Abstract
Purpose
Cumulative evidence supports the clear relationship of obstructive sleep apnea (OSA) with cardiovascular disease (CVD). And, adherence to continuous positive airway pressure (CPAP) treatment alleviates the risk of CVD in subjects with OSA. Vascular endothelial growth factor (VEGF), a potent angiogenic cytokine regulated by hypoxia-inducible factor, stimulates the progression of CVD. Thus, whether treatment with CPAP can actually decrease VEGF in patients with OSA remains inconclusive. The purpose of the present study was to quantitatively evaluate the impact of CPAP therapy on VEGF levels in OSA patients.
Methods
We systematically searched Web of Science, Cochrane Library, PubMed, and Embase databases that examined the impact of CPAP on VEGF levels in OSA patients prior to May 1, 2017. Related searching terms were “sleep apnea, obstructive,” “sleep disordered breathing,” “continuous positive airway pressure,” “positive airway pressure,” and “vascular endothelial growth factor.” We used standardized mean difference (SMD) to analyze the summary estimates for CPAP therapy.
Results
Six studies involving 392 patients were eligible for the meta-analysis. Meta-analysis of the pooled effect showed that levels of VEGF were significantly decreased in patients with OSA before and after CPAP treatment (SMD = − 0.440, 95% confidence interval (CI) = − 0.684 to − 0.196, z = 3.53, p = 0.000). Further, results demonstrated that differences in age, body mass index, apnea–hypopnea index, CPAP therapy duration, sample size, and racial differences also affected CPAP efficacy.
Conclusions
Improved endothelial function measured by VEGF may be associated with CPAP therapy in OSA patients. The use of VEGF levels may be clinically important in evaluating CVD for OSA patients. Further large-scale, well-designed long-term interventional investigations are needed to clarify this issue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An epidemiological study has implicated that obstructive sleep apnea (OSA), characterized by the presence of collapse of airway during sleep that leads to intermittent hypoxia, is a common condition affecting 4% of middle age population [1]. Cumulative evidence supports the clear relationship of OSA with cardiovascular disease (CVD). That is attributed to the repetitive sympathetic activation, which may induce the regulation of neural, humoral, and inflammatory responses and promote endothelial dysfunction [2]. And, available data is in favor of adherence to continuous positive airway pressure (CPAP) treatment, alleviating the risk of CVD in subjects with OSA [3].
Vascular endothelial growth factor (VEGF), a potent angiogenic cytokine regulated by hypoxia-inducible factor, stimulates the progression of CVD [4]. Several studies have highlighted that expression of VEGF were increased not only in mice exposed to intermittent hypoxia [5] but also in OSA patients [6]. Previous researches by Schulz [6] and Imagawa [7] indicated that concentrations of VEGF were elevated in OSA patients, which were linked with the severity indexed by the apnea–hypopnea index.
Given the potentially serious prognosis of untreated OSA patients, it is crucial to emphasize the role of CPAP in the risk of CVD, with a regulation of VEGF [8]. However, the outcomes of CPAP have revealed conflicting results. The authors have described an additional beneficial effect after 1 year of adherent CPAP treatment in 10 OSA patients but not in non-compliant patients. It is speculated that CPAP could decrease VEGF levels via the reduction of nocturnal hypoxia [8]. Similarly, significantly reduced serum VEGF levels in OSA patients were presented after improvement of nocturnal hypoxia through oxygen administration [9]. Conversely, regardless of exposure to a short-term CPAP therapy, other investigators reported unchanged VEGF levels [10]. Further, it was reported that 1-week withdrawal from CPAP treatment led to a return in daytime urinary noradrenaline, reflecting increased sympathetic activity, and unfortunately, this was not accompanied by increased circulating VEGF levels [11]. Moreover, plasma values of VEGF rose after improvement of the nocturnal hypoxia by nasal CPAP over several months, reflecting endothelial restoration process [12].
Thus, whether treatment with CPAP can actually decrease VEGF in patients with OSA remains inconclusive. The purpose of the present study was to quantitatively evaluate the impact of CPAP therapy on VEGF levels in OSA patients.
