601–608. https://doi.org/10.1093/sleep/34.5.601
Long NH, Thanasilp S, Thato R (2016) A causal model for fatigue in lung cancer patients receiving chemotherapy. Eur J Oncol Nurs 21:242–247. https://doi.org/10.1016/j.ejon.2015.10.010
Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, Sadeh A, Spira AP, Taylor DJ (2015) The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med 13(Suppl 1):S4–s38. https://doi.org/10.1080/15402002.2015.1046356
Lee PH, Suen LK (2017) The convergent validity of Actiwatch 2 and ActiGraph Link accelerometers in measuring total sleeping period, wake after sleep onset, and sleep efficiency in free-living condition. Sleep Breath 21:1522–1709
Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy Scale: development and validation of the general measure. J Clin Oncol 1993:11:570–579. http://www.facit.org/FACITOrg/Questionnaires
Neubauer DN, Pandi-Perumal SR, Spence DW, Buttoo K, Monti JM (2018) Pharmacotherapy of insomnia. J Cent Nerv Syst Dis 10:1179573518770672. https://doi.org/10.1177/1179573518770672
Buysse DJ (2013) Insomnia. JAMA 309(7):706–716. https://doi.org/10.1001/jama.2013.193
Morris BA, Thorndike FP, Ritterband LM, Glozier N, Dunn J, Chambers SK (2015) Sleep disturbance in cancer patients and caregivers who contact telephone-based help services. Support Care Cancer 23(4):1113–1120. https://doi.org/10.1007/s00520-014-2458-y
Savard J, Hervouet S, Ivers H (2013) Prostate cancer treatments and their side effects are associated with increased insomnia. Psycho-Oncology 22(6):1381–1388. https://doi.org/10.1002/pon.3150
Ohayon MM, Roth T (2001) What are the contributing factors for insomnia in the general population? J Psychosom Res 51(6):745–755. https://doi.org/10.1016/S0022-3999(01)00285-9
Akman T, Yavuzsen T, Sevgen Z, Ellidokuz H, Yilmaz AU (2015) Evaluation of sleep disorders in cancer patients based on Pittsburgh Sleep Quality Index. Eur J Cancer Care 24(4):553–559. https://doi.org/10.1111/ecc.12296
Miaskowski C, Lee K, Dunn L, Dodd M, Aouizerat BE, West C, Paul SM, Cooper B, Wara W, Swift P (2011) Sleep-wake circadian activity rhythm parameters and fatigue in oncology patients prior to the initiation of radiation therapy. Cancer Nurs 34(4):255–268. https://doi.org/10.1097/NCC.0b013e3181f65d9b
Savard J, Simard S, Blanchet J, Ivers H, Morin CM (2001) Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep 24(5):583–590
Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ (1997) Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord 42(2–3):209–212
Berk M (2009) Sleep and depression—theory and practice. Aust Fam Physician 38(5):302–304
Mercadante S, Girelli D, Casuccio A (2004) Sleep disorders in advanced cancer patients: prevalence and factors associated. Support Care Cancer 12(5):355–359. https://doi.org/10.1007/s00520-004-0623-4
Trudel-Fitzgerald C, Savard J, Ivers H (2014) Longitudinal changes in clusters of cancer patients over an 18-month period. Health Psychol 33(9):1012–1022. https://doi.org/10.1037/a0033497
Clevenger L, Schrepf A, Degeest K, Bender D, Goodheart M, Ahmed A, Dahmoush L, Penedo F, Lucci J III, Thaker PH, Mendez L, Sood AK, Slavich GM, Lutgendorf SK (2013) Sleep disturbance, distress, and quality of life in ovarian cancer patients during the first year after diagnosis. Cancer 119(17):3234–3241. https://doi.org/10.1002/cncr.28188
The National Institutes of Mental Health (2018) Depression. https://www.nimh.nih.gov/health/topics/depression/index.shtml. Accessed 29/5 2018
Walsh D, Rybicki L (2006) Symptom clustering in advanced cancer. Support Care Cancer 14(8):831–836. https://doi.org/10.1007/s00520-005-0899-z
