Abstract
Background
Reducing the need for diagnostic sleep studies for obstructive sleep apnea (OSA) would reduce direct and opportunity costs while expediting time to treatment for this common and morbid disorder. We sought to determine if an established sleep apnea screening questionnaire (STOP-BANG) and wrist-worn overnight oximetry data could provide high positive predictive value for the presence of OSA.
Methods
We conducted a prospective observational study of consecutive unattended sleep study patients at a single facility. Patients were referred for sleep testing after chart review by a sleep physician. We assessed area under the receiver-operating characteristic curve (ROC AUC) and positive predictive value (PPV) of STOP-BANG score and oxygen desaturation index (ODI) for a respiratory disturbance index (RDI) ≥15/h.
Results
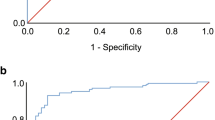
Among 234 test patients, 65 % had an RDI ≥15/h. STOP-BANG had poor ability to discriminate these patients (ROC AUC 0.62). ODI added significant diagnostic information to the STOP-BANG score, increasing the ROC AUC to 0.86. Having the ODI, the STOP-BANG score no longer contributed significant diagnostic information, and the ODI alone discriminated as well as the combination (ROC AUC 0.86). Forty nine percent had an ODI ≥7/h, which had PPV of 92 % (95 % confidence interval (CI), 86 to 96 %). In the validation sample of 1,196 consecutive patients, ODI ≥ 7/h had a PPV of 97 % (95 % CI, 95 to 97 %).
Conclusions
Among patients with a high prevalence of OSA, high ODI is common and its presence has high PPV for OSA. These data suggest that overnight oximetry prior to sleep testing could significantly reduce the number of patients requiring sleep studies, thereby reducing costs and time to treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a common condition, and the benefits of OSA treatment include reduced daytime sleepiness [1], improved reaction time [2], and lower blood pressure [3]. The current standard of care for OSA requires an overnight sleep study to confirm the presence of OSA prior to initiating treatment. This sleep study can be an in-laboratory attended polysomnogram (PSG) or in selected patients at high suspicion for OSA a home sleep test (HST). While HST is much less expensive than PSG, both types of studies require setup of technically complex diagnostic equipment and require expert interpretation of the acquired data. Cost, convenience, and access to PSG and HST services also remain significant barriers to diagnosing and therefore treating OSA.
Among persons with very high pre-test probability for a disease, diagnostic testing often adds little value, as most tests will be positive for disease, and many negative tests will be false negatives. Therefore, if an OSA screening test could confidently identify those with very high pre-test probability for OSA, such a test could potentially reduce the need for diagnostic sleep studies. We previously tested the STOP-BANG sleep apnea screening questionnaire [4] in our sleep study referral population and found that a maximal STOP-BANG score of 8 points had a positive predictive value of 85 % (95 % confidence interval 76–92 %) for OSA, defined as a respiratory disturbance index (RDI) of ≥15/h [5]. We concluded that the STOP-BANG score was insufficient to omit diagnostic sleep studies, as 15 % (and perhaps up to 24 %) of persons with a maximal STOP-BANG questionnaire score would actually not have significant OSA. Furthermore, only a small proportion (7.9 %) of our referral population had the maximum STOP-BANG score of 8 points.
In this study, we hypothesized that overnight oximetry data would add significant diagnostic information to STOP-BANG questionnaire scores. We prospectively tested this hypothesis in the sleep apnea referral clinical at the Minneapolis Veterans Affairs (VA) Sleep Apnea Clinic.
Methods
Study participants
We conducted a single-institution, prospective observational study that included both test and validation cohorts.
