Abstract
Purpose
Fibroblast activating protein (FAP) is highly expressed in the synovial tissues of rheumatoid arthritis (RA) patients. The aim of this study was to determine the feasibility of PET imaging with an Al[18F] F-NOTA-labeled FAP inhibitor 04(18F-FAPI-04) for the evaluation of arthritic progression and therapeutic response in experimental arthritis.
Methods
Fibroblast-like synoviocytes (FLSs) were obtained from patients with RA or osteoarthritis (OA), and the relationship between 18F-FAPI-04 uptake and the inflammatory activity of RA FLSs was investigated. Collagen-induce arthritis (CIA) mice models were established and treated with methotrexate (MTX) or etanercept (ETC). Then, PET imaging was performed 24 h following 18F-FAPI-04 injection. The imaging results were compared by assessing macroscopic arthritis scores and histological staining.
Results
18F-FAPI-04 uptake was obvious in RA FLSs that characterizing FAP activation. The higher the uptake of 18F-FAPI-04, the more severity of the inflammatory phenotype in RA FLS. Furthermore, the uptake of 18F-FAPI-04 in inflamed joints could be found even before the deformity of the parental joints could be observed by histological examination. Both MTX and ETC were effective in inhibiting the progression of arthritis in CIA mice was confirmed by macroscopic, histological, and radiographic pathology scores. Importantly, 18F-FAPI-04 uptake declined accordingly in CIA models following MTX and ETC treatment.
Conclusions
These findings suggest that PET imaging of 18F-FAPI-04 can be used to monitor treatment response in RA, and is more sensitive in disease speculation than macroscopic arthritis scoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic, autoimmune disease characterized by erosive and symmetrical arthritis [1]. The affected joints are mainly small joints that were manifested by excessive synovitis. Without effective intervention, the lesion synovium can invade the joint, resulting in joint destruction and dysfunction.
Treatment strategies for RA include disease-modifying antirheumatic drugs (DMARDs), non-steroidal anti-inflammatory drugs (NSAIDs), biological agents, glucocorticoids, phytopharmaceutical preparations, surgery, plasma exchange, and immunosorbent [2]. Due to the heterogeneity of the disease and individual differences, to monitor the activity of the disease and the therapeutic effect is very important for the remission of the patient’s disease [3, 4].
Fibroblast-like synoviocytes (FLSs) are the main pathological effector whose abnormal activation could lead to pannus formation and cartilage erosion [5]. At present, the parameters for diagnosis and evaluation of the therapeutic effect of RA include joint swelling and pain, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, inflammatory indicators, and changes in imaging data [6]. However, there is still no intuitive indicator to monitor the changes in RA FLSs during the treatment periods.
Fibroblast activation protein (FAP) is mainly expressed on the cell surface as type II dipeptidase, which can hydrolyze the surrounding collagen and mediate cell invasion and migration [7]. Therefore, isotopically labeled FAP inhibitors (FAPI) have been extensively studied in tumor diagnosis and efficacy testing [8, 9]. Among these inhibitors, FAPI-04 has been widely modified and developed as a diagnostic probe for tumors and some autoimmune diseases due to its high affinity for FAP [10,11,12]. Previous studies demonstrated that FAP was highly expressed in activated FLSs from collagen-induce arthritis (CIA) models and RA patients [13, 14]. Recently, we found that Al [18F]F-NOTA-FAPI-04 (18F-FAPI-04) can be initially applied for the diagnosis of RA [10]. Whether 18F-FAPI-04 PET imaging could be utilized to monitor the therapeutic effect in RA remains to be determined. In this study, 18F-FAPI-04 PET imaging was further explored to detect its efficacy in monitoring the pathological progression and remission in CIA models.
Materials and Methods
Cell Culture
FLSs were isolated from the synovial tissue of patients with RA or OA who underwent joint replacement surgery as described previously [15]. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Shandong Medicinal Biotechnology Center research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Briefly, the synovial tissue was washed 3 times with PBS containing 1% penicillin–streptomycin. Then, it was cut to mince packs and digested with type II and type III collagenase in a 37 degrees 5% CO2 incubator for 6 h. Dulbecco’s minimum essential medium (DMEM) was added and blown well, then filtered through 70 μm. The filtered liquid was centrifuged to collect the cell sediment and cultured with DMEM medium containing 15% FBS. Four to six generations of FLS were used for this study.
