Abstract
Purpose
We determined whether hypoxia parameters are associated with C-reactive protein (CRP), mean platelet volume (MPV), white matter hyperintensity (WMH), and the severity of obstructive sleep apnea (OSA), and also evaluated whether hypoxia parameters, CRP, MPV, and WMH differ in patients with similar apnea-hypopnea index (AHI) scores.
Methods
A total of 297 patients, who were evaluated using polysomnography, were assessed retrospectively. The measured hypoxia parameters included total sleep time with oxygen saturation <90% (ST90), percentage of cumulative time with oxygen saturation <90% (CT90), and lowest oxygen saturation (min SaO2). The patients were divided into subgroups according to their CT90 values, and patients with different AHI severities were divided into subgroups according to their ST90 and min SaO2 levels.
Results
Hypoxia parameters are associated with CRP, MPV, WMH, and the severity of OSA (P < 0.05). The hypoxia parameters differed in all subgroup analyses of similar AHI groups (P < 0.001), and CRP differed only in severe OSA (P < 0.008, P < 0.001). In subgroup analyses of similar AHI groups, MPV and WMH were not significantly different (P > 0.05). Above the hypoxia threshold (CT90 ≥ 10%) of CRP, MPV increased significantly and the presence of WMH increased twofold.
Conclusions
These data suggest that increased hypoxia severity may mediate increased inflammation and activation of platelets and contribute to the pathogenesis of WMH in patients with OSA. In addition, patients with severe OSA may show significant variability in inflammation and vascular risk. Further prospective data are needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
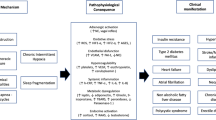
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of upper airway partial or complete collapse during sleep, resulting in hypopnea or apnea. The severity of OSA is traditionally stratified using the apnea-hypopnea index (AHI), regardless of the duration or morphology of apnea or hypopnea episodes [1]. Thus, AHI does not completely reflect the severity of hypoxia [2, 3]. In addition, patients who have similar AHI may have different clinical symptoms and outcomes. Deeper and longer oxygen desaturation events presumably contribute more to physiological stress and harmful health consequences than shallower and shorter events. In addition, patients with severe hypoxia, despite having a similar AHI, may have more severe physiological stress and cardiovascular consequences and could even die [3]. On the other hand, the American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events classifies the severity of OSA according to the AHI, and it is recommended that nocturnal hypoxia be classified using the lowest oxygen saturation (min SaO2) value [1]. Due to the lack of a more precise definition, there is no universally accepted quantitative clinical test to measure the intensity and/or severity of chronic intermittent hypoxia [4].
An association among OSA, cardiovascular disease, and coagulation abnormalities has been suspected for many years, although the mechanisms that mediate this association are still not completely understood. However, episodic collapse of the upper airway leads to chronic intermittent hypoxia, triggering oxidative stress and chronic inflammation and giving rise to detrimental effects on cardiovascular, neurocognitive, and metabolic functions [5]. The inflammatory processes leading to endothelial dysfunction also induce atherosclerosis in OSA [5, 6]. C-reactive protein (CRP) is a prototypic marker of inflammation and has an active role in atherogenesis by promoting expression of adhesion molecules. CRP levels may be an independent predictor of future cardiovascular events in apparently healthy subjects and patients who are diagnosed with cardiovascular disease [7]. Hypoxemia and increased oxidative stress are important mechanisms related to increased systemic inflammation in OSA [6]. An association between OSA and systemic inflammatory markers has been reported in several studies [2, 8].
Chronic intermittent hypoxia inducing sympathetic hyperactivity as well as high blood pressure and hemodynamic changes, including hypercoagulability and platelet activation, have been demonstrated in OSA. The cause of increased platelet activation remains unclear, but the severity of OSA seems to influence platelet aggregation as a function of nocturnal hypoxia [9]. Platelets play an important role in thrombosis, inflammation, and atherogenesis and activated platelets function in cardiovascular disease and complications [10]. Platelet volume is a recent marker of platelet activation and function, and mean platelet volume (MPV) is an indicator of platelet activation [11]. Increased MPV is associated with cardiovascular disease and vascular risk factors [10]. Furthermore, previous studies have demonstrated that high MPV is associated with OSA [12, 13].