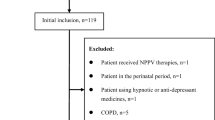
The test cohort consisted of consecutive patients who underwent unattended sleep studies at the Minneapolis VA Medical Center between February 2013 and April 2013. The validation cohort consisted of 1,196 consecutive patients who had undergone unattended, portable sleep studies at our institution between July 2011 and November 2012 and whose findings have been previously published [5]. Sleep study referrals in both the test and validation cohorts were handled identically and included a review of the patient’s chart by an experienced sleep physician. Based on this chart review, patients at high clinical suspicion for uncomplicated OSA were directly referred for an unattended sleep study. Patients with suspected complicated sleep-disordered breathing or other sleep disorders were referred for sleep clinic evaluation and/or formal in-laboratory polysomnography. Those patients directly referred to unattended sleep studies formed the basis of this study.
This study was reviewed and approved by the local institutional review board, and requirement for informed consent was waived.
Data collection
Sleep studies in the test and validation cohorts were performed using the WatchPAT 200 (Itamar Medical, Franklin, MA). This technology has been validated by formal PSG, and there is high correlation between peripheral arterial tonometry (PAT)- and PSG-derived respiratory indices [6, 7]. Patients with cardiac arrhythmias such as chronic atrial fibrillation were not eligible for WatchPAT unattended studies and were not included in this analysis. Patients in the test cohort also wore high-resolution, wrist-worn, battery-powered overnight oximetry study devices (WristOx 3150, Nonin Medical, Plymouth, MN). These were set to record data every 2 s through a reusable sensor (Nonin Soft Sensor 8000S, Nonin Medical, Plymouth, MN).
Data from the devices were downloaded the next morning, and automated reports were obtained. The oximetry oxygen desaturation index (OxODI) desaturation event definition was a change in pulse oxyhemoglobin saturation (SpO2) of ≥4 % for ≥10 s, as automatically scored by computerized analysis (Nonin nVISION software, Nonin Medical, Plymouth, MN).
The STOP-BANG questionnaire [4] was administered to all participants undergoing WatchPAT unattended sleep studies. The STOP-BANG questionnaire was administered to patients by a respiratory therapist. The therapist also measured the neck circumference in the sleep clinic, while body mass index (BMI) was extracted from the electronic medical record. Patients were not aware of their unattended sleep study results until after the STOP-BANG questionnaires were completed.
Statistical methods
Sample characteristics are described as the mean ± standard deviation or as proportions (percentages). Relationships between variables are reported as Pearson’s r statistic.
Multivariable logistic regression was employed to test the hypothesis that the OxODI could provide additional diagnostic information when combined with STOP-BANG scores. The dependent variable was moderate-to-severe OSA, defined as a RDI ≥15/h. The area under the receiver-operating characteristic curve (ROC AUC) is reported as a measure of overall discrimination of subjects with or without an RDI ≥15/h. DeLong’s method was used to compare ROC AUCs [8]. Positive predictive values (PPV) with 95 % confidence intervals were calculated for selected cut points to assess the performance for identifying subjects that could reasonably forego a diagnostic sleep study. We sought to identify a cut point that could provide >90 % PPV for an RDI ≥15/h. The PPV of the selected OxODI cut point was confirmed in the validation sample of 1,196 previously studied patients.
Stata 12.1 (StataCorp, College Station, TX) was used for all statistical analyses.
Results
Of 244 consecutive patients undergoing unattended WatchPAT sleep studies, PAT data were successfully obtained in 237 (97.1 %). Three of these patients did not have complete STOP-BANG data, leaving 234 patients to test our hypothesis. WristOx data were successfully obtained in 100 % of these patients.
Demographic, comorbidity, and sleep symptom characteristics are provided in Table 1. Patients had a mean age of 56 years, had a mean body mass index of 32 kg/m2, and were predominantly male (95 %), reflecting the nature of our VA unattended sleep study population. Hypertension was common (51 %), but few had heart failure, as such patients are preferentially referred for formal polysomnography. The most commonly reported sleep symptom was tiredness (87 %), with 45 % having an Epworth Sleepiness Score >10 points. Most (74 %) reported loud snoring and 54 % reported observed apneas.