Methods and materials
Literature search and selection
We systematically searched Web of Science, Cochrane Library, PubMed, and Embase databases prior to May 1, 2017, on original English language literatures, which were limited to human studies. All searches included free text and corresponding MeSH terms, and the combination of following search terms were used: (1) “obstructive sleep apnea,” “sleep apnea, obstructive,” “sleep apnea syndrome,” “OSA,” “sleep apnea,” “sleep apnea or sleep apnoea,” “sleep disordered breathing,” “SDB”; (2) “continuous positive airway pressure,” “CPAP,” “positive airway pressure,” “PAP”; (3) “Vascular endothelial growth factor,” “VEGF”. Besides, we manually searched for additional researches from the reference lists of relevant publications. Two investigators independently reviewed related studies based on title and abstract that included empirical data linking to the treatment outcome on VEGF in OSA. A third researcher should make consensus if any disagreement between the two reviewers was aroused.
Studies were included if they satisfied the following criteria: (1) observational studies or randomized control trails; (2) the study populations were limited to adults (age ≥ 18); (3) OSA subjects were diagnosed for the first time and never received any form of treatment before except for CPAP; (4) OSA was diagnosed based on standard polysomnography; (5) the concentrations of VEGF needed to be reported both before and after CPAP; and (6) sufficient data were presented that allowed for a meta-analysis. Exclusion criteria were as follows: (1) studies which disagreed with the inclusion criteria would be excluded; (2) non-English literature; (3) abstracts, case reports, editorials, expert opinions, letters, animal studies, and reviews without original data; and (4) unpublished data from conference. When multiple studies reported effects using the same patient group, the research with the largest population was included. If the studies did not provide adequate data, the corresponding author was contacted; after two no-response attempts, the studies were also ruled out.
Data extraction and analysis
Two authors independently extracted the data from each study including first author’s name, the year of publication, study design, country of the study, number of patients, duration of CPAP therapy, source of VEGF, values of VEGF before and after CPAP treatment, and patients’ characteristics.
The meta-analysis was conducted using Stata statistical software (Version 12.0, Stata Corporation, College Station, TX, USA). Standardized mean difference (SMD) was used for analyzing the summary estimates, considering VEGF measured and reported differently. Q and I2 statistics were considered statistical heterogeneity among individual studies.
If there was evidence of statistical heterogeneity indicated by p < 0.10 or I2 > 50%, then a randomized-effects model was applied to combine effect size. Otherwise, a fixed-effects model was conducted to estimate the pooled effects. Sensitivity analysis was performed to explore the influence of a single study on overall efficacy of CPAP of this meta-analysis.
Potential publication bias was presented using funnel plot and tested by “Begg test” and “Egger test”. A p < 0.05 was considered as statistically significant for the overall effect size.
Quality assessment
The Cochrane risk of bias tool [13] was used to independently assess study quality by two reviewers. Each study was evaluated from six aspects: random sequence generation, allocation concealment, blinding of the participant, blinding of outcome measures, incomplete data, and selective reporting. The discrepancy was resolved by a third reviewer.
Pool analysis
The heterogeneity test revealed that there were significant differences among individual studies (chi squared = 250.83, p = 0.000, I2 = 98.0%). Therefore, a randomized-effects model was used for the pooled analysis. Meta-analysis of the pooled effect showed that levels of VEGF were significantly decreased in patients with OSA before and after CPAP treatment (SMD = − 0.440, 95% confidence interval (CI) = − 0.684 to − 0.196, z = 3.53, p = 0.000) (Fig. 2).
Publication bias and sensitivity analysis
The funnel plot (Fig. 3) showed that small publication bias might exist. However, Begg’s tests (p = 0.734) and Egger’s tests (p = 0.147) suggested no evidence to support publication bias in our meta-analysis. Furthermore, sensitivity analysis revealed that omitting any one of the studies at a time did not influence the overall result of the pooled analysis, confirming temperate overall results (Fig. 4).
Subgroup analysis
In order to explore factors which may lead to heterogeneity in the effectiveness of CPAP, we performed subgroup analysis. Potential factors such as baseline BMI, severity of OSA, CPAP therapy duration (< 3 and ≥ 3 month), sample size (< 60 and ≥ 60), sample (plasma and serum), racial differences (Asian and none Asian), and study design (RCTs and non-RCTs) were accessed. With regard to changes in VEGF before and after CPAP treatment, it was likely that an AHI ≥ 30 events/h, a BMI < 35 kg/m2, a CPAP therapy duration ≥3 months, an Asian location, serum sample, and non-RCTs were the factors leading to heterogeneity (Table 3).