In brain magnetic resonance imaging (MRI), cerebral white matter hyperintensities (WMH) are hyperintense lesions in the periventricular or subcortical areas on T2-weighted or fluid-attenuated inversion recovery (FLAIR) sequences [14]. Although the pathological correlates of WMH are heterogeneous, the presence of focal myelinolysis, axonal loss, and gliosis associated with vessel wall hyalinosis suggests that chronic hypoperfusion may contribute to the development of WMH [15]. In addition, the association of vascular risk factors with WMH in OSA may explain why the pathogenesis of WMH is vascular in nature [16]. WMH are an important public health issue because they are associated with stroke, dementia, and death and may also be associated with OSA and CRP [16–18].
In this study, patients within the AHI severity categories were divided into subgroups based on total sleep time with oxygen saturation <90% (ST90), percentage of cumulative time with oxygen saturation <90% (CT90), and min SaO2. Then, we determined whether hypoxia parameters are associated with CRP, MPV, WMH, and severity of OSA and also evaluated whether hypoxia parameters, CRP, MPV, and WMH differ in patients with varying OSA severity but similar AHI scores.
Materials and methods
Study design and patients
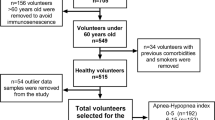
This study was performed at Baskent University Alanya Research and Medical Center (Alanya, Turkey). The medical charts of patients who underwent a complete polysomnography (PSG) test at our sleep disorder center (accredited by the Turkish Sleep Association) between October 2008 and October 2015 were evaluated. In total, 297 patients whose medical records contained CRP levels, complete blood counts, and MRI scans were included after application of exclusion criteria. Laboratory tests, CRP and MPV values, and the presence of WMH on MRI scans were collected retrospectively from the patients’ medical records. Patients aged ≥18 years who had no anemia, no active infection, and CRP levels <10 mg/L were included in the study. Age, sex, body mass index (BMI), Epworth Sleep Scale (ESS), and hypoxia parameters, including min SaO2, ST90, CT90, and sleep time, were calculated and recorded from the patients’ PSG records. The presence of medical co-morbidities including history of hypertension, diabetes mellitus, coronary heart disease, hyperlipidemia, cerebrovascular disease, and smoking status was recorded. Patients were excluded if they had central sleep apnea syndrome, narcolepsy, previous treatment for OSA (continuous positive airway pressure, surgery, and/or oral device), age <18 years, chronic obstructive pulmonary disease, bronchial asthma, dementia, renal failure, hepatic damage, malignancy, head trauma, or a brain tumor. Participants who had high CRP levels (≥10 mg/L) were excluded, because high CRP levels can result from non-specific inflammation and therefore might not provide a positive predictive value [7].
Thus, 223 OSA patients and 74 control subjects were included. Participants were divided into the following four groups based on their AHI: (1) normal/simple snoring, AHI < 5; (2) mild OSA, AHI 5 to <15; (3) moderate OSA, AHI 15 to <30; and (4) severe OSA, AHI ≥ 30. All of the participants were divided into the CT90 < 10% or CT90 ≥ 10% hypoxia categories according to Zhang et al. [19]. In addition, the patients within the AHI severity categories were divided into subgroups according to the lowest and highest ST90 and lowest and highest min SaO2.
This study was evaluated and determined to be exempt from the requirement for informed consent by the Baskent University Institutional Review Board due to its retrospective study design.