Animals
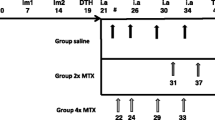
All applicable Medicinal Biotechnology Center Animal Welfare Committee for the care and use of animals were followed. Eight-week-old male DBA/1 mice were obtained from Hua Fukang Biotechnology Co., Ltd (Beijing, China), housed in a pathogen-free environment, and given free access to food and water. The collagen (CII, Chondrex, Redmond, WA, USA) was dissolved in 0.1 M acetic acid at a concentration of 2 mg/mL and emulsified with complete Freund’s adjuvant (Chondrex) equally. The DBA/1 mice received 0.1 mL CII emulsion at the base of the tail. A booster injection of 0.1 mL of CII in complete Freund’s adjuvant (IFA; Chondrex) was given on day 21. After the booster injection, arthritis developed. Paw inflammation was graded on a scale of 0 to 4 as follows:0, paws with no swelling or focal redness; 1, mild swelling limited to the middle part of the foot or ankle joint; 2, mild swelling spreading from ankle joint to mid-foot; 3, mild swelling spreading from ankle to toe; and 4, severe swelling including ankle, foot, and toe [16]. The CIA mice were randomly divided into three groups (n = 9) for treatment effect evaluation. The vehicle group received volume-matched vehicle control, methotrexate (MTX) group were given MTX treatment with the dose of 5 mg/kg, and etanercept (ETC) group were given ETC treatment with the dose of 5 mg/kg. From days 22 to days 46, each mouse was administered intraperitoneally three times in a week. Additionally, microcalipers were used to measure the thickness of the arthritic hind paws every 4 days.
Synthesis of the Radioactive Tracer
Al [18F] F-NOTA-FAPI-04 (supplementary Fig. 1) was synthesized as previously described [10]. Briefly, QMA SepPak light cartridge (Waters Corporation, USA) was used to capture the [18F]F−. Then, the mixture of [18F]F−, saline, AlCl3, acetonitrile, and NOTA-FAPI-04 was heated at 110 ℃ for 10 min. The radiotracer was filtered through a 0.22 μm sterile filter after removing the free [18F]F−.
PET Imaging
The small-animal PET scanner (IRIS PET/CT, Inviscan, France) was used to perform Micro-PET/CT imaging as previously described [10]. Briefly, the micro-PET/CT imaging study was conducted 60 min after injections of 3.7–7.4 MBq 18F-FAPI-04. A 10-min scan was performed after the mice were anesthetized with isoflurane. Three-dimensional ordered subset expectation maximization (Monte Carlo-based 3D OSEM) was used to reconstruct the obtained images. Osi-rix MD software was used to process images and regions of interest (ROIs), and average signal levels were measured in each ROI. Quantitative data are expressed as %ID/mL (percentage injected dose per mL).
RT-qPCR
Total RNA was extracted using an RNA extraction kit (Vazyme, Nanjing, China). Reverse transcription was performed using a HiScript II Reverse Transcriptase kit (Vazyme). For each sample, 1 µg RNA was used for cDNA synthesis. qPCR was performed using Taq Pro Universal SYBR qPCR Master Mix (Vazyme) in a LightCycler 480 II (Roche, Basel, Switzerland). Relative gene expression was calculated using the 2−ΔΔCT method and normalized as indicated [17]. Primer sequences were as follows. FAP, forward: ATGAGCTTCCTCGTCCAATTCA; reverse: AGACCACCAGAGAGCATATTTTG; GAPDH, forward, GCACCGTCAAGGCTGAGAAC; reverse, TGGTGAAGACGCCAGTGGA.
ELISA
IL-6, IL-8, and FAP are measured according to the manufacturer’s instructions. For the detection of IL-6 and IL-8 in the supernatant, FLSs culture medium was collected and centrifuged at 355 × g. A lysate of FLS cells was prepared using RIPA. The lysate was centrifuged at 12,000 rpm and the supernatant was harvested for FAP analysis.
HE Staining, Immunofluorescence Staining, and Toluidine Blue Staining
The hind knee joints of CIA mice were fixed in 4% paraformaldehyde and then decalcified in 10% EDTA for 3 weeks. Following embedding in paraffin, the tissues were sliced into 4-μm sections. The H&E staining was conducted using a kit from Solarbio (Beijing, China). The tissue sections were first routinely processed and then fluorescently stained with appropriate primary (anti-FAP, 1:300; Invitrogen, # PA5-99,313) and secondary antibodies [10]. The nuclei were then stained with DAPI (Beyotime, Beijing, China). A scanning confocal microscope (FV3000, Olympus, Japan) was used to capture images. Toluidine blue staining was performed using toluidine blue solution (0.5% toluidine blue, 1% borax).
