Abstract
Purpose
No definitive associations or causal relationships have been determined between obstructive sleep apnea-hypopnea (OSAH) and sleep bruxism (SB). The purpose of this study was to investigate, in a population reporting awareness of both OSAH and SB, the associations between each specific breathing and jaw muscle event.
Methods
Polysomnography and audio–video data of 59 patients reporting concomitant OSAH and SB history were analyzed. Masseteric bursts after sleep onset were scored and classified into three categories: (1) sleep rhythmic masticatory muscle activity with SB (RMMA/SB), (2) sleep oromotor activity other than RMMA/SB (Sleep-OMA), and (3) wake oromotor activity after sleep onset (Wake-OMA).
Spearman’s rank correlation coefficient analyses were performed. Dependent variables were the number of RMMA/SB episodes, RMMA/SB bursts, Sleep-OMA, and Wake-OMA; independent variables were apnea-hypopnea index (AHI), arousal index(AI), body mass index(BMI), gender, and age.
Results
Although all subjects had a history of both SB and OSAH, sleep laboratory results confirmed that these conditions were concomitant in only 50.8 % of subjects. Moderate correlations were found in the following combinations (p < 0.05); RMMA/SB episode with AI, RMMA/SB burst with AI and age, Sleep-OMA burst with AHI, and Wake-OMA burst with BMI.
Conclusions
The results suggest that (1) sleep arousals in patients with concomitant SB and OSAH are not strongly associated with onset of RMMA/SB and (2) apnea-hypopnea events appear to be related to higher occurrence of other types of sleep oromotor activity, and not SB activity. SB genesis and OSAH activity during sleep are probably influenced by different mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some patients report concomitant complaints related to sleep bruxism (SB) and obstructive sleep apnea-hypopnea (OSAH) [1–6]. However, the strength and specificity of this association during sleep remain debated in the literature. [7, 8]. In a previous paper, we reported from a nonconsecutive case series our attempt to determine causality between SB and OSAH using a temporal analysis of co-occurrences of jaw muscle activity and apnea-hypopnea [5]. In summary, our results showed that almost half the SB events (i.e., rhythmic masticatory muscle activity—RMMA) occurred after sleep apnea-hypopnea events, suggesting a weak association, and that whereas some SB-related RMMA were concomitant to OSAH, others were probably not related to apnea-hypopnea. Hence, no definite conclusion could be drawn on the strength of the association or on causality between OSAH and SB [9, 10]. Various methodological factors may explain these discrepancies.
For example, the use of different methods to assess SB presence (e.g., questionnaire alone vs. advanced sleep recording and scoring methods) is probably a major cause of discrepancy [1–8]. A recent sleep laboratory study in a large general population sample with wide age range failed to show any strong correlations between sleep breathing variables and SB [11]. However, that study was performed without audio–video recording to confirm the specificity of SB. In fact, recording and scoring of jaw muscle activity during sleep revealed that various types of muscle activity are not specific to SB events. These include myoclonic contraction, swallowing, sighing, and idiopathic or natural jaw muscle activity [12, 13]. To assess the specificity of the putative association between OSAH and SB, it is critical to perform a discriminant analysis to distinguish typical RMMA in SB from other types of orofacial motor activity using polysomnography and audio–video recording (PSG–AV).
In this study, because we know that a weak temporal sequence of OSAH triggers RMMA [5], we hypothesized that the number of AH events will correlate with the number of SB events, further supporting a weak association between OSAH and SB. The analysis was performed in subjects who reported concomitant SB tooth grinding and sleep apnea-hypopnea complaints, in other words, patients with suspected concomitant OSAS and SB.
Methods
Subject characteristics and study design
Fifty-nine Japanese patients (47 male, 12 female) with a mean age of 44.8 years (SD 10.8, range 22–72) reporting awareness of breathing cessation and tooth grinding as well as signs and symptoms of OSAH and SB participated in this study. Subjects spent one night in a sleep clinic to confirm the presence of OSAH and SB. Patients with OSAH reported (1) unintentional sleepiness episodes during daytime, unrefreshing sleep, or poor sleep quality; (2) sudden awakenings with breathing cessation, gasping, or choking during sleep; and (3) bed partner’s reports of loud snoring and/or breathing cessations during sleep. Patients with SB reported (1) awareness of tooth grinding sounds or tooth clenching during sleep, as reported by sleep partners or family members; (2) jaw muscle discomfort, fatigue, or pain and jaw lock upon awaking; and (3) clinician-observed tooth wear. Subjects were excluded if they had history of major neurologic, psychiatric, or sleep disorders (e.g., REM behavior disorder, periodic leg movements during sleep) or used psychoactive medication, had a temporomandibular disorder (e.g., orofacial pain, mouth opening limitation), or were edentate.
