Abstract
Purpose
Our goal in this study is to determine the prevalence and associated factors of obstructive sleep apnea (OSA) in morbidly obese patients undergoing bariatric surgery.
Methods
This descriptive study was conducted at King Chulalongkorn Memorial Hospital from 2007 to 2015. Data of morbidly obese patients who underwent bariatric surgery were included using ICD-10 code for principle diagnosis “morbid obesity” (E668) and ICD-9 code for “bariatric surgery” (4389, 4438, 4439).
Results
Baseline characteristics of 238 patients who met the inclusion criteria demonstrated 49.2% male, mean age of 33.9 ± 10.8 years, and mean BMI of 52.6 ± 11.6. Sleeve gastrectomy and Roux-en Y gastric bypass surgery were performed in 51.5 and 48.5%; respectively. High risk for OSA using STOP-Bang as a screening questionnaire (≥3 points) was 92.7%. The prevalence of OSA using respiratory disturbance index (RDI) ≥ 5 was demonstrated at 85.7%. Mild, moderate, and severe OSA was observed in 8.8, 15.3, and 75.9%, respectively. Snoring, STOP-Bang score ≥ 3, fatty liver, and BMI were significantly correlated with OSA compared to the group without OSA with the odds ratio of 17.04 (p = <0.0001, 95% CI = 6.67–43.49), 16 (p = 0.01, 95% CI = 1.95–131.11), 4.75 (p = 0.001, 95% CI = 1.82–12.37), and 1.04 (p = 0.045, 95% CI = 1.0009–1.09), respectively. Comparison between non-severe and severe OSA groups demonstrated dyslipidemia and BMI to be correlated with OSA severity (odds ratio = 3.06, 95% CI 1.36–6.89, p = 0.007 and odds ratio = 1.07, 95% CI 1.03–1.13, p = 0.001, respectively).
Conclusions
Obstructive sleep apnea is frequently observed in morbidly obese patients undergoing bariatric surgery and the severity tends to be severe. Snoring, STOP-Bang score ≥ 3, fatty liver, and BMI were significantly correlated with OSA. Dyslipidemia and BMI were demonstrated to be associated factors for severity of OSA in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Across the globe, obesity is a growing major health concern. In 2008, database from WHO demonstrated that 35% of people older than 20 years were overweight (more than 1400 million people) and 11% were obese. This trend was predicted to be increased in all age group [1]. In Thailand, database from department of health in 2009 and 2010 has shown that 22.2% and 36.5% of Thai people older than 15 years were obese, respectively [2]. Obesity can be treated using multi-modality approach such as lifestyle modification, medication, and bariatric surgery. Currently, bariatric surgery is a fast-growing and effective approach to control weight in morbidly obese patients who failed lifestyle modification.
As shown in various studies, intraoperative and postoperative complications in the bariatric surgery have been linked with obstructive sleep apnea (OSA). A 2012 systematic review demonstrates statistically significant relationship between OSA and perioperative complications [3].
Obstructive sleep apnea prevalence has been demonstrated to be high in this population [4–8]. Various parameters have been found as predictors for OSA including BMI, neck circumference, and Epworth sleepiness scale (ESS) score [4–9]. However, the data of OSA prevalence as well as the associated factors are limited in Asian countries.