The mean STOP-BANG score was 5.3 ± 1.4 points, mean OxODI was 9.6 ± 9.7/h, and mean RDI was 22.8 ± 14.6/h. STOP-BANG scores were weakly correlated with the RDI (r = 0.23; p = 0.0003) and OxODI (r = 0.28; p < 0.0001), whereas the OxODI had a strong correlation with the RDI (r = 0.86; p < 0.0001).
In the test sample, 65 % had an RDI ≥15/h. Consistent with our previously published findings, the STOP-BANG score alone had poor ability to discriminate these patients (ROC AUC 0.62; 95 % CI, 0.55 to 0.68). Multivariable logistic regression indicated that the OxODI added significant diagnostic information to the STOP-BANG score (odds ratio 1.51; p < 0.001) increasing the ROC AUC to 0.86 (95 % CI, 0.81 to 0.90). Having the OxODI, the STOP-BANG score no longer contributed significant diagnostic information (odds ratio 0.95; p = 0.71), and the OxODI alone discriminated as well as the combination (ROC AUC 0.86; 95 % CI, 0.81 to 0.90). Therefore, we dropped the STOP-BANG score from subsequent analyses of PPV.
Higher OxODI was associated with higher PPV for an RDI ≥15/h (Table 2). In the test sample, 49 % (115 out of 234) had an OxODI ≥7/h, and 106 out of these 115 had an RDI ≥15/h (PPV of 92 %; 95 % CI, 86 to 96 %). Higher OxODI cut points had slightly better PPV, but the number of patients exceeding the diagnostic threshold progressively decreased.
In the test sample, OxODI was highly correlated (r = 0.95, p < 0.0001) with the ODI derived from the WatchPAT device (PATODI). In the validation sample (n = 1,196) that had a similar percentage with RDI ≥15/h (68 %) and PATODI ≥7/h (52 %), a PATODI ≥7/h had a PPV of 97 % (95 % CI, 95 to 97 %) for an RDI ≥15/h.
Discussion
In our sleep clinic referral population, an ODI ≥7/h had a very high positive predictive value (92 and 97 % in the test and validation samples, respectively) for identifying those with significant OSA. Approximately 50 % of our patients had an ODI ≥7/h and might therefore reasonably be treated with autotitrating continuous positive airway pressure (AutoCPAP) based on oximetry alone, without having to undergo a diagnostic sleep study.
A proposed algorithm incorporating overnight oximetry to streamline OSA care is diagramed in Fig. 1. Although we have focused on a cut point of ODI ≥7/h, we note that altering the cut point to an even higher cut point of ODI ≥8 or ≥9/h further increases positive predictive value and would still be present in a substantial portion of those undergoing unattended sleep studies. This strategy could reduce cost by eliminating both the direct costs of the sleep study and the opportunity costs of lost time off work due to the overnight sleep study. Overnight oximetry requires almost no patient instruction, the device automatically powers on upon insertion of the fingertip into the reusable flexible oximetry probe, and can more easily be mailed to patients for use without in-person instruction. Because overnight oximetry can easily be done in the patient’s home, our strategy could reduce the direct and opportunity costs of travel to and from sleep centers.
A proposed algorithm to streamline sleep apnea diagnosis and treatment, using overnight oximetry as an intermediate step prior to unattended sleep studies. Our data suggest that approximately 50 % of patients could forego unattended sleep studies using an oxygen desaturation index (ODI) cut point of ≥7/h to direct patients to autotitrating continuous positive airway pressure (AutoCPAP) without a sleep study, with a high degree of clinical certainty that their respiratory disturbance index (RDI) is ≥15/h. We note that sleep clinic consultation, in-lab polysomnogram, unattended sleep studies, and clinical follow-up remain important components of this proposed algorithm
While home sleep testing (HST) is currently expanding, most sleep centers still require patients come to the sleep clinic for personal HST setup instruction, in order to minimize rates of failed data acquisition. HST therefore remains challenging in patients with limited access to transportation or logistic travel challenges such as highly rural populations. Furthermore, our strategy could reduce time to OSA treatment, as overnight oximetry can typically be scheduled and completed in less time than unattended or in-lab polysomnography.