PSG
All of the study participants underwent PSG at a sleep laboratory using a computerized PSG device (E series, 44 channels; Compumedics, Victoria, Australia). During the PSG study (16 channels), the following parameters were documented: four-channel electroencephalogram, electrooculogram, submental and leg electromyogram, electrocardiogram, nasal airflow using a nasal pressure cannula, airflow at the nose and mouth (thermistors), chest and abdominal respiratory movements, oxygen saturation (pulse oximetry), snoring microphone, and body position. All of the studies were interpreted by a sleep specialist who was blinded to the participants’ characteristics. Apnea was defined as cessation of airflow for ≥10 s with continued effort (obstructive) or lack of effort (central) to breathe. Hypopnea was defined as >50% decrease in a valid measure of airflow without a requirement for associated oxygen desaturation or arousal, and with less airflow reduction in association with oxygen desaturation >3% or an arousal of at least 10 s. The ST90 was recorded in minutes, and min SaO2 and CT90 were recorded as percentages. The min SaO2 value was the lowest oxygen saturation during sleep. Sleep staging was performed according to the American Academy of Sleep Medicine criteria [1].
Laboratory measurements
Blood samples in phlebotomy tubes containing no anticoagulant were centrifuged at 3000 g for 10 min and subsequently analyzed. Serum CRP was measured using latex-enhanced immunoturbidimetry with monoclonal anti-CRP antibodies (Architect C 800, Abbott Diagnostic Systems, Abbott Park, IL, USA) (CRP reference level, ≤8 mg/L).
Blood samples in ethylenediaminetetraacetic acid (EDTA) tubes were analyzed using electrical impedance and optical fluorescence methods in an automated hematology analysis device (CELL-DYN Ruby, Diagnostic Systems, Abbott Park, IL, USA). The hematologic parameters measured included leukocyte count (reference range, 4.5 to 11 × 109/L), neutrophil count (2 to 6.9 × 109/L), lymphocyte count (0.6 to 3.4 × 109/L), hemoglobin (female, 12 to 16.5 mg/dL; male, 13.5 to 17.50 mg/dL), platelet count (140 to 440 × 109/L), MPV (0 to 99.9 femtoliters [fL]), and red cell distribution width (RDW) (11.6 to 17%).
The presence of WMH was evaluated by MRI of the whole brain. All of the MRI brain scans (1.0 Tesla, Siemens Magnetom Vision Plus, Siemens, Munich, Germany) were performed with the orbitomeatal line as a reference. The scans included ≥3 sequences as follows: sagittal T1-weighted, axial T2-weighted, and axial FLAIR images. The slice thickness was 5 mm, the gap was 1 mm, and no intravenous contrast was used. All of the MRI scans were reviewed and scored by a radiologist who was blinded to the clinical details. The scan results were reinvestigated for the presence of WMH.
Statistical analysis
Data analysis was performed using statistical software (IBM SPSS Statistics for Windows, Version 22.0, IBM Corp., Armonk, NY, USA). Continuous variables were reported as means ± standard deviations or medians (ranges). Categorical variables were reported as numbers (%). The normality of continuous variables was evaluated using Kolmogorov Smirnov tests. The similarities among groups were evaluated using variance Levene’s tests. Differences in continuous variables between two groups were evaluated using independent samples t tests or Mann-Whitney tests. Comparisons between more than two groups were evaluated using one-way analysis of variance or Kruskal-Wallis tests. Pairwise comparisons were evaluated using Tukey’s tests and Siegel Castellan’s tests. Categorical variables were compared using Pearson’s χ2 tests (chi-square tests). The relationships between continuous variables were determined using Spearman’s rank correlation. Multiple stepwise linear regression analyses were used to determine the factors affecting ST90 and minSaO2. The best MPV cutoff value for discriminating the hypoxia threshold (CT90 ≥ 10%) was determined using ROC curve analysis. The area under the curve (AUC), sensitivity, specificity, and negative and positive predictive values were calculated. Statistical significance was defined as P ≤ 0.05.
