Abstract
Purpose
Positron emission tomography (PET)/computed tomography (CT) using 18F-fluoromisonidazole (FMISO) has been used as an imaging tool for tumour hypoxia. However, it remains unclear whether they are useful when scanning is performed earlier, e.g. at 2-h post-injection with a high sensitivity PET scanner. This study aimed to investigate the relationship between quantitative values in 18F-fluoromisonidazole (18F-FMISO)-PET obtained at 2- and 4-h post-injection in patients with head and neck cancer.
Procedures
We enrolled 20 patients with untreated locally advanced head and neck cancer who underwent 18F-FMISO-PET/CT scan between August 2015 and March 2018 at our institute. Image acquisition was performed 2 h and 4 h after 18F-FMISO administration using a combined PET/CT scanner. The SUVmax, SUVmean, SUVpeak, tumour-to-blood ratio (TBR), tumour-to-muscle ratio (TMR), metabolic tumour volume (MTV), and total lesion hypoxia (TLH) were measured in the region of interest of the primary tumour. We evaluated the between-image Spearman’s rank correlation coefficients and percentage differences in the quantitative values. The locations of the maximum uptake pixel were identified in both scans, and the distance between them was measured.
Results
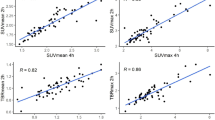
The mean (SD) SUVmax at 2 h and 4 h was 2.2(0.7) and 2.4(0.8), respectively. The Spearman’s rank correlation coefficients (ρ) and mean (SD) of the percentage differences of the measures were as follows: SUVmax (0.97; 7.0 [5.1]%), SUVmean (0.97; 5.2 [5.8]%), SUVpeak (0.94; 5.3 [4.7]%), TBR (0.96; 14.2 [9.8]%), TMR (0.96; 14.7 [8.4]%), MTV (0.98; 39.9 [41.3]%), and TLH (0.98; 40.1 [43.4]%). There were significant between-scan correlations in all quantitative values. The mean (SD) distance between the two maximum uptake pixels was 7.3 (5.3) mm.
Conclusions
We observed a high correlation between the quantitative values at 2 h and 4 h. When using a combined high-quality PET/CT, the total examination time for FMISO-PET can be shortened by skipping the 4-h scan.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radiation therapy is crucially involved in managing head and neck squamous cell carcinoma (HNSCC). Although there have been therapeutic and technological advances, some patients present with persistent tumours after irradiation or develop locoregional failure, which results in significant morbidity and mortality [1]. Numerous factors affect tumour sensitivity to radiation therapy. Hypoxia, which is among the tumour microenvironments, is associated with poor sensitivity to radiation therapy. In 1953, Gray et al. reported that oxygen concentration strongly influenced the biological effects of ionizing radiation [2]. Several studies have reported that tumour hypoxia in head and neck cancer is associated with radioresistance; moreover, it is an adverse prognostic factor [3, 4]. 18F-fluoromisonidazole (FMISO) is used for non-invasive tumour hypoxia evaluation; further, its uptake is associated with patient prognosis [5,6,7,8].
Several quantitative values in 18F-FMISO positron emission tomography (PET) at 4-h post-injection have been reported as reproducible [9]. However, their usefulness after prior scanning, for example, at 2-h post-injection, remains unclear. Only one study has reported that images acquired at 4-h post-injection provided higher contrast than those acquired at 2-h post-injection [10]. The total examination time in 18F-FMISO-PET can be shortened if the quantitative values at 2- and 4-h post-injection are equivalent.
We hypothesized that using a combined PET/computed tomography (CT) scanner, which has detectors that are more sensitive and yields high-quality images, could provide novel findings. This study aimed to assess the correlation of quantitative values obtained using 18F-FMISO-PET with a high-quality PET/CT system at 2- and 4-h post-injection in patients with head and neck cancer.
Materials and Methods
Patients
We enrolled 20 patients with untreated locally advanced head and neck cancer (LAHNC) who underwent 18F-FMISO-PET/CT scanning between August 2015 and October 2018 at our institute. This study was approved by the institutional review board of our hospital (Approval number C1053-1). All the patients provided written informed consent. Table 1 presents the characteristics of the patients. The median age of the patients was 68 years (range: 40–84 years); there were 14 men and 6 women. The 20 patients with LAHNC presented with the following: maxillary sinus cancer (n = 1), nasopharyngeal cancer (n = 7), oropharyngeal cancer (n = 4), hypopharyngeal cancer (n = 7), and laryngeal cancer (n = 1), without other metastatic diseases. The histology of 19 and 1 patient showed characteristics of squamous cell carcinoma and adenocarcinoma, respectively. Based on the Classification of Malignant Tumours, 7th edition of Union for International Cancer Control (UICC), we classified 2, 9, 2, and 7 patients as stage II, III, IVA, and IVB, respectively.