Materials and methods
Protocol
From 2007 to 2015, we collected the data of patients with morbid obesity who underwent bariatric surgery at King Chulalongkorn Memorial Hospital, Bangkok, Thailand, by searching from electronic medical record. We used ICD-10 code for principle diagnosis “morbid obesity” (E668) and ICD-9 code for “bariatric surgery” (4389, 4438, 4439) to search name, hospital number, and admission number of patients. Criteria to define morbid obesity were BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with metabolic comorbidities including OSA, type 2 diabetes mellitus, hypertension, or dyslipidemia using the WHO recommendations [10]. After the review of medical records, data were collected including polysomnographic data and clinical data such as height; weight; BMI; neck circumference; underlying comorbidity including type 2 diabetes mellitus, hypertension, dyslipidemia, fatty liver disease, coronary artery disease, renal disease, and cerebrovascular disease; and symptoms including snoring, nocturia, headache, apnea, and dry throat. STOP-Bang was utilized as a screening questionnaire for OSA. Score of at least 3 was used to define high risk for OSA [11]. This STOP-Bang questionnaire was validated in Thai language [12]. Epworth sleepiness scale (ESS) was utilized to demonstrate degree of sleepiness in our study. Score of at least 10 was used to define excessive daytime sleepiness [13]. This ESS questionnaire was also validated in Thai language [14]. The most recent diagnostic criteria of OSA from International Classification of Sleep Disorders (3rd edition) using one of the two criteria were utilized in our study [15].
-
1.
Respiratory disturbance index (RDI) ≥ 5 events per hour plus one of OSA-related symptoms or OSA-related comorbidities
-
2.
Respiratory disturbance index (RDI) ≥ 15 events per hour.
Statistical analysis
Characteristic data were presented in mean ± standard deviation or median and range and frequencies (percent). Chi-squared or Fisher’s exact test was used to compare categorical variables. T test or Mann-Whitney U test was used to compare continuous variables. Association factors were determined by logistic regression analysis. The results were expressed as odds ratio (OR), 95% confidence intervals (CI), and p value. Univariate analysis with forward stepwise selection was used to determine the appropriate multivariate model. Multivariate analysis was performed on factors that were significant in the univariate analysis. P value of less than 0.05 was considered statistically significance. The SPSS statistical software was used for statistical analysis. The study was approved by institutional review board, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB No. 571/57).
Results
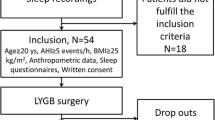
There were 255 patients underwent bariatric surgery during the period of 2007 to 2015. In 17 patients, the medical record was not available. The analysis included total of 238 patients (Fig. 1) in which all except 27 patients underwent polysomnography. There were 175 patients who had complete report of polysomnography in medical record and 36 patients who did not have complete report. In 36 patients, 29 patients had partial polysomnography report, and 7 patients were documented to have OSA and currently using PAP but polysomnography report was not available. Prevalence of OSA in our study was reported to be high at 85.7%. Using the available polysomnographic data, OSA severity was classified to mild OSA, moderate OSA, and severe OSA in 8.8, 15.3, and 75.9%, respectively. STOP-Bang score ≥ 3 points was noted at 92.7%. This finding coincided with high prevalence of OSA reported from polysomnography. Sleep gastrectomy and Roux-en Y gastric bypass were performed in 51.5 and 48.5%, respectively.
For clinical characteristics of the patients, percentage of male sex, mean age, BMI, and neck circumference were 49.2%, 33.9 ± 10.8 years, 52.6 ± 11.6 kg/m2, and 18.2 ± 2.5 inches., respectively. Hypertension (60.9%), fatty liver disease (60.5%), and dyslipidemia (53.8%) were among the most common comorbidities observed in our study. Snoring (85.3%), morning headache (37.4%), and nocturia (28.2%) were among the three most common OSA-related symptoms. In 175 patients who underwent polysomnography and had complete official report, 42.4% of the patients underwent baseline polysomnography and 57.6% underwent split-night polysomnography. Mean total AHI, total RDI, mean oxygen saturation, and nadir oxygen saturation were 71.1 ± 48.2, 71.8 ± 47.7, 88.2 ± 10.7, and 72.2 ± 15.4, respectively. Sleep-related hypoventilation was defined by an increase in PCO2 or surrogate to the level above 55 mmHg for more than 10 mins or an increase in PCO2 or surrogate above 10 mmHg compared to awake baseline value with the level above 50 mmHg for more than 10 mins [16]. Sleep-related hypoventilation was shown in 52% of this group.