Results
Comparisons of the demographic characteristics of the OSA and control groups are shown in Table 1. The odds ratio (OR) of WMH in the OSA group compared with the control group was 3.2 (95% confidence interval [CI] 1.84–5.71; P < 0.001). Univariate analyses revealed significant correlations between ST90 and age (r = 0.370), BMI (r = 0.360), AHI (r = 0.808), ESS (r = 0.152), CRP (r = 0.433), MPV (r = 0.346), and WMH (ESS, P = 0.009; all other parameters, P < 0.001). Univariate analyses also revealed significant inverse correlations between min SaO2 and age (r = −0.404), BMI (r = −0.385), AHI (r = −0.771), ESS (r = −0.149), MPV (r = −0.387), and WMH (ESS, P = 0.010; all other parameters, P < 0.001). After adjusting for confounders, multiple linear regression revealed that ST90 was independently positively correlated with AHI (r = 1.356, P < 0.001), BMI (r = 1.081, P = 0.038), and CRP (r = 2.772, P = 0.033), and negatively correlated with min SaO2 (r = −2.926, P < 0.001). After adjusting for confounders, multiple linear regression analyses revealed that min SaO2 was independently negatively correlated with AHI (r = −0.197, P < 0.001), BMI (r = −0.224, P = 0.007), age (r = −0.087, P = 0.005), CRP (r = −0.430, P = 0.038), MPV (r = −1.226, P < 0.001), and ST90 (r = −1.054, P < 0.001).
All participants were divided into the CT90 < 10% and CT90 ≥ 10% hypoxia subgroups (Table 2). The CT90 values of approximately half of the patients with severe OSA (51; 52% of patients) were ≥10%. The OR of WMH in the CT90 ≥ 10% group was 2.21 (95% CI, 1.17–4.16; P = 0.0145). According to receiver-operating characteristic (ROC) analysis, a MPV value >8.34 fL could predict a threshold CT90 ≥ 10% with a sensitivity of 77% and a specificity of 57% (AUC, 0.714; 95% CI, 0.659–0.765; P < 0.001).
In the subgroup analysis of ST90 and min SaO2 within the AHI severity categories, hypoxia parameters (ST90, CT90, and min SaO2) were significantly different in all OSA severity categories, and CRP values were significantly different only in the severe OSA group. MPV and WMH were not different in the subgroup analysis of hypoxia parameters in similar AHI groups (Tables 3 and 4).
Discussion
The main goal of this study was to evaluate the associations between hypoxia parameters (ST90, CT90, and min SaO2) and CRP, MPV, and WMH on brain MRI with regard to OSA severity. This study revealed that ST90 and min SaO2 were significantly correlated not only with CRP and MPV (P < 0.001) but also with WMH on brain MRI. In addition, a CT90 value ≥10% was reported to have clinical relevance [19, 27]. Above this threshold, CRP and MPV were significantly elevated and WMH was increased twofold in patients with OSA (Table 2). MPV can be used as a moderate quality indicator of hypoxia threshold (CT90 ≥ 10%) at the optimal cutoff value 8.34 fL, with a sensitivity of 77% and a specificity of 57%. Furthermore, in subgroup analyses of the ST90 and min SaO2 values in the different OSA severity categories, hypoxia parameters were different in all similar AHI groups and CRP levels significantly differed only in the severe OSA group (Tables 3 and 4). These data suggest that increased severity hypoxia may mediate increased inflammation and platelet activation and contribute to the pathogenesis of WMH in patients with OSA. In addition, patients with severe OSA may show significant variability in inflammation and vascular risk.
Previous studies have also reported an association between hypoxia parameters and CRP levels in patients with OSA [2, 8]. In one study, hypoxia was measured by pulse oximetry during sleep in the homes of middle-aged community residents [8]. In another study, ST90 was only evaluated in patients with severe OSA [2]. In this study, ST90 and min SaO2 were independently correlated with CRP (P < 0.033 and P < 0.038, respectively). In addition, in a subgroup analysis of the hypoxia parameters in different OSA severities, CRP levels only significantly differed in the severe OSA group (Tables 3 and 4). CRP is an important inflammatory marker and predicts the vascular risk [7]. These findings suggest that despite similar AHI values, patients with severe hypoxia had a greater level of inflammation and increased vascular risk. However, variable results have been reported in previous studies [20, 21]. Guilleminault et al. reported that BMI was associated with CRP in OSA patients; however, the patients in that study were fewer and older than those in our study [20]. The Wisconsin Sleep Cohort Study reported that CRP levels were associated with BMI but not with OSA severity. The authors suggested that obesity might be the primary reason for the relationship between OSA and CRP levels [21]. The discrepancy with our results may be due to differences in subject characteristics and the methods used to assess OSA. This study included a greater percentage of males (66%) than the Wisconsin Sleep Cohort Study (55%), which may have affected the results, as obesity is associated with elevated levels of CRP and the association is stronger in women [22].