Although we hypothesized that a combination of STOP-BANG scores and ODI data would provide high positive predictive value, we found that the ODI alone was sufficient to confidently predict the presence of moderate to severe OSA. The STOP-BANG questionnaire did not improve the diagnostic discrimination of ODI alone, likely due to the weak correlation between STOP-BANG scores and the RDI used to detect the presence of OSA.
While other non-veteran sleep clinics may not have quite the same high prevalence of RDI ≥15/h or ODI ≥7/h, even if 25 % of persons with OSA could be identified by overnight oximetry alone, when extrapolated to the large global population of persons with undiagnosed OSA, eliminating the need for even 25 % of sleep studies could have substantial financial and public health implications.
Our concept of using overnight oximetry to reduce the need for sleep studies has been proposed in the past. In a 1999 publication entitled “Nocturnal oximetry for the diagnosis of the sleep apnea hypopnea syndrome: a method to reduce the number of polysomographies?”, Chiner and colleagues studied 275 consecutive patients referred for PSG at a university sleep center in Spain [9]. In their sample, in which 78 % had an apnea–hypopnea index ≥15/h, an ODI ≥5/h (present in 51 % of their sample) had a PPV of 93 % (95 % CI, 88 to 96 %) for predicting sleep apnea and ODI ≥10/h (present in 43 % of their sample) had PPV of 96 % (95 % CI, 92 to 99 %). Although Chiner and colleagues used an older tabletop oximetry device (Nellcor N-200, Hayward, CA) that recorded data every 6 s and required manual review (whereas we used a wrist-worn device that recorded data every 2 s with automated analysis), our results are strikingly similar. Other older studies have likewise suggested overnight oximetry can be useful in reducing the need for sleep studies in populations referred for sleep studies [10–12] and more recently in preoperative patients [13].
Despite the existence of these data for some time now, the use of oximetry to preclude PSG has not been widely applied, perhaps due to the historical need for PSG-based titration to determine what effective CPAP pressure each patient required. In the modern era of autotitration CPAP, which appears to provide equivalent or superior treatment outcomes when compared to PSG-based CPAP [14, 15], we suggest that these older data and our current data using portable, wrist-worn oximetry should receive renewed attention and clinical consideration.
While we feel our data are compelling and can justify a local change in practice at our facility, we acknowledge that our results should be confirmed in other VA and non-VA settings. In particular, our sleep apnea program serves very few women, young persons, and minority populations, so we cannot extrapolate our findings to such populations. We also must emphasize that all patients undergoing unattended sleep studies were referred to us and briefly screened by an experienced sleep physician, so this approach should not be extrapolated to primary care settings without further study.
In summary, data from our VA unattended sleep study clinic suggest that a diagnostic–treatment algorithm using wrist-worn oximetry to forgo PSG/HST in selected patients, following chart review by an experienced sleep physician, could substantially reduce the direct and opportunity costs associated with making a diagnosis of OSA while also facilitating faster time to treatment for this common and morbid condition.