Redeker NS, Lev EL, Ruggiero J (2000) Insomnia, fatigue, anxiety, depression, and quality of life of cancer patients undergoing chemotherapy. Sch Inq Nurs Pract 14(4):275–298
Alvaro PK, Roberts RM, Harris JK (2013) A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep 36(7):1059–1068
Ruivo Marques D, Clemente V, Allen Gomes A, de Azevedo MHP (2018) Profiling insomnia using subjective measures: where are we and where are we going. Sleep Med 42:103–104. https://doi.org/10.1016/j.sleep.2017.12.006
Zorick F (1994) Insomnia. In: Kryger MH, Roth T, WC D (eds) Principles and practice of sleep medicine. W.B Saunders Co, Philadelphia, pp 483–485
Landry GJ, Best JR, Liu-Ambrose T (2015) Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci 7:166. https://doi.org/10.3389/fnagi.2015.00166
Grandner MA, Kripke DF, Yoon I-Y, Youngstedt SD (2006) Criterion validity of the Pittsburgh Sleep Quality Index: investigation in a non-clinical sample. Sleep Biol Rhythms 4(2):129–139. https://doi.org/10.1111/j.1479-8425.2006.00207.x
Silberfarb PM, Hauri PJ, Oxman TE, Lash S (1985) Insomnia in cancer patients. Soc Sci Med 20(8):849–850
Kay DB, Karim HT, Soehner AM, Hasler BP, James JA, Germain A, Hall MH, Franzen PL, Price JC, Nofzinger EA, Buysse DJ (2017) Subjective-objective sleep discrepancy is associated with alterations in regional glucose metabolism in patients with insomnia and good sleeper controls. Sleep 40(11). https://doi.org/10.1093/sleep/zsx155
Manconi M, Ferri R, Sagrada C, Punjabi NM, Tettamanzi E, Zucconi M, Oldani A, Castronovo V, Ferini-Strambi L (2010) Measuring the error in sleep estimation in normal subjects and in patients with insomnia. J Sleep Res 19(3):478–486. https://doi.org/10.1111/j.1365-2869.2009.00801.x
American Academy of Seep Medicine (2014) International Classification of Sleep Disorders. 3rd edn. Darien IL, USA
Acknowledgments
We would like to thank all the participants for their voluntary contribution to the study. We would like to thank Dr. Nguyen Thi Hoai Nga, Dr. Le Thanh Duc, Dr. Nguyen Trung Kien, Ms. Quach Thi Viet Huong, and Ms. Le Thi Tuyen from Vietnam national Cancer Hospital; Mr. Nguyen Cong Binh, Dr. Le Thi Le Quyen, Dr. Nguyen Trong Hieu, Dr. Le Thu Ha, Ms. Nguyen Phuong Thao, and Ms. Nguyen Hong Van from Hanoi Oncology Hospital; Prof. Mai Trong Khoa and Dr. Nguyen Van Thai from The Nuclear Medicine and Oncology Center of Bach Mai hospital for their great assistant during the data collection. We are also thankful to Dr. Paul Lee from the School of Nursing, The Hong Kong Polytechnic University for providing the Actigraph devices and helping us analyze data from Actigraph.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study obtained ethical approval from the Human Subject Ethics Board, The Hong Kong Polytechnic University (Hong Kong SAR), and Hanoi School of Public Health (Vietnam).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The institutions where work was performed:
1. Vietnam National Cancer Hospital, 43 Quan Su St., Hoan Kiem district, Hanoi, Vietnam
2. Bach Mai Hospital, 78 Giai Phong St., Dong Da district, Hanoi, Vietnam
3. Hanoi Oncology Hospital, 42A Thanh Nhan, Hanoi, Vietnam
Rights and permissions
About this article
Cite this article
Hoang, H.T.X., Molassiotis, A., Chan, C.W. et al. New-onset insomnia among cancer patients undergoing chemotherapy: prevalence, risk factors, and its correlation with other symptoms. Sleep Breath 24, 241–251 (2020). https://doi.org/10.1007/s11325-019-01839-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-019-01839-x