Results of searching results
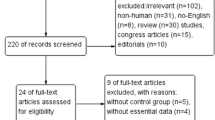
As shown in Fig. 1, our initial literature search yielded 461 relevant publications and a total of 455 remained after duplicate entries were excluded. On further screening, nine papers were considered to be potentially relevant and 446 records were removed based on reasons listed in Fig. 1. Of the nine publications, requisite data were not available in three articles. Finally, six studies involving a total of 496 subjects were eligible for our meta-analysis. We developed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol [14]. Details of the literature search are outlined in Fig. 1. Basic characteristics of the six eligible studies are presented in Table 1. There were five observational studies and one randomized clinical trial among all included records (Tables 1, 2, and 3).
Discussion
In the present meta-analysis, we quantitatively explored the impact of CPAP treatment on VEGF in adults with OSA. A remarkable finding from our study is that response to CPAP, a reduction of hypoxia-responsive angiogenic marker VEGF, was indicated. Also, significant results were observed in the subgroup analysis.
Notably, the majority of data suggest that patients with OSA are exposed to increasing risk for CVD [17]. Repetitive hypoxic events during sleep may be partly responsible for the link. Thus, it may be significantly clinical to report a decline of VEGF concentration with treatment of sleep apnea and potentially reduced angiogenesis, which indicated a better endothelial function. However, it should be pointed out that there has no consistent conclusion regarding the effects of CPAP on circulating VEGF levels, meanwhile, it remains unclear that CPAP alleviates the vascular injury to what extent. Previous publications [8, 15] had confirmed that increased VEGF could be normalized by appropriate CPAP intervention, particularly in OSA patients. Nevertheless, some investigators suggested that short follow-up duration of CPAP may have given insufficient time to affect vascular outcomes [10]. Especially for those patients with prior diagnosis of severe OSA, an immediate return of significant hypoxemia was accompanied with withdrawal from CPAP therapy. And, similar results were demonstrated in the study of Valipour, in which differences in patients with an AHI of greater than 15/h, subjects with an AHI of less than 5/h, and OSA patients undergoing CPAP were analyzed, with no significant differences among groups [18]. In agreement with previous study, Philips demonstrated that the sympathetic activity changes during CPAP withdrawal, with no change in plasma values of VEGF [11]. On the contrary, improvement of the nocturnal hypoxia by nasal CPAP was accompanied with an increase of VEGF over several months, which reflected an endothelial restoration process and a decrease of endothelial damage [12]. The small number of participants, the absence of a control group, the measurement of VEGF, the compliance with the therapy [19], and the duration of the treatment [20] may explain the inconsistent findings. According to the present analysis, we supposed that it is academically rational that CPAP therapy, to some extent, could reduce VEGF levels. We tentatively put forward a beneficial action on the endothelium via the attenuation of the hypoxia-related damage, resulting from lower oxidative stress.
It should be noted that in most studies of our analysis, CPAP intervention duration was short. And, the duration of CPAP therapy ranging from one night to 1 year may be an important contributor to the susceptibility of primary studies to confounding. We actually believed that it is possible that the limited adherence to therapy may be insufficient to drive protection [21, 22]. However, adequate CPAP adherence remains a challenging issue [23]. Possible improved outcomes at better CPAP adherence time need to be supported by more large-scale well-designed clinical trials [24]. In reality, significantly reduced VEGF levels were interpreted in patients who complied with 1 year of CPAP application [8]; another group reported increased circulating VEGF after 3 months with CPAP [12]. Furthermore, according to different treatment durations, a subgroup analysis was performed to understand how long it will take to reduce VEGF levels by effective CPAP therapy, reflecting that VEGF levels changed significantly and decreased after 3 months. Nevertheless, it is still unclear that how long it is adequate to provoke changes in the endothelial restoration process [11, 25].