This study was approved by the ethics committee of Hokkaido University Hospital (No. 010-0033). Written informed consent was obtained from all subjects prior to participation. Ten of the 59 subjects also participated in our previous study [5].
Polygraphic sleep recording
The 59 subjects slept for one night in a sleep laboratory, and polysomnographic (PSG) recording and scoring were performed, as described below. The recording variables included electroencephalograms (EEGs) at C3-M2, C4-M1, O1-M2, and O2-M1; right and left electrooculogram; electromyogram (EMG) of the submental, masseter, and anterior tibialis muscle; and electrocardiogram. Body positions were detected using a position sensor equipped with a 3D accelerometer. Sleep breathing variables included airflow (with a nasal/oral thermistor), chest and abdominal effort, and SpO2 measured by pulse oximetry. Recordings were performed with an Alice 5 PSG system (Philips Electronics, Amsterdam, the Netherlands). PSG–AV and masseter recordings were used for SB scoring. The masseter side selected for analysis was randomized across subjects: right side for 30 subjects and left side for 29 subjects.
All recording signals were amplified and analogue-to-digital (A/D)-converted at a 2-kHz sampling frequency. All scorings were performed offline using commercial software (Alice Sleepware, Philips Electronics) and LabChart 5 (ADInstruments, Bella Vista, NSW, Australia). Masseter EMG data were high-pass-filtered at 20 Hz and converted to absolute values using LabChart 5. Sleep stages were scored according to standard criteria [14].
Assessment of breathing and masseter muscle activity
Sleep apnea and hypopnea events were scored according to standard criteria, with an apnea-hypopnea index (AHI) threshold of 5 events/h or more [14, 15].
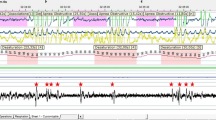
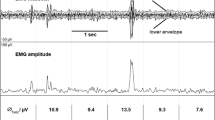
EMG bursts of sleep rhythmic masticatory muscle activity with SB (RMMA-SB) and other oromotor activities were assessed according to published criteria [13–19]. The amplitude threshold was set at twice the baseline activity [13]. Bursts with a greater amplitude than the value with duration exceeding 0.25 s were selected after onset of sleep until wake-up in the morning.
Other oromotor activities observed during wake stage were classified as wake oromotor activity after sleep onset (Wake-OMA). The sleep-related oromotor activity (OMA) (e.g., swallowing, coughing, or face scratching), other than RMMA/SB, was classified as sleep oromotor activity (Sleep-OMA) based on PSG–AV scoring. Unidentified bursts, i.e., face hidden by a blanket, were excluded from the analysis.
SB events were estimated by localizing RMMA in masseter EMG traces. EMG events separated by 3-s intervals were recognized as RMMA/SB episodes if they corresponded to one of the three following patterns: phasic (three or more masseter EMG bursts, each lasting 0.25 to 2.0 s), tonic (at least one masseter EMG burst longer than 2.0 s), or mixed (both masseter burst types). Then EMG bursts included in RMMA/SB episodes were scored as RMMA/SB bursts (Fig. 1) .
Inclusion criteria for SB diagnosis based on PSG–AV recoding was the presence of >2 RMMA/SB episodes/h and/or >25 RMMA/SB bursts/h [15, 17].
Statistical analysis
Spearman’s rank correlation coefficient analyses were performed on data from the 59 subjects. Dependent variables were the number of RMMA/SB episode, RMMA/SB bursts, Sleep-OMA bursts, and Wake-OMA bursts per hour. Independent variables were (1) the AHI or total number of apnea and hypopnea events(AH events) per hour of sleep; (2) the arousal index (AI = total number of wake arousals during sleep period including sleep-related micro-arousals (3–15 s)/h) [20]; (3) body mass index (BMI); (4) gender, with male–female classification treated as a categorical factor using dummy variables (female = 0, male = 1); and (5) age.
For the correlation analysis, statistical significance was set at p < 0.05. Correlations with AHI, AI, BMI, age, and gender were investigated for each number of bursts. Analyses were performed using Microsoft Office Excel 2007 (Microsoft Co.) and Statcel 2 (OMS Publishing Inc., Tokorozawa, Japan).
Results
Polysomnographic sleep data analysis
Sleep macrostructure data are presented in Table 1.