Statistical Analysis
GraphPad Prism 8.0 Software (GraphPad Software, Inc., San Diego, CA) was used for statistical analysis. The quantitative values were expressed as the mean ± standard error of the mean (SEM). Descriptive statistics were performed to evaluate the normality of the data distribution before the analysis of significant differences. Student’s t-test was used to conduct the comparison between two groups. One-way or two-way analysis of variance (ANOVA) was used to perforrm the comparison between multiple groups, followed by the Tukey multiple comparison test. Statistically significant differences between experimental groups were indicated by P values below 0.05.
Result
18F-FAPI-04 Uptake in RA FLSs Is Positively Correlated with Its Inflammatory Phenotype
FLSs were isolated from the synovial tissues in patients with RA or OA who underwent joint replacement surgery. Besides the fact that FAP expression was significantly higher in RA than in OA FLSs at the basal levels, FAP could also be induced by inflammatory factors more significantly in RA FLSs than in OA FLSs, (Fig. 1A, supplementary Fig. 2A). In our previous study, the specificity of 18F-FAPI-04 has been verified through in vitro and in vivo blocking experiments, which will not be shown here [10]. Since 18F-FAPI-04 could specifically target FAP, FLSs were exposed by 18F-FAPI-04 for 60 min and were subsequently subjected to analyzing the uptake values. As shown in Fig. 1B, there was no significant difference in 18F-FAPI-04 uptake in OA FLSs with or without TNF-α. However, the uptake of 18F-FAPI-04 was significantly higher in RA FLSs than in OA FLSs. Furthermore, TNF-α stimulation significantly elevated the uptake of 18F-FAPI-04 in RA FLSs. Therefore, these facts suggest that the uptake of 18F-FAPI can characterize the variation of FAP expression in RA FLSs.
The expression of FAP in closely related to the inflammatory phenotype of RA FLSs. OA FLSs and RA FLSs were obtained from the patients who underwent joint replacement. FLSs was treated with TNF-α (10 ng/mL) (A, B), MTX (1 μM) or ETC (10 μg/mL) for 24 h (E–H). A, E Total protein was extracted and FAP expression was analyzed by ELISA. B, F RA FLSs were treated with.18F-FAPI-04 (100 kBq) for 60 min. Then, the cells were lysed, and radioactivity was measured with a gamma counter. C, D, G, H Cell culture supernatants were collected to detect the expression of IL-6 and IL-1β. RA FLSs were lysed to detect the content of FAP by ELISA. Date was expressed as mean ± SEM (n = 6). The data was analyzed using one-way ANOVA. *P < 0.05, ***P < 0.001
To further clarify whether 18F-FAPI-04 uptake can predict the status of RA FLS activation, we investigated the relationship between pro-inflammatory cytokine secretion and FAP expression. As shown in Fig. 1C and D, the secreted levels of IL-6 and IL-1β were positively correlated with the expression levels of FAP. Next, we explored whether 18F-FAPI-04 could monitor the response of RA FLSs towards MTX or ETC. As expected, both MTX and ETC can inhibit FAP expression (Fig. 1E, supplementary Fig. 2B), 18F-FAPI-04 uptake (Fig. 1F), and the production of pro-inflammatory cytokine (Fig. 1G, H). Therefore, the above results suggest that 18F-FAPI-04 could be used to monitor RA FLSs activation.
18F-FAPI-04 Imaging in CIA Can Visually Characterize the Degree of Arthritis and FAP Expression
To investigate whether 18F-FAPI-04 can be used to characterize the arthritic degree, CIA models with different arthritis scores were selected to perform PET imaging. There was a physiological as well as a pathological uptake of 18F-FAPI-04, shown in Fig. 2A. Since 18F-FAPI-04 is excreted mainly through urine and bile [18], high uptake of 18F-FAPI-04 occurs in the kidney, bladder, and large intestine. More importantly, it is evident that the uptake of 18F-FAPI-04 was high in inflamed joints. Additionally, its uptake values were significantly and positively correlated with arthritis scores (Fig. 2B). Then, we sectioned the knee joints with arthritis scores of 0, 1, 2, 3, and 4 to detect the expression levels of FAP. It can be seen from Fig. 2C that the severity degree of arthritis was positively correlated with the expression levels of FAP. In addition, 18F-FAPI-04 uptake was highly positively correlated with the fluorescence intensity of FAP expression (Fig. 2D). Therefore, 18F-FAPI-04 can be used to characterize the severity of arthritis.