In this study, we found that OSA severity, ST90, and min SaO2 significantly correlated with MPV (P < 0.001). In a subgroup analysis of hypoxia parameters in similar AHI groups, MPV did not differ significantly (Tables 3 and 4). In addition, a higher MPV was an independent predictor of hypoxia. MPV can be used as a moderate quality indicator of hypoxia threshold (CT90 ≥ 10%) at the best cutoff value, 8.34 fL, with a sensitivity of 77% and a specificity of 57%. These findings suggest that a hypoxia above this threshold indicates an increased MPV turnover. Larger MPVs are enzymatically more active and associated with platelet reactivity and platelet activity, which increase in vascular diseases [10, 11]. Previous results showed that MPV is associated with the desaturation index, and another study reported that MPV is associated with ST90 and min SaO2 only in patients with severe OSA [12, 13]. However, another study reported an inverse correlation between MPV and min SaO2, but no correlation between MPV and OSA severity [23]; the sample size of that study was smaller (n = 98) than that in our study.
In this study, patients with OSA had a threefold increased risk of exhibiting WMH compared with the control group. In addition, the risk of exhibiting WMH was 2.2-fold higher in the CT90 ≥ 10% group than the CT90 < 10% group. Hypoxia above the threshold (CT90 ≥ 10%) may be important in the pathogenesis of WMH in patients with OSA. Nevertheless, WMH was not different in the subgroup analysis of hypoxia parameters according to the different severity of OSA in similar AHI groups (Tables 3 and 4). Earlier studies have reported that moderate to severe OSA resulted in a twofold increased risk of exhibiting WMH [17, 18]. However, some previous studies did not demonstrate a relationship between OSA and WMH. One study was limited by its small sample size and another by the older age of its subjects [24, 25]. The pathogenesis of WMH suggests that it represents ischemic tissue developing from inflammation, atherosclerosis, and repeated episodes of cerebrovascular shearing stress [15]. Microglial activation may occur during chronic cerebral hypoperfusion and could contribute to further tissue damage. Oligodendrocytes and neurons may be more susceptible to hypoperfusion under chronic low-grade inflammation, and lesions may progress at a more rapid rate [26].
Previous studies observed that a CT90 ≥ 10% and ST90 > 36 min is an important threshold for predicting surgical outcome [19, 27]. These parameters could reflect the severity of intermittent hypoxia during sleep, which may be associated with destabilized respiratory control, decreased respiration chemoreceptor sensitivity, and impaired pharyngeal dilator muscle function. Nocturnal intermittent hypoxia above this threshold may disrupt the normal structured autonomic and hemodynamic responses to sleep [28–30]. In the current study, OSA patients above this threshold have more hypoxia, inflammation, vascular risk factors, and WMH, as compared to those with an OSA under this threshold (CT90 < 10%) (Table 2). It could be hypothesized that neurophysiologic compensation mechanisms are irreversibly damaged in these patients. Together, these data suggest that approximately half of the severe OSA patients are in the irreversible stage, and any treatment modality (CPAP and/or surgery) may avoid further damage but have only symptomatic utility and not reverse abnormalities in the neurologic structures.
The strength of this study lies in the subgroup analyses of CRP, MPV, WMH, and hypoxia parameters among patients with similar AHI values. In addition, participants were divided into CT90 < 10% or CT90 ≥ 10% hypoxia categories. The limitations of this study include its retrospective design, inherent problems of selection bias, and that it was a single-institutional analysis, which could lead to referral bias. In addition, vascular factors, such as coronary heart disease, hypertension, hyperlipidemia, diabetes mellitus, smoking, and obesity, were not strictly excluded. The lack of measurements of other platelet-activating factors, such as thromboxane A2 and β-thromboglobulin, and thrombotic or endothelial dysfunction factors, such as tissue plasminogen activator, the von Willebrand factor, and homocysteine, is also a limitation. Comparing our results with levels of other thrombotic and endothelial dysfunction markers may improve the understanding of OSA pathophysiology. Furthermore, MRI scans and blood tests were not performed immediately after PSG, and we did not evaluate the localization or number of WMH.