Sleep breathing data
Most detected and scored AH events were obstructive apnea-hypopnea (91.5 %). The mean AHI was 16.2 (SD 16.3). Forty-one (69 %) of the subjects were diagnosed with OSAH using AHI ˃5/h (range 0–60) (Fig. 2). Although all subjects had a history compatible with sleep apnea, one subject showed no AH event during PSG recording.
A Venn diagram representation of subject distribution based on RMMA/SB and OSAH sleep laboratory diagnosis. NB: 41patients were diagnosed as OSAH, with an AHI exceeding 5/h. Forty-six patients were diagnosed as SB, with an RMMA/SB episode index exceeding 2/h and/or number of RMMA/SB bursts exceeding 25/h. Dx diagnosis, OSAH obstructive sleep apnea-hypopnea, RMMA rhythmic masticatory muscle activity, SB sleep bruxism
Masseter EMG data
Overall masseter EMG bursts (regardless of type) had a mean index of 65.8/h (Table 2). Scorable bursts were classified into RMMA/SB bursts within RMMA/SB episodes, Sleep-OMA bursts, or Wake-OMA bursts. Mean number for each burst index per category was 20.6/h (31.3 %), 22.1/h (33.6 %), and 17.5/h (26.6 %), respectively. Of all masseter EMG traces, 7.8 % were scored as unidentified bursts (mean = 5.1/h) due to the face hidden by a blanket.
The mean RMMA/SB episode index (episodes/h) was 5.1/h. Forty-six subjects presented more than 2 episodes/h with RMMA/SB bursts, and only 13 subjects presented more than 25 bursts/h. In all, 46 subjects (78.0 %) were diagnosed with SB, having both ˃2 episodes/h and/or 25 bursts/h [15, 17, 18].
Concomitant conditions
Of the 46 SB patients with high RMMA/SB frequency, 30 showed AHI ˃5/h and were considered as having concomitant OSAH and SB (Fig. 2). The distributions of the number of each EMG burst type and the AHI for the 59 participants are shown in Fig. 3. Although all 59 subjects reported a concomitant history of both SB and OSAH, only 57 had at least one or both conditions confirmed by sleep laboratory data. Of the total sample, 50.8 % had both diagnoses, 18.6 % had OSAH diagnosis alone, and 27.1 % had SB diagnosis alone. As a negative finding, 3.3 % had neither diagnosis.
Correlation analysis
Correlation coefficients and p values for each number of bursts and episodes with AHI, AI, BMI, age, and gender are shown in Table 3. AHI did not show a significant correlation with RMMA/SB episodes nor with RMMA/SB bursts. RMMA/SB episodes were significantly correlated with AI. RMMA/SB bursts were significantly correlated with AI and age. Sleep-OMA bursts were significantly correlated with AHI. Wake-OMA bursts were significantly correlated with BMI. However, these correlations were moderate.
Discussion
Self-reported and clinical diagnoses of concomitant SB and OSAH are poorly confirmed by sleep laboratory recordings. These results also suggest that (1) sleep arousals in patients with concomitant SB and OSAH are weakly associated with RMMA/SB onset and (2) apnea-hypopnea events appear to be related to a higher occurrence of other types of sleep OMA and are not dominant with RMMA-SB activity. We therefore did not confirm our hypothesis: the number of AH events will correlate with the number of SB events, and RMMA-SB and OSAH activity genesis during sleep are probably influenced by different mechanisms as discussed below (e.g., not arousal-related to RMMA/SB but to other types of OMA that may be due to oral dryness-mouth breathing, fluctuation in oxygen level, etc).
Discrepancy between self-reports and clinical diagnosis and sleep laboratory diagnosis
Previous studies suggested that fewer than 50 % of subjects with awareness of tooth grinding, based on questionnaires, were confirmed as SB patients in a sleep laboratory [11, 17]. In the present sleep laboratory study using the above criteria (see “Methods”), SB was confirmed in 78.0 % of self-reported and clinically observed SB patients and OSAH was confirmed in the sleep laboratory in 69 % of patients.
The discrepancy between self-reports and clinically observed conditions and sleep laboratory diagnosis was acceptable in the present study. However, this discrepancy would be critical when co-occurring AH-OSAH and RMMA-SB are investigated. We found concomitant activity in only half the subjects, regardless of self-reported awareness and history.