Data Acquisition
Image acquisition was performed at 2 h and 4 h after 18F-FMISO administration. All images were acquired for 20 min in 3-dimensional mode using a dedicated PET/CT scanner (Discovery IQ, GE Healthcare) with 5 circular BGO (Bi4Ge3O12) detectors. The sensitivity was 22 kcps/MBq, which was 2.4 times higher than our conventional PET/CT scanner. Image reconstruction was performed using VuePoint HD. The axial resolution was full width at half maximum of 5.6 mm, at 1 cm from the centre of the field of view.
Image Analysis
18F-FMISO-PET images were quantitatively analysed. Although a threshold for the hypoxia definition remains unestablished, we defined a volume of interest where the standardized uptake value (SUV) was ≥ “blood mean + 0.5 × standard deviation (SD)”, which was modified from a previous study [9], as the metabolic tumour volume (MTV) for calculating PET parameters. We calculated the SUVmax, SUVmean, SUVpeak, tumour-to-blood ratio (TBR), tumour-to-muscle ratio (TMR), metabolic tumour volume (MTV), and total lesion hypoxia (TLH) from the volume of interest containing the primary tumour. The SUVmax was calculated as the maximal single voxel of activity concentration/(injected dose/body weight). The SUVmean was calculated as the average SUV within the MTV, while the SUVpeak was calculated as the maximum average SUV within a 1-cm3 spherical volume. The TBR was derived as the maximum tumour concentration divided by the blood concentration in the cervical artery measured in the axial image at the hyoid level. We did not perform invasive blood sampling for TBR analysis. The TMR was defined as the tumour uptake divided by the uptake of the posterior cervical muscle in the axial image at the hyoid level. We analysed the average value of each 10 muscle lesions and the blood uptake in the region of interest (ROI) with a diameter of 0.4 cm. Subsequently, the physiological accumulation region identified in other modalities was manually excluded from the MTV. The TLH was calculated as the MTV × SUVmean.
CT and 18F-FMISO-PET images were co-registered on a workstation (MIM, version 6.5.3). The locations of the maximum uptake pixels on the FMISO2h and FMISO4h images were identified, and the distance between them was measured to evaluate the location reproducibility of 18F-FMISO uptake.
Statistical Analysis
Regarding the statistical analysis of the reproducibility of 18F-FMISO uptake and MTV, we evaluated the Spearman’s rank correlation coefficients of these quantitative values. We also calculated the percentage difference as the difference between both values divided by their average values. A correlation coefficient of more than 0.7 indicates a high correlation. Statistical significance was set at an αvalue of 0.05. All statistical analyses were performed using Microsoft Excel software, version 16.11.
Results
The mean (SD) 18F-FMISO injection dose was 327.5 (132.8) MBq (range: 166.3–601.0 MBq); moreover, the time interval between injection and scan start was 126.0 (11.9) min (range: 114–167 min) for FMISO2h and 245.9 (13.4) min (range: 226–263 min) for FMISO4h.
Table 2 shows the SUVmax, SUVmean, SUVpeak, TBR, TMR, MTV, and TLH. The Spearman’s rank correlation coefficients (ρ) of the parameters and the mean (SD) of the percentage difference were as follows: SUVmax (0.97; 7.0 [5.1]%), SUVmean (0.97; 5.2 [5.8]%), SUVmean (0.94; 5.3 [4.7]%), TBR (0.96; 14.2 [9.8]%), TMR (0.96; 14.7 [8.4]%), MTV (0.98; 39.9 [41.3]%), and TLH (0.98; 40.1 [43.4]%) (Fig. 1). There was a high between-scan correlation of the quantitative values (P < 0.01).
The mean (SD) distance between the maximum uptake locations of both scans was 7.3 (5.3) mm (range: 0.9–22.1 mm). This distance was smaller than the PET/CT scanner resolution in 11 out of 20 patients. Figure 2 presents a selected axial image of the representative cases.