Abbreviations
- BMI:
-
Body mass index
- CPAP:
-
Continuous positive airway pressure
- HST:
-
Home sleep test
- ODI:
-
Oxygen desaturation index
- OSA:
-
Obstructive sleep apnea
- PAT:
-
Peripheral arterial tonometry
- PPV:
-
Positive predictive value
- PSG:
-
Polysomnogram
- RDI:
-
Respiratory disturbance index
- ROC AUC:
-
Receiver-operating characteristic area under curve
- VA:
-
Veterans Affairs
References
Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ (2006) Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 19, CD001106
Tregear S, Reston J, Schoelles K, Phillips B (2010) Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep 33:1373–1380
Fava C, Dorigoni S, Dalle Vedove F, Danese E, Montagnana M, Guidi GC, Narkiewicz K, Minuz P (2014) Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest 145:762–771
Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM (2008) STOP Questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 108:812–821
Kunisaki KM, Brown KE, Fabbrini AE, Wetherbee EE, Rector TS (2014) STOP-BANG questionnaire performance in a Veterans Affairs unattended sleep study program. Ann Am Thorac Soc 11:192–197
Pittman SD, Ayas NT, MacDonald MM, Malhotra A, Fogel RB, White DP (2004) Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep 27:923–933
Choi JH, Kim EJ, Kim YS, Choi J, Kim TH, Kwon SY, Lee HM, Lee SH, Shin C, Lee SH (2010) Validation study of portable device for the diagnosis of obstructive sleep apnea according to the new AASM scoring criteria: Watch-PAT 100. Acta Otolaryngol 130:838–843
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Chiner E, Signes-Costa J, Arriero JM, Marco J, Fuentes I, Sergado A (1999) Nocturnal oximetry for the diagnosis of the sleep apnoea hypopnoea syndrome: a method to reduce the number of polysomnographies? Thorax 54:968–971
Sériès F, Marc I, Cormier Y, La Forge J (1993) Utility of nocturnal home oximetry for case finding in patients with suspected sleep apnea hypopnea syndrome. Ann Intern Med 119:449–453
Lévy P, Pépin JL, Deschaux-Blanc C, Paramelle B, Brambilla C (1996) Accuracy of oximetry for detection of respiratory disturbances in sleep apnea syndrome. Chest 109:395–399
Magalang UJ, Dmochowski J, Veeramachaneni S, Draw A, Mador MJ, El-Solh A, Grant BJ (2003) Prediction of the apnea-hypopnea index from overnight pulse oximetry. Chest 124:1694–1701
Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y (2012) Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg 114:993–1000
Kuna ST, Gurubhagavatula I, Maislin G, Hin S, Hartwig KC, McCloskey S, Hachadoorian R, Hurley S, Gupta R, Staley B, Atwood CW (2011) Noninferiority of functional outcome in ambulatory management of obstructive sleep apnea. Am J Respir Crit Care Med 183:1238–1244
Rosen CL, Auckley D, Benca R, Foldvary-Schaefer N, Iber C, Kapur V, Rueschman M, Zee P, Redline S (2012) A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the home PAP study. Sleep 35:757–767
Acknowledgments
This study supported by an Upper Midwest Veterans Integrated Service Network 23 Strategic Initiative Award. The funding agency had no role in design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; nor decision to submit the manuscript for publication. We thank the Veterans Integrated Service Network (VISN) 23 for supporting this project. We thank the Minneapolis VA Sleep Clinic staff for assistance with data collection, logistic support for this study, and for their dedication to the sleep health of veterans.
Conflict of interest
The authors declare that they have no competing interests.
Guarantor
KMK and TSR had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. The lead author KMK affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of VISN 23, the Minneapolis VA Health Care System, the U.S. Department of Veterans Affairs, the U.S. Government, or the University of Minnesota.
Author contributions
KMK conceived and designed the study, directed the statistical analyses, interpreted the data, drafted the manuscript, and approved the final version.
OG contributed to collection and interpretation of the data, revised the manuscript critically for important intellectual content, and approved the final version.
EEW contributed to the conception and design of the study, contributed to interpretation of the data, revised the manuscript critically for important intellectual content, and approved the final version.
TSR contributed to the conception and design of the study, performed the statistical analyses, interpreted the data, revised the manuscript critically for important intellectual content, and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kunisaki, K.M., Bohn, O.A., Wetherbee, E.E. et al. High-resolution wrist-worn overnight oximetry has high positive predictive value for obstructive sleep apnea in a sleep study referral population. Sleep Breath 20, 583–587 (2016). https://doi.org/10.1007/s11325-015-1251-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-015-1251-6