18F-FAPI-04 uptake, arthritis score and FAP expression in CIA mice. CIA mice with different scores were selected for PET imaging. These mice underwent micro-PET/CT imaging 1 h after intravenous injection of 3.7–7.4 MBq of 18F-FAPI-04. A Representative 18F-FAPI-04 images of CIA mice with different arthritis scores. B Joint sections were used to detect the FAP expression by immunofluorescence (200 ×). C Correlation of 18F-FAPI-04 uptake and arthritis score. D Correlation of 18F-FAPI-04 uptake and FAP fluorescence intensity of CIA tissue sections
18F-FAPI-04 PET Imaging Is More Sensitive than Traditional Methods in Monitoring Arthritic Progression
In this study, the body weight, hind paw thickness, and arthritis score were recorded every 4 days. Furthermore, the 18F-FAPI-04 uptake value of each joint was obtained by PET imaging. There was no significant difference in weight change among the groups (supplementary Fig. 3). As shown in Fig. 3A and B, the differences of arthritis scores and paw thickness became obvious and significant from the 12th day following treatment in therapeutic group when compared to its parental controls. However, such differences reached its significant level on the 8th day following treatment when the arthritic progression was detected by PET imaging as shown by SUV (Fig. 3C). These results suggested that the detection of FAP expression by PET imaging was more sensitive to monitor the response of RA to treatment.
Overall evaluation and quantification of 18F-FAPI-04 uptake in CIA mice. CIA mice were divided into vehicle control group, MTX group (5 mg/kg) and ETC group (5 mg/kg). All drug injection was administered intraperitoneal three times per week from day 32 to day 56. Arthritis score (A), paw thickness (B), and 18F-FAPI-04 uptake (C) were evaluated every 4 days. Data was expressed as mean ± SEM (n = 6). The data was analyzed using one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001
The Uptake of 18F-FAPI-04 Was Applicable in Monitoring the Therapeutic Response in Arthritis Models Suggested More Severity of Synovial Hyperplasia and Cartilage Destruction
As expected, 4 weeks following treatment with MTX or ETC showed significant improvements in arthritis scores and paw thickness, which was accompanied by the decreased signals in 18F-FAPI-04 PET imaging (Fig. 4A). Furthermore, HE staining showed these treatments could effectively inhibit synovial hyperplasia (Fig. 4B) and alleviate cartilage destruction (Fig. 4C). Consistently, FAP expression in synovial tissues was significantly reduced (Fig. 4D).
The value of 18F-FAPI-04 PET imaging in monitoring the therapeutic response in CIA mice. Mice treated with vehicle, MTX (5 mg/kg) or ETC groups (5 mg/kg) underwent PET imaging and histological evaluation at day 56 after the first immunization. (A) Representative 18F-FAPI-04 images of CIA mice received MTX or ETC treatment. HE staining (B) (100 ×), toluidine blue staining (C) (100 ×) and immunofluorescence (D) (200 ×) were performed
Discussion
The etiology of RA is complex that involves genetics, infections, sex hormones, and other factors [19]. RA is characterized by excessive inflammation, synovial hyper-proliferation and cartilage destruction. Therefore, the main goal of RA treatment was to control the progression of inflammation, ameliorating joint damage, and reducing the occurrence of complications [20]. However, to date, long-term remission rates in patients with RA remain low [21]. Synovial inflammation persists in some patients even when clinical remission is achieved, leading to the destruction of bone and cartilage. Therefore, effective monitoring of synovial inflammatory status in long-term treatment is important to determine disease activity and guide medication regimens.
CRP, ESR, and DAS28 serve as the diagnosis parameters to evaluate the activity and efficacy of RA. However, CRP and ESR are often disturbed by a variety of factors unrelated to inflammation, such as age, gender, anemia, and therapeutic drugs [22]. Recently, ultrasound and MRI have also highlighted their value in the diagnosis of synovial lesions, but neither is specific [23, 24]. Therefore, all of these indices have limitations, and developing sensitive and robust evaluation methods is important for the long-term management of RA patients.