Table 1 demonstrated clinical characteristics of our patients comparing the groups with and without OSA. The polysomnographic characteristics of these patients comparing between patients with non-severe OSA and severe OSA were demonstrated in Table 2. Association between variables and OSA/severity of OSA were shown in Table 3. Snoring, STOP-Bang score ≥ 3, fatty liver, and BMI were significantly correlated with OSA compared to those in the group without OSA with the odds ratio of 17.04 (p = <0.0001, 95% CI = 6.67–43.49), 16 (p = 0.01, 95% CI = 1.95–131.11), 4.75 (p = 0.001, 95% CI = 1.82–12.37), and 1.04 (p = 0.045, 95% CI = 1.0009–1.09), respectively. Comparison between non-severe and severe OSA group demonstrated dyslipidemia and BMI to be correlated with OSA severity (odds ratio = 3.06, 95% CI 1.36–6.89, p = 0.007 and odds ratio = 1.07, 95% CI 1.03–1.13, p = 0.001, respectively).
Discussion
Obstructive sleep apnea is prevalent in bariatric surgery population demonstrated at 85.7% in this first report in Thai population. Our data is resembled to previous studies in both Asian and Western countries. The prevalence of OSA in various previously published reports was reviewed in Table 4, ranging from 70 to 95% similar to our finding (85.7%) [4, 5, 7, 8]. However, prevalence of severe OSA was observed to be higher (75.9%) in our data when comparison was made with previously published paper ranging from 27.8 to 49%. We utilized RDI > 30 events per hour as a criteria for severe OSA and other studies utilized AHI instead. Theoretically, this may result in higher percentage of severe OSA reported in our study. However, if we were to use AHI > 30 events per hour instead, prevalence of severe OSA was still demonstrated to be high at 74.4%. The relatively higher BMI observed in our study (52.6 ± 11.6) compared to previously published paper (ranging from 42 ± 7.3 to 52) may be a potential explanation. We previously published our data demonstrating that the obese OSA patients compared to non-obese group had more severe OSA reflecting by higher RDI [17].
Our study showed that hypertension, fatty liver disease, dyslipidemia, and type 2 diabetes mellitus were the most common comorbidities coincided with OSA. Snoring was the most common symptom of OSA and significantly correlated with OSA. Previous studies demonstrated several variables to be correlated with OSA and severe OSA. In 2007, Lee YH’s study in multiethnic bariatric surgery population in Singapore demonstrated that neck circumference was a strong predictor for severe OSA [18]. In 2010, Yeh PS’s study in Taiwan demonstrated BMI, neck circumference, and ESS were predictors of OSA in bariatric surgery population [7]. However, in 2001, Serafini FM did not demonstrate BMI, ESS, or snoring to be correlated with severity of OSA in morbidly obese patients undergoing bariatric surgery in USA [9]. In our study, we demonstrated that snoring, STOP-Bang ≥3, fatty liver disease, and BMI significantly correlated with OSA. In addition, dyslipidemia and BMI were significant predictors for severe OSA. Our results are similar to previous reports studied in Asian population. Our data supports the use of STOP-Bang as a screening questionnaire in this bariatric surgery population. STOP-Bang was previously demonstrated to be a reasonable screening questionnaire in surgical setting as well as in the outpatient’s setting [16].
Despite our study demonstrated interesting finding, the nature of retrospective study resulted in some missing and incomplete data. Since we assumed that the patients not undergoing polysomnography did not have OSA, the prevalence may have been underestimated if some of those patients in fact had OSA. Prospective study in bariatric surgery population on OSA prevalence, postoperative outcome including the change in cardiometabolic indicators, and the effect of OSA treatment are warrant.
Conclusion
Prevalence of OSA in morbidly obese patients undergoing bariatric surgery is high and the severity tends to be severe. Snoring, STOP-Bang ≥3, fatty liver disease, and BMI were significantly correlated with OSA. Dyslipidemia and BMI were demonstrated to be associated factors for severity of OSA in this population.