Relationship between RMMA/SB, Sleep-OMA, and AH event
Previous studies suggested that sleep arousal could be associated with the onset of both sleep OSAH and SB [21, 22]. In previous reviews, RMMA-related arousal with autonomic sympathetic activation was speculated to be part of sleep preservation, to oropharyngeal lubrication and re-opening of the upper airway [9, 23, 24]. In a young population of SB subjects, an association was found between sleep arousal and RMMA genesis for about 80 % of events, and in a general population of all ages, this percentage dropped to about 50 % [11] and [25]. Other mechanisms therefore appear to be involved in RMMA/SB genesis in SB subjects. In the present study, a low correlation was found between the number of RMMA/SB bursts and the arousal index, which does not support a predominant role of sleep arousal in the presence of concomitant AH and RMMA.
Considering the positive association between OMA and AHI as well as previous findings that most AH events and arousals occur in a close temporal relationship [26], it is possible that the genesis of co-occurring SB and OSAH in this subset of patients may be due to different mechanisms during sleep.
Association between SB and OSAH
We previously investigated the causality of the genesis of RMMA/SB and AH events in a temporal association study [5]. We found that although most RMMA/SB events occurred after AH events in patients with concomitant OSAH and SB, some also occurred before. However, the cause should precede the effect in a strong causality assessment. This temporal association does not support a direct cause-and-effect relationship, but rather an indirect association. Therefore, mechanisms other than a dominant role of sleep arousal might explain the co-occurrence. The presence of other movements that were not specific to SB could provide an alternative explanation [9, 12, 13]. SB may have co-occurred with periodic limb movements in a subgroup of individuals [27]. Furthermore, masseter activity was frequently observed with sleep-related respiratory activity in patients with OSAH, but in only about 1/3 of events associated with sleep arousal [28]. These findings suggest that when EMG bursts of masticatory muscle are observed during sleep, activity such as swallowing, yawning, or body movements may influence the probability of observing OMA that is nonspecific to RMMA/SB during sleep in OSAH patients [12, 13]. In the present study, a low association was found between SB incidence and AH (i.e., concomitant in only half the subjects), along with a low association with sleep arousal. We therefore concluded that these OSAH patients presented a secondary form of SB [5] and that the SB mechanism may differ between OSAH patients and normal healthy subjects.
Limitations
Whereas this study provides new evidence to challenge the frequent clinical observation that OSAH and SB co-occur, several limitations should be noted to avoid misleading interpretations or extrapolations. First, we did not consider autonomic heart rate variability or sympathetic nerve activity. Second, the cross-sectional study design did not allow drawing definite conclusions about cause-and-effect relationships among the variables. To overcome these limitations, a longitudinal intervention study design is required. Third, the weak association might be partially due to the inclusion of subjects who self-reported signs and symptoms of OSAH and/or SB but who were not diagnosed as OSAS and/or SB by PSG–AV in our single night of sleep analysis. Fourth, all subjects were patients in a sleep clinic, and no control subjects were included to assess the strength and specificity of the association. Fifth, influence of female–male distribution needs to be further evaluated. Sixth, in this study, we did not analyze AHI or EMG bursts for each sleep stage (N1, N2, N3, REM). The addition of this data in future studies could increase the accuracy of the findings. Seventh, we did not assess upper airway resistance syndrome (UARS) or snoring. Future studies should include UARS to better challenge the relationship between breathing and SB. These limitations should be controlled for in future studies.
References
Ohayon MM, Li KK, Guilleminault C (2001) Risk factors for sleep bruxism in the general population. Chest 119:53–61
Yoshida KA (1998) Polysomnographic study on masticatory and tongue muscle activity during obstructive and central sleep apnea. J Oral Rehabil 25:603–609
Inoko Y, Shimizu K, Morita O, Kohno M (2004) Relationship between masseter muscle activity and sleep-disordered breathing. Sleep Biol Rhythm 2:67–8
Phillips BA, Okeson J, Paesani D, Gilmore R (1986) Effect of sleep position on sleep apnea and parafunctional activity. Chest 90:424–9
Saito M, Yamaguchi T, Mikami S et al (2014) Temporal association between sleep apnea–hypopnea and sleep bruxism events. J Sleep Res 23:196–203