As a serine peptidase, FAP is strongly expressed in cancer-associated fibroblasts in more than 90% of epithelial tumors and contributes to progression and prognostic deterioration in various solid cancers [25]. This makes FAP a promising target for radionuclide-based tumor diagnosis and treatment, as well as the diagnosis of malignant diseases associated with extracellular matrix remodeling. However, in addition to tumors, FAP has been found to be highly expressed in acute myocardial infarction, interstitial lung diseases, and some autoimmune diseases [26,27,28]. Recent studies show that FAP is expressed in all subpopulations of synovial cells and knockdown of FAP can effectively mitigate the progression of arthritis. However, the application of FAP-based tracers in autoimmune diseases such as RA remains poorly known.
Our previous study demonstrated the specific and sensitive uptake and localization of 18F-FAPI-04 in the synovial tissues from CIA mice RA patients. More importantly, 18F-FAPI-04 imaging better reflected the degree of synovial hyperplasia compared to 18F-FDG [10, 14, 29]. Therefore, this study aimed to investigate whether PET imaging with 18F-labeled FAP inhibitor could be used to monitor RA therapy response. To mimic therapeutic response, MTX and etanercept (ETC) were used. MTX is the anchor drug for RA treatment and it could alleviate the inflammatory phenotype of RA FLSs and macrophages [30, 31]. Besides, the soluble ETC is often used in patients with poor response to conventional DMARDs or severely active RA [32]. Our study shows that both MTX and ETC can suppress the inflammatory phenotype of RA FLS. The activity of RA FLSs can be weighed by 18F-FAPI-04 PET imaging. More importantly, the sensitivity of 18F-FAPI-04 PET imaging in predicting the progression or remission was significantly better than the classical diagnosis parameters such as arthritis score and paw thickness during treatment of CIA mice with MTX and ETC. This suggests the use of 18F-FAPI-04 as a tracer for quantitative PET imaging of inflamed synovium for non-invasive, specific, and objective monitoring.
Conclusion
18F-FAPI-04 imaging of the inflamed synovium allows whole-body visual quantification of RA specifically to monitor the response to treatment. It is more sensitive in disease monitoring compared to standard methods of clinical arthritis scoring.
Data Availability
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mormile I et al (2021) The N-Formyl peptide receptors and rheumatoid arthritis: a dangerous liaison or confusing relationship? Front Immunol 12:685214
Aletaha D, Smolen JS (2018) Diagnosis and Management of rheumatoid arthritis: a review. JAMA 320(13):1360–1372
Scherer HU, Häupl T, Burmester GR (2020) The etiology of rheumatoid arthritis. J Autoimmun 110:102400
Zhao J et al (2021) Molecular and cellular heterogeneity in rheumatoid arthritis: mechanisms and clinical implications. Front Immunol 12:790122
Li GF, Qin YH, Du PQ (2015) Andrographolide inhibits the migration, invasion and matrix metalloproteinase expression of rheumatoid arthritis fibroblast-like synoviocytes via inhibition of HIF-1α signaling. Life Sci 136:67–72
Maradit-Kremers H et al (2007) Raised erythrocyte sedimentation rate signals heart failure in patients with rheumatoid arthritis. Ann Rheum Dis 66(1):76–80
Hamson EJ et al (2014) Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteomics Clin Appl 8(5–6):454–463
Altmann A, Haberkorn U, Siveke J (2021) The latest developments in imaging of fibroblast activation protein. J Nucl Med 62(2):160–167
Toms J et al (2020) Targeting fibroblast activation protein: radiosynthesis and preclinical evaluation of an (18)F-labeled FAP inhibitor. J Nucl Med 61(12):1806–1813
Ge L et al (2022) Preclinical evaluation and pilot clinical study of [(18)F]AlF-NOTA-FAPI-04 for PET imaging of rheumatoid arthritis. Eur J Nucl Med Mol Imaging 49(12):4025–4036
Kuyumcu S et al (2021) Safety of fibroblast activation protein-targeted radionuclide therapy by a low-dose dosimetric approach using 177Lu-FAPI04. Clin Nucl Med 46(8):641–646
Dorst DN et al (2022) Targeting of fibroblast activation protein in rheumatoid arthritis patients: imaging and ex vivo photodynamic therapy. Rheumatology (Oxford) 61(7):2999–3009
Bauer S et al (2006) Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res Ther 8(6):R171