Details of the exact mechanism on the link between OSA and vascular injury have been extensively investigated in majority of studies, unfortunately, it was still not fully understood. It should be acknowledged that, as a primary treatment for OSA, there is evidence to suggest that CPAP treatment could not only reduce sympathetic activity [26] but also lead to a favorable effect on endothelial function [27]. Unfortunately, the causative association of chronic intermittent hypoxia and inflammation in the process of CVD remains unclear [28]. As a pro-angiogenic factor, the production and release of VEGF can mobilize endothelial progenitor cells and may enhance the recruitment of these cells to the injured vascular tissue [29]. The ischemic tissue or organ compensates for the decreased oxygen via the upregulation of blood supply. Particularly, in precapillary pulmonary vessels, vascular remodeling was increasing when animals were exposed to chronic hypoxia [30]. Apart from vascular inflammation, we also focus on the lack of the inhibitory effect induced by nitric oxide on VEGF gene expression [31]. Also, based on repeated intermittent nocturnal hypoxemic insults, increased transcription of VEGF gene can be stimulated by hypoxia-inducible factor [32]. And, findings need to be interpreted that the CPAP withdrawal model could lead to dramatic return in OSA patients, with a considerable rise in blood pressure, catecholamine excretion, and reduced endothelial function [12]. We hypothesis that it occurred because of lower oxidative stress during sleep in those without sufficient CPAP treatment.
Additionally, in most of included reports, VEGF was determined in serum rather than in plasma, which may account for the discrepant findings. It was indicated that serum VEGF reflects platelet and leukocyte release in vitro, contributing to the ascent VEGF concentrations [33]. Although plasma was recommended by some investigators for VEGF analysis [34], there still existed a robust linear relation of VEGF concentration between serum and plasma in OSA patients [35].
Several limitations of the current study must be acknowledged. First, although we did an exhaustive literature search and enrolled six studies into our meta-analysis, the sample size of each study was relatively small. Multicenter, large-scale studies are required to explore the effect of CPAP on improving endothelial function. Second, because of self-control study design in most studies, the presence of methodological heterogeneity seemed impossible. Due to differences in adherent and non-adherent individuals rather than their use of CPAP, analyses based on nonrandomized comparisons may be responsible for confounding. Moreover, it would be unethical to leave diagnosed OSA untreated in order to report potential changes in VEGF. Third, especially significant heterogeneity such as variability in AHI, duration of therapy was observed, which may affect the accuracy of the conclusion. It is still possible that VEGF could be affected by long-term CPAP use rather than short-term. However, the publication bias and sensitivity analysis implicated that all studies enrolled into the final analysis were relatively objective and reliable. Fourth, most of them did not refer to the factors in details including medication, food, and hormonal supplementation which may affect VDGF levels. In fact, they concerned more on diseases and any other sleep-related disorders, heavy alcohol exposure, or concurrent treatment which may affect OSA outcomes. Nevertheless, we should still focus on these confounders in future research. In addition, only papers published in English were enrolled; it may cause potential publication bias. Finally, different studies utilized a variety of measurement techniques for VEGF. Accounting for VEGF measured and reported differently, we used SMD to report the summary estimates instead of the absolute level.
Conclusion
Improved endothelial function measured by VEGF may be associated with CPAP therapy in OSA patients. Further large-scale, well-designed long-term interventional investigations addressing effect of CPAP on VEGF expression in OSA and outcome in terms of CVD are needed to answer these questions.
References
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Shamsuzzaman AS, Gersh BJ, Somers VK (2003) Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 290:1906–1914
Anandam A, Patil M, Akinnusi M, Jaoude P, El-Solh AA (2013) Cardiovascular mortality in obstructive sleep apnoea treated with continuous positive airway pressure or oral appliance: an observational study. Respirology 18:1184–1190
Wang Y, Huang Q, Liu J, Wang Y, Zheng G, Lin L, Yu H, Tang W, Huang Z (2017) Vascular endothelial growth factor A polymorphisms are associated with increased risk of coronary heart disease: a meta-analysis. Oncotarget 8:30539–30551
Da RD, Forgiarini LF, Baronio D, Feijó CA, Martinez D, Marroni NP (2012) Simulating sleep apnea by exposure to intermittent hypoxia induces inflammation in the lung and liver. Mediat Inflamm 2012:879419
Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F (2002) Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med 165:67–70