Discussion
We observed a high correlation between the quantitative values of 18F-FMISO-PET/CT scans at 2- and 4-h post-injection, with few patients presenting a moderate difference in volumetric parameters.
18F-FMISO-PET images obtained at 4-h post-injection were considered as more suitable for reproducibility evaluation compared with images acquired less than 3 h due to slow blood clearance of 18F-FMISO and low contrast [5,6,7, 11,12,13,14]. Additionally, Gagel et al. [15] and Zimmy et al. [16] compared pO2 measurements with 2-h 18F-FMISO PET data to pO2 probe measurement in head and neck cancer. From these studies, it is equivocal as to which timepoint is better for 18F-FMISO imaging. A previous study by Okamoto et al. [9] compared the quantitative values of two 18F-FMISO-PET/CT images obtained at 48-h intervals, including SUVmax, TBR, TMR, and hypoxic volume (HV), and reported high reproducibility of these quantitative values at 4 h post-injection. Another previous study [10] directly compared the 18F-FMISO images acquired at 2- and 4-h post-injection. It was concluded that images acquired after 4 h had a higher contrast than those acquired after 2 h. The discrepancy between this study and ours could arise from two aspects: the difference in analysis method which uses absolute value or correlations and the sensitivity of PET scanners. The present study found a high correlation between quantitative values obtained at 2 h and 4 h using a current combined PET/CT scanner, which has highly sensitive detectors and can yield high-quality images. Further examination is needed for the evaluation of the two scanners. Specifically, there was a high between-scan correlation in quantitative values, including SUVmax, TBR, and TMR. Moreover, there was a high between-scan correlation in volumetric values, including MTV and TLH. Further studies with a larger population should confirm the reproducibility of two 18F-FMISO PET/CT images acquired at intervals of more than 2-h post-injection. This could shorten the total examination time in 18F-FMISO-PET, which results in a decrease in the patient burden caused by this examination.
18F-FMISO has an imidazole ring and is reductively activated in hypoxic cells [17]. It is the most common PET radiotracer for tumour hypoxia imaging; however, its high lipophilicity and slow clearance kinetics require up to 2- or 3-h post-injection to obtain contrast [18]. The biological half-life of this tracer is 12–13 h [19, 20]. A previous study concluded that images acquired after 4 h of uptake and washout provided higher contrast compared with images acquired after 2 h [10]. In the present study, the correlation of SUVmax and the subtraction evaluation were indicative of the reproducibility of FMISO2h and FMISO4h.
A previous study reported that the oxygen enhancement ratio was approximately 3 under 20 mmHg [21], which decreased to 2.0–2.8 at an oxygen tension of 3–20 mmHg. Therefore, compared with normoxic areas, areas of FMISO accumulation are radioresistant. Regarding oxygen tension in areas of FMISO accumulation, an animal study using pig liver reported reduced FMISO when pO2 was less than 15 mmHg [22]. In that study, the SUV of FMISO was 1.57, which corresponded to a pO2 of 20 mmHg. Various thresholds of TBR, TMR, and SUVmax have been provisionally determined [23]. The present study used an SUV ≥ “blood mean + 0.5 ×SD” as the threshold since it is adaptive and suitable for hypoxic volume delineation. We did not draw off the volume using a SUVmax threshold of 50 %, 60 %, or 1.6. A universal threshold separating the background from the hypoxic region is required to define the target for dose escalation. Future large-scale studies should examine the optimal cut-off value of 18F-FMISO-PET uptake for estimating the hypoxic state and predicting therapy outcome for HNC.
The hypoxic area in a tumour is characterized by chronic and acute hypoxia [24, 25]. Acute hypoxia can change every few hours or days and therefore could alter the 18F-FMISO uptake distribution. Mönnich et al. [26] reported that acute hypoxia did not affect PET imaging reproducibility in simulations. In the present study, there were no significant differences in SUVmax, TBR, TMR, MTV, and TLH. This observed reproducibility is suggestive of the insignificant effect of transient acute hypoxia on 18F-FMISO studies at two intervals. Future studies should assess whether acute hypoxia can be captured at this PET resolution.