Hosoya H, Kitaura H, Hashimoto T et al (2014) Relationship between sleep bruxism and sleep respiratory events in patients with obstructive sleep apnea syndrome. Sleep Breath 18(4):837–44
Okeson JP, Phillips BA, Berry DT, Cook YR, Cabelka JF (1991) Nocturnal bruxing events in subjects with sleep-disordered breathing and control subjects. J Craniomandib Disord 5:258–64
Sjöholm TT, Lowe AA, Miyamoto K, Fleetham JA, Ryan CF (2000) Sleep bruxism in patients with sleep-disordered breathing. Arch Oral Biol 45:889–96
Carra MC, Huynh N, Lavigne G (2012) Sleep bruxism a comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin N Am 56:387–413
Balasubramaniam R, Klasser GD, Cistulli PA, Lavigne GJ (2014) The link between sleep bruxism, sleep disordered breathing and temporomandibular disorders: an evidence-based review. J Dent Sleep Med 1:27–37
Maluly M, Andersen ML, Dal-Fabbro C et al (2013) Polysomnographic study of the prevalence of sleep bruxism in a population sample. J Dent Res 92(7 Suppl):97S–103S
Dutra KMC, Pereira JR, Rompré PH, Huynh N, Fleming N, Lavigne GJ (2009) Oro-facial activities in sleep bruxism patients and in normal subjects: a controlled polygraphic and audio–video study. J Oral Rehabil 36:86–92
Yamaguchi T, Abe S, Rompré PH, Manzini C, Lavigne GJ (2012) Comparison of ambulatory and polysomnographic recording of jaw muscle activity during sleep in normal subjects. J Oral Rehabil 39:2–10
Iber C, Ancoli-Israel S, Chesson A et al (2007) American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine, Westchester
American Academy of Sleep Medicine (2014) International classification of sleep disorders 3rd ed. online version, IL: American Academy of Sleep Medicine;2014. (http://www.aasmnet.org/store/product.aspx?pid=849)
Lavigne GJ, Rompre PH, Montplaisir JY (1996) Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res 75:546–552
Rompré PH, Daigle-Landry D, Guitard F, Montplaisir JY, Lavigne GJ (2007) Identification of a sleep bruxism subgroup with a higher risk of pain. J Dent Res 86:837–42
American Academy of Sleep Medicine (2005) International classification of sleep disorders 2nd ed. American Academy of Sleep Medicine. Westchester, IL
Koyano K, Tsukiyama Y (2009) Clinical approach to diagnosis of sleep bruxism. In: Lavigne GJ, Cistulli PA, Smith MT (eds) Sleep medicine for dentists: a practical overview. Quintessence, Chicago
(2013) EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. J Dent Res 92(7 Suppl):97S–103S. doi: 10.1177/0022034513484328
Huynh N, Kato T, Rompré PH et al (2006) Sleep bruxism is associated to micro-arousals and an increase in cardiac sympathetic activity. J Sleep Res 15:339–46
Bradley TD, Tkacova R, Hall MJ, Ando S, Floras JS (2003) Augmented sympathetic neural response to simulated obstructive apnoea in human heart failure. Clin Sci (Lond) 104:231–238
Maluly M1, Andersen ML, Dal-Fabbro C, Garbuio S, Bittencourt L, de Siqueira JT, Tufik S. Polysomnographic study of the prevalence of sleep bruxism in a population sample
Lavigne GJ, Kato T, Kolta A, Sessle BJ (2003) Neurobiological mechanisms involved in sleep bruxism. Crit Rev Oral Biol Med 14:30–46
Lavigne GJ, Manzini C, Huyuh N (2011) Sleep bruxism. In: Kryger NH, Roth T, Dement C (eds) Principal and practice of sleep medicine, 5th edn. Elsevier Saunders, Philadelphia, pp 1128–39
Catcheside PG, Jordan AS (2013) Reflex tachycardia with airway opening in obstructive sleep apnea. Sleep 36:819–821
van der Zaag J, Naeije M, Wicks DJ, Hamburger HL, Lobbezoo F (2014) Time-linked concurrence of sleep bruxism, periodic limb movements, and EEG arousals in sleep bruxers and healthy controls. Clin Oral Investig 18:507–13
Kato T, Katase T, Yamashita S et al (2013) Responsiveness of jaw motor activation to arousals during sleep in patients with obstructive sleep apnea syndrome. J Clin Sleep Med 9:759–65
Acknowledgments
We would like to thank Natsue Maruyama, Mari Niimi, and Miku Matsumura for their invaluable cooperation, and Margaret McKyes for linguistic revision. This study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 24390427). GL is a Canada Research Chair in Pain, Sleep, and Trauma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
GL holds governmental grant on pain, sleep, and trauma plus free access to oral appliances for research purposes (Narval, Somnomed), and this is not in conflict with the present paper content.
Author contributorship
All authors made substantial contributions to this study.
Rights and permissions
About this article
Cite this article
Saito, M., Yamaguchi, T., Mikami, S. et al. Weak association between sleep bruxism and obstructive sleep apnea. A sleep laboratory study. Sleep Breath 20, 703–709 (2016). https://doi.org/10.1007/s11325-015-1284-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-015-1284-x