Imagawa S, Yamaguchi Y, Higuchi M, Neichi T, Hasegawa Y, Mukai HY, Suzuki N, Yamamoto M, Nagasawa T (2001) Levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea–hypopnea syndrome. Blood 98:1255–1257
Lavie L, Kraiczi H, Hefetz A, Ghandour H, Perelman A, Hedner J, Lavie P (2002) Plasma vascular endothelial growth factor in sleep apnea syndrome: effects of nasal continuous positive air pressure treatment. Am J Respir Crit Care Med 165:1624–1628
Teramoto S, Kume H, Yamamoto H, Ishii T, Miyashita A, Matsuse T, Akishita M, Toba K, Ouchi Y (2003) Effects of oxygen administration on the circulating vascular endothelial growth factor (VEGF) levels in patients with obstructive sleep apnea syndrome. Intern Med 42:681–685
Maeder MT, Strobel W, Christ M, Todd J, Estis J, Wildi K, Thalmann G, Hilti J, Brutsche M, Twerenbold R, Rickli H, Mueller C (2015) Comprehensive biomarker profiling in patients with obstructive sleep apnea. Clin Biochem 48:340–346
Phillips CL, Yang Q, Williams A, Roth M, Yee BJ, Hedner JA, Berend N, Grunstein RR (2007) The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. J Sleep Res 16:217–225
Munoz-Hernandez R, Vallejo-Vaz AJ, Sanchez Armengol A, Moreno-Luna R, Caballero-Eraso C, Macher HC, Villar J, Merino AM, Castell J, Capote F, Stiefel P (2015) Obstructive sleep apnoea syndrome, endothelial function and markers of endothelialization. Changes after CPAP. PLoS One 10:e0122091
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2016) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349:g7647
Turnbull CD, Rossi VA, Santer P, Schwarz EI, Stradling JR, Petousi N, Kohler M (2017) Effect of OSA on hypoxic and inflammatory markers during CPAP withdrawal: Further evidence from three randomized control trials. Respirology 22:793–799
Ciftci TU, Kokturk O, Demirtas S, Gulbahar O, Bukan N (2011) Consequences of hypoxia-reoxygenation phenomena in patients with obstructive sleep apnea syndrome. Ann Saudi Med 31:14–18
Drager LF, Polotsky VY, Lorenzi-Filho G (2011) Obstructive sleep apnea an emerging risk factor for atherosclerosis. Chest 140:534–542
Valipour A, Litschauer B, Mittermayer F, Rauscher H, Burghuber OC, Wolzt M (2004) Circulating plasma levels of vascular endothelial growth factor in patients with sleep disordered breathing. Respir Med 98:1180–1186
Waradekar NV, Sinoway LI, Zwillich CW, Leuenberger UA (1996) Influence of treatment on muscle sympathetic nerve activity in sleep apnea. Am J Respir Crit Care Med 153:1333–1338
Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK (2000) Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 100:2332–2335
Barbé F, Duráncantolla J, Sánchezdelatorre M, Martínezalonso M, Carmona C, Barceló A, Chiner E, Masa JF, Gonzalez M, Marín JM (2012) Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 307:2161
Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunstrom E (2016) Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med 194:613–620
Weaver TE, Grunstein RR (2008) Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 5:173–178
Khan SU, Duran CA, Rahman H, Lekkala M, Saleem MA, Kaluski E (2017) A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea. Eur Heart J. https://doi.org/10.1093/eurheartj/ehx597
Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V, Neal B (2017) Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA 318:156–166
Ziegler MG, Mills PJ, Loredo JS, Ancoliisrael S, Dimsdale JE (2001) Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest 120:887–893
Lattimore JL, Wilcox I, Skilton M, Langenfeld M, Celermajer DS (2006) Treatment of obstructive sleep apnoea leads to improved microvascular endothelial function in the systemic circulation. Thorax 61:491–495
Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, Schulz R (2006) Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med 173:897–901
Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM (2000) Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res 86:1198–1202
Voelkel NF, Tuder RM (2000) Hypoxia-induced pulmonary vascular remodeling: a model for what human disease? J Clin Invest 106:733–738
Schulz R, Schmidt D, Blum A, Lopes-Ribeiro X, Lücke C, Mayer K, Olschewski H, Seeger W, Grimminger F (2000) Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: response to CPAP therapy. Thorax 55:1046–1051
Shweiki D, Itin A, Soffer D, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845
Webb NJA, Bottomley MJ, Watson CJ, Brenchley PEC (1998) Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin Sci 94:395–404
Jelkmann W (2001) Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Biochem 47:617
Gozal D, Lipton AJ, Jones KL (2002) Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep 25:59–65
Funding
This work was supported by grant 2017-1-87 for Youth Research Fund from Fujian Provincial Health Bureau.
Author information
Authors and Affiliations
Contributions
Jia-Chao Qi and LiangJi Zhang contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Qi, JC., Zhang, L., Li, H. et al. Impact of continuous positive airway pressure on vascular endothelial growth factor in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath 23, 5–12 (2019). https://doi.org/10.1007/s11325-018-1660-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-018-1660-4