Our final goal is determining the dose escalation for a hypoxic region to improve the radiotherapy outcome. There have been numerous reports on parameters regarding the hypoxic region as a prognostic factor [27, 28]. Moreover, there have been several studies on dose painting based on the spatial distribution of hypoxia [29, 30]. These studies have shown that hypoxia-targeted radiotherapy using dose painting is a feasible technique for increasing tumour control without a concomitant increase in normal tissue complications. A small pilot study described the outcome of patients with HPV-related oropharyngeal cancer whose radiation dose was de-escalated from 70 to 60 Gy in grossly involved lymph nodes [31]. In de-escalation treatment strategies, functional imaging for selectively de-escalating nodes to 60 Gy has been confirmed as safe. These dose painting planning requires the feasibility and reproducibility of functional imaging.
In the FDG-PET protocol, imaging at 2-h post-injection was considered to yield good contrast and high diagnostic ability compared with imaging at 1-h post-injection [32]. However, scans performed at later times show degraded image quality due to physical F-18 decay. In routine clinical FDG-PET examinations, a 1-h uptake phase protocol is adopted since its diagnostic ability is not inferior. Therefore, despite the good contrast of FMISO, which peaks after 4 h, the high correlation could allow a shorter 2-h imaging protocol upon clinical application. Regarding the absolute value, SUV was non-significant, while TBR or TMR had significant differences mainly due to decreased background activity resulting from decay and washout. Whether a 4-h timepoint has improved tumour-to-background contrast is not necessarily relevant from a biological perspective. These differences in TBR or TMR could allow qualitative FMISO2h evaluation and may also be useful in quantitative analysis. The shortened protocol could allow differentiation of the hypoxic tumour region from the background; however, care must be taken when extracting the volume and using volumetric parameters for dose painting.
Conclusion
Our preliminary data demonstrated a higher correlation between quantitative values obtained at 2- and 4-h post-injection. Using the novel high-quality PET/CT, the total examination time in FMISO-PET can be shortened by skipping the scan at 4 h.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Blanchard P, Baujat B, Holostenco V, Bourredjem A, Baey C, Bourhis J, Pignon JP, MACH-CH Collaborative group (2011) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumor site. Radiother Oncol 100:33–40
Gray LH, Conger AD, Ebert M, Hornsey S, Scott OCA (1953) Concentrating of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 26:638–648
Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW (1997) Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 38:285–289
Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, Becker A, Adam M, Molls M, Dunst J, Terris DJ, Overgaard J (2005) Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol 77:18–24
Rajendran JG, Schwartz DL, O’Sullivan J et al (2006) Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res 12:5435–5441
Rischin D, Peters L, Fisher R, Macann A, Denham J, Poulsen M, Jackson M, Kenny L, Penniment M, Corry J, Lamb D, McClure B (2005) Tirapazamine, cisplatin, and radiation versus fluorouracil, cisplatin, and radiation in patients with locally advanced head and neck cancer: a randomized phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02). J Clin Oncol 23:79–87
Koh WJ, Bergman KS, Rasey JS, Peterson LM, Evans ML, Graham MM, Grierson JR, Lindsley KL, Lewellen TK, Krohn KA, Griffin TW (1995) Evaluation of oxygenation status during fractionated radiotherapy in human nonsmall cell lung cancers using [F-18] fluoromisonidazole positron emission tomography. Int J Radiat Oncol Biol Phys 33:391–398
Eschmann SM, Paulsen F, Reimold M, Dittmann H, Welz S, Reischl G, Machulla HJ, Bares R (2005) Prognostic impact of hypoxia imaging with 18F-misonidazole PET in non–small cell lung cancer and head and neck cancer before radiotherapy. J Nucl Med 46:253–260