In conclusion, these data suggest that increased hypoxia severity may mediate increased inflammation and platelet activation and contribute to the pathogenesis of WMH in patients with OSA. In addition, patients with severe OSA may show significant variability in inflammation and vascular risk. On the other hand, inflammation, platelet activation, and WMH increase on brain MRI in patients with hypoxia above a certain threshold (CT90 ≥ 10%); therefore, vascular risk factors increase even more in these hypoxic patients. Based on the present and previous studies, AHI combined with hypoxia parameters may enable better identification of prognostic information and selection of individualized treatment options in patients with OSA. Further prospective data are needed.
References
Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV, American Academy of Sleep Medicine (2015) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.2. Darien IL: American Academy of sleep medicine. J Clin Sleep Med 15(11):1253–1254
Zhang XB, Zen HQ, Lin QC, Chen GP, Chen LD, Chen H (2014a) TST, as a polysomnographic variable, is superior to the apnea hypopnea index for evaluating intermittent hypoxia in severe obstructive sleep apnea. Eur Arch Otorhinolaryngol. doi:10.1007/s00405-014-3044-0
Kulkas A, Tiihonen P, Julkunen P, Mervaala E, Töyräs J (2013) Novel parameters indicate significant differences in severity of obstructive sleep apnea with patients having similar apnea-hypopnea index. Med Biol Eng Comput. doi:10.1007/s11517-013-1039-4
Bostanci A, Turhan M, Bozkurt S (2015) Factors influencing sleep time with oxygen saturation below 90% in sleep-disordered breathing. Laryngoscope. doi:10.1002/lary.24942
Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A et al (2008) Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation scientific statement from the American Heart Association Council for high blood pressure research professional education committee, council on clinical cardiology, stroke council, and council on cardiovascular nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. doi:10.1161/CIRCULATIONAHA.107.189375
Song D, Fang G, Greenberg H, Liu SF (2015) Chronic intermittent hypoxia exposure-induced atherosclerosis: a brief review. Immunol Res. doi:10.1007/s12026-015-8703-8
Ridker PM (2007) C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 49(21):2129–2138
Muraki I, Tanigawa T, Yamagishi K, Sakurai S, Ohira T, Imano H et al (2010) Nocturnal intermittent hypoxia and C reactive protein among middle-aged community residents: a cross-sectional survey. Thorax. doi:10.1136/thx.2009.128744
Rahangdale S, Yeh SY, Novack V, Stevenson K, Barnard MR, Furman MI, Frelinger AL, Michelson AD, Malhotra A (2011) The influence of intermittent hypoxemia on platelet activation in obese patients with obstructive sleep apnea. J Clin Sleep Med 7(2):172–178
Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP (2009) Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost 9(3):177–190
Martin JF, Shaw T, Heggie J, Penington DG (1983) Measurement of the density of human platelets and its relationship to volume. Br J Haematol 54(3):337–352
Varol E, Ozturk O, Gonca T, Has M, Ozaydin M, Erdogan D et al (2010) Mean platelet volume is increased with severe obstructive sleep apnea. Scand J Clin Lab Invest. doi:10.3109/00365513.2010.520733
Nena E, Papanas N, Steiropoulos P, Zikidou P, Zarogoulidis P, Pita E et al (2012) Mean platelet volume and platelet distribution width in non-diabetic subjects with obstructive sleep apnoea syndrome: new indices of severity? Platelets. doi:10.3109/09537104.2011.632031
Ovbiagele B, Saver JL (2006) Cerebral white matter hyperintensities on MRI: current concepts and therapeutic implications. Cerebrovasc Dis 22(2–3):83–90
Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F et al (1993) Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 43(9):1683–1689