Dorst DN et al (2021) Targeting of fibroblast activation protein in rheumatoid arthritis patients: imaging and ex vivo photodynamic therapy. Rheumatology (Oxford) 61(7):2999–3009
Song G et al (2019) CD109 regulates the inflammatory response and is required for the pathogenesis of rheumatoid arthritis. Ann Rheum Dis 78(12):1632–1641
Ge L et al (2022) ATF6α contributes to rheumatoid arthritis by inducing inflammatory cytokine production and apoptosis resistance. Front Immunol 13:965708
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
Ballal S et al (2021) Biodistribution, pharmacokinetics, dosimetry of [(68)Ga]Ga-DOTA.SA.FAPi, and the head-to-head comparison with [(18)F]F-FDG PET/CT in patients with various cancers. Eur J Nucl Med Mol Imaging 48(6):1915–1931
Figus FA et al (2021) Rheumatoid arthritis: extra-articular manifestations and comorbidities. Autoimmun Rev 20(4):102776
Radu AF, Bungau SG (2021) Management of rheumatoid arthritis: an overview. Cells 10(11):2857
Murray K et al (2021) Long-term remission and biologic persistence rates: 12-year real-world data. Arthritis Res Ther 23(1):25
Adamis D, van Gool WA, Eikelenboom P (2021) Consistent patterns in the inconsistent associations of Insulin-like growth factor 1 (IGF-1), C-reactive protein (C-RP) and interleukin 6 (IL-6) levels with delirium in surgical populations A systematic review and meta-analysis. Arch Gerontol Geriatr 97:104518
Wakefield RJ et al (2005) Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol 32(12):2485–2487
Wang X, Qian G, Duan H (2020) Diagnostic value of musculoskeletal ultrasound in rheumatoid finger arthritis. J Coll Physicians Surg Pak 30(6):617–621
Mona CE et al (2022) Correlation of (68)Ga-FAPi-46 PET Biodistribution with FAP expression by immunohistochemistry in patients with solid cancers: interim analysis of a prospective translational exploratory study. J Nucl Med 63(7):1021–1026
Varasteh Z et al (2019) Molecular imaging of fibroblast activity after myocardial infarction using a (68)Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J Nucl Med 60(12):1743–1749
Röhrich M et al (2022) Fibroblast activation protein-specific PET/CT imaging in fibrotic interstitial lung diseases and lung cancer: a translational exploratory study. J Nucl Med 63(1):127–133
Schmidkonz C et al (2020) Disentangling inflammatory from fibrotic disease activity by fibroblast activation protein imaging. Ann Rheum Dis 79(11):1485–1491
Laverman P et al (2015) Immuno-PET and Immuno-SPECT of rheumatoid arthritis with radiolabeled anti-fibroblast activation protein antibody correlates with severity of arthritis. J Nucl Med 56(5):778–783
Iwamoto N et al (2021) Methotrexate alters the expression of microRNA in fibroblast-like synovial cells in rheumatoid arthritis. Int J Mol Sci 22(21):11561
Municio C et al (2018) Methotrexate limits inflammation through an A20-dependent cross-tolerance mechanism. Ann Rheum Dis 77(5):752–759
Zhao S, Mysler E, Moots RJ (2018) Etanercept for the treatment of rheumatoid arthritis. Immunotherapy 10(6):433–445
Funding
This study was funded by National Natural Science Foundation of China (Grant No. 82072850, 81772760, 82101903, 81901666, 82171801, 82271842), Natural Science Foundation of Shandong Province (Grant No. ZR2020YQ55), Key Research and Development project of Shandong Province (No. 2021ZDSYS27), The Innovation Project of Shandong Academy of Medical Sciences (2021), The Youth Innovation Technology Plan of Shandong University (Grant No. 2019KJK003), and Academic Promotion Programme of Shandong First Medical University (Grant No. 2019LJ001).
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing or revision of the article, and all authors approved the final publication version. In the study, Jinxiang Han had full access to all data and is responsible for the accuracy and integrity. Jinxiang Han and Lin Wang conceptualized and designed the study. Luna Ge and Lin Wang wrote the manuscript. Acquisition of data: Qingyun Zhang, Xuehong Lin, Mengxue Lü, Dandan Shi, Ruojia Zhang, Haojun Shi, Kai Cheng. Yuang Zhang, and Jihong Pan and Guanhua Song analyzed and interpreted data. Luna Ge, Lin Wang, Weiqi Wang, Xiaofan Zhang, and Zhurui Shao revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Q., Lin, X., Wang, W. et al. Evaluation of 18F-FAPI-04 Imaging in Assessing the Therapeutic Response of Rheumatoid Arthritis. Mol Imaging Biol 25, 630–637 (2023). https://doi.org/10.1007/s11307-023-01817-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-023-01817-6