References
World Health Organization (2014) Obesity and overweight fact sheet. Publishing WHO, Charlotte http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed 10 October 2014
Department of health (2013) Database of nutritional status in Thai people older than 15 years in 2009 and 2013. Publishing anamai, Thailand http://www.anamai.moph.go.th/main.php?filename =Statistics. Accessed 10 October 2014
Vasu TS, Grewal R, Doghramji K (2012) Obstructive sleep apnea syndrome and perioperative complications: a systematic review of the literature. J Clin Sleep Med 8(2):199–207. doi:10.5664/jcsm.1784
Lee YH, Johan A, Huat Wong KK, Edwards N, Sullivan C (2009) Prevalence and risk factors for obstructive sleep apnea in a multiethnic population of patients presenting for bariatric surgery in Singapore. Sleep Med 10:226–232. doi:10.1016/j.sleep.2008.01.005
Lopez PP, Stefan B, Schulman CL, Byers PM (2008) Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg 74:834–838
Khan A, King WC, Patterson EJ, Laut J, Raum W, Courcoulas AP et al (2013) Assessment of obstructive sleep apnea in adults undergoing bariatric surgery in the longitudinal assessment of bariatric surgery-2 (LABS-2) study. J Clin Sleep Med 9:21–29. doi:10.5664/jcsm.2332
Yeh PS, Lee YC, Lee WJ, Chen SB, Ho SJ, Peng WB et al (2010) Clinical predictors of obstructive sleep apnea in Asian bariatric patients. Obes Surg 20:30–35. doi:10.1007/s11695-009-9854-2
Daltro C, Gregorio PB, Alves E, Abreu M, Bomfim D, Chicourel MH et al (2007) Prevalence and severity of sleep apnea in a group of morbidly obese patients. Obes Surg 17:809–814. doi:10.1007/s11695-007-9147-6
Serafini FM, Anderson WM, Rosemurgy AS, Strait T, Murr MM (2000) Clinical predictors of sleep apnea in patients undergoing bariatric surgery. Obes Surg 11:28–31
World Health Organisation (1999) 10 facts on obesity. Publishing WHO, Charlotte http://www.who.int/features/factfiles/obesity/facts/en/. Accessed 10 October 2014
Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM (2008) STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 108(5):812–821. doi:10.1097/ALN.0b013e31816d83e4
Banhiran W, Durongphan A, Saleesing C, Chongkolwatana C (2014) Diagnostic properties of the STOP-Bang and its modified version in screening for obstructive sleep apnea in Thai patients. J Med Assoc Thai 2014 97(6):644–654
Johns MW (1991) A new method for measuring daytime sleeping: the Epworth sleepiness scale. Sleep 14:540–545
Banhiran W, Assanasen P, Nopmaneejumruslers C, Metheetrairut C (2011) Epworth sleepiness scale in obstructive sleep disordered breathing: the reliability and validity of the Thai version. Sleep Breath 15(3):571–577. doi:10.1007/s11325-010-0405-9
Darien IL (2014) International classification of sleep disorders, 3nd edn. American Academy of Sleep Medicine, Westchester
Iber C, Ancoli-Israel S, Chesson A, Quan SF (2015) The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. American Academy of Sleep Medicine Version 2, Darien, IL
Chirakalwasan N, Teerapraipruk B, Simon R, Hirunwiwatkul P, Jaimchariyatam N, Desudchit T et al (2013) Comparison of polysomnographic and clinical presentations and predictors for cardiovascular-related diseases between non-obese and obese obstructive sleep apnea among Asians. J Clin Sleep Med 9(6):553–555. doi:10.5664/jcsm.2748
Nagappa M, Liao P, Wong J, Auckley D, Ramachandran SK, Memtsoudis S et al (2015) Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One 10(12):1–21. doi:10.1371/journal.pone.0143697
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kositanurit, W., Muntham, D., Udomsawaengsup, S. et al. Prevalence and associated factors of obstructive sleep apnea in morbidly obese patients undergoing bariatric surgery. Sleep Breath 22, 251–256 (2018). https://doi.org/10.1007/s11325-017-1500-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-017-1500-y