Debette S, Markus HS (2010) The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systemic review and meta-analysis. BMJ 341:c3666. doi:10.1136/bmj.c3666
Kim H, Yun CH, Thomas RJ, Lee SH, Seo HS, Cho ER et al (2013) Obstructive sleep apnea as a risk factor for cerebral white matter change in middle-aged and older general population. Sleep. doi:10.5665/sleep.2632
Nishibayashi M, Miyomoto M, Miyamoto T, Suzuki K, Hirata K (2008) Correlation between severity of obstructive sleep apnea and prevalence of silent cerebrovascular lesions. J Clin Sleep Med 4(3):242–247
Zhang J, Li Y, Cao X, Xian J, Tan J, Dong J, Ye J (2014b) The combination of anatomy and physiology in predicting the outcomes of velopharyngeal surgery. Laryngoscope. doi:10.1002/lary.24510
Guilleminault C, Kirisoglu C, Ohayon MM (2004) C-reactive protein and sleep-disordered breathing. Sleep 27(8):1507–1511
Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E (2007) Correlates of serum C-reactive protein (CRP)–no association with sleep duration or disordered breathing. Sleep 30(8):991–996
Khera A, Vega GL, Das SR, Ayers C, McGuire DK, Grundy SM, de Lemos JA (2009) Sex differences in the relationship between C-reactive protein and body fat. J Clin Endocrinol Metab. doi:10.1210/jc.2008-2406
Kurt OK, Yildiz N (2013) The importance of laboratory parameters in patients with obstructive sleep apnea syndrome. Blood Coagul Fibrinolysis. doi:10.1097/MBC.0b013e32835d53d4
Davies CW, Crosby JH, Mullins RL, Traill ZC, Anslow P, Davies RJ et al (2001) Case control study of cerebrovascular damage defined by magnetic resonance imaging in patients with OSA and normal matched control subjects. Sleep 24(6):715–720
Ding J, Nieto FJ, Beauchamp NJ Jr, Harris TB, Robbins JA, Hetmanski JB et al (2004) Sleep-disordered breathing and white matter disease in the brainstem in older adults. Sleep 27(3):474–479
Wakita H, Tomimoto H, Akiguchi I, Matsuo A, Lin JX, Ihara M et al (2002) Axonal damage and demyelination in the white matter after chronic cerebral hypoperfusion in the rat. Brain Res 924(1):63–70
Turhan M, Bostanci A, Bozkurt S (2015) Predicting the outcome of modified tongue base suspension combined with uvulopalatopharyngoplasty. Eur Arch Otorhinolaryngol. doi:10.1007/s00405-014-3311-0
Skelly JR, Bradford A, O'Halloran KD (2010) Intermittent hypoxia impairs pharyngeal dilator muscle function in male but not female rats. Adv Exp Med Biol 669:285–287. doi:10.1007/978-1-4419-5692-7_58
Kimoff RJ, Sforza E, Champagne V, Ofiara L, Gendron D (2001) Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med 164(2):250–255
Bradford A, McGuire M, O'Halloran KD (2005) Does episodic hypoxia affect upper airway dilator muscle function? Implications for the pathophysiology of obstructive sleep apnoea. Respir Physiol Neurobiol. doi:10.1016/j.resp.2005.04.001
Authors’ contributions
A. Yilmaz Avci and S. Avci designed the study. A. Yilmaz Avci and H. Lakadamyali acquired and interpreted the data. A. Yilmaz Avci, S. Avci, and U. Can revised the manuscript for important intellectual content.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding sources were used.
Financial disclosure
The authors declared that this study received no financial support.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Because this was a retrospective study, no ethical committee approval was taken. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Oral informed consent was taken from all the participants.
Rights and permissions
About this article
Cite this article
Yilmaz Avci, A., Avci, S., Lakadamyali, H. et al. Hypoxia and inflammation indicate significant differences in the severity of obstructive sleep apnea within similar apnea-hypopnea index groups. Sleep Breath 21, 703–711 (2017). https://doi.org/10.1007/s11325-017-1486-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-017-1486-5