Okamoto S, Shiga T, Yasuda K, Ito YM, Magota K, Kasai K, Kuge Y, Shirato H, Tamaki N (2013) High reproducibility of tumor hypoxia evaluated by 18F-fluoromisonidazole PET for head and neck cancer. J Nucl Med 54:201–207
Abolmaali N, Haase R, Koch A, Zips D, Steinbach J, Baumann M, Kotzerke J, Zöphel K (2011) Two or four hour [18F] FMISO-PET in HNSCC. Nuklearmedizin 50:22–27
Lin Z, Mechalakos J, Nehmeh S, Schoder H, Lee N, Humm J, Ling CC (2008) The influence of changes in tumor hypoxia on dose-painting treatment plans based on 18F-FMISO positron emission tomography. Int J Radiat Oncol Biol Phys 70:1219–1228
Dirix P, Vandecaveye V, De Keyzer F et al (2009) Dose painting in radiotherapy for head and neck squamous cell carcinoma: value of repeated functional imaging with 18F-FDG PET, 18F-fluoromisonidazole PET, diffusion-weighted MRI, and dynamic contrast-enhanced MRI. J Nucl Med 50:1020–1027
Lee NY, Mechalakos JG, Nehmeh S, Lin Z, Squire OD, Cai S, Chan K, Zanzonico PB, Greco C, Ling CC, Humm JL, Schöder H (2008) Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys 70:2–13
Nehmeh SA, Lee NY, Schröder H, Squire O, Zanzonico PB, Erdi YE, Greco C, Mageras G, Pham HS, Larson SM, Ling CC, Humm JL (2008) Reproducibility of intratumor distribution of 18F-fluoromisonidazole in head and neck cancer. Int J Radiat Oncol Biol Phys 70:235–242
Gagel B, Reinartz P, Dimartino E et al (2004) pO2 polarography versus positron emission tomography ([18F] fluoromisonidazole, [18F]-2-fluoro-29-deoxyglucose): an appraisal of radiotherapeutically relevant hypoxia. Strahlenther Onkol 180:616–622
Zimmy et al (2006) FDG-a marker of tumour hypoxia? A comparison with [18F]fluoromisonidazole and pO2-polarography in metastatic head and neck cancer. Eur J Nucl Med Mol Imaging 33:1426–1431
Juchau MR et al (1989) Bioactivation in chemical teratogenesis. Annu Rev Pharmacol Toxicol 29:165–167
Biskupiak JE, Krohn KA et al (1993) Second generation hypoxia agents. J Nucl Med 34:411–413
Gray AJ, Dische S, Adams GE, Flockhart IR, Foster JL (1976) Clinical testing of the radiosensitiser Ro-07-0582 I. dose tolerance, serum and tumour concentrations. Clin Radiol 27:151–157
Saunders ME, Dische S, Anderson P et al (1978) The neurotoxicity of misonidazole and its relationship to dose, half-life and concentration in the serum. Br J Cancer Suppl 3:268–270
Hall EJ (2006) Radiobiology for the radiologist, 6th edn. Lippincott Williams and Wilkins, Philadelphia, pp 88–89
Piert M, Manchulla HJ, Becker G et al (2000) Dependency of the [18F]Fluoromisonidazole uptake on oxygen delivery and tissue oxygenation in the porcine liver. Nucl Med Biol 27:693–700
Fleming N et al (2015) Imaging tumor hypoxia with positron emission tomography. Br J Cancer 112:238–250
Pajonk F, Vlashi E, McBride WH (2010) Radiation resistance of cancer stem cells: the 4 R’s of radiobiology revisited. Stem Cells 28:639–648
Bayer C, Shi K, Astner ST, Maftei CA, Vaupel P (2011) Acute versus chronic hypoxia: why a simplified classification is simply not enough. Int J Radiat Oncol Biol Phys 80:965–968
Mönnich D, Troost EG, Kaanders JH, Oyen WJ, Alber M, Thorwarth D (2012) Modelling and simulation of the influence of acute and chronic hypoxia on [18F]fluoromisonidazole PET imaging. Phys Med Biol 57:1675–1684
Lee N, Nehmeh S, Schoder H et al (2009) Prospective trial incorporating pre-/mid-treatment [18F]-misonidazole positron emission tomography for head-and-neck cancer patients undergoing concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys 75:101–108
Walsh JC, Lebedev A, Aten E, Madsen K, Marciano L, Kolb HC (2014) The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal 21:1516–1554
Yasuda K, Onimaru R, Okamoto S, Shiga T, Katoh N, Tsuchiya K et al (2013) [18F]fluoromisonidazole and a new PET system with semiconductor detectors and a depth of interaction system for intensity modulated radiation therapy for nasopharyngeal cancer. Int J Radiat Oncol, Biol, Phys 85:142–147
Chang JH, Wada M, Anderson NJ, Joon DL, Lee ST, Gong SJ et al (2013) Hypoxia-targeted radiotherapy dose painting for head and neck cancer using 18F-FMISO PET: a biological modeling study. Acta Oncol (Madr) 52:1723–1729
Lee N, Schoder H, Beattie B, Lanning R, Riaz N, McBride S, Katabi N, Li D, Yarusi B, Chan S, Mitrani L, Zhang Z, Pfister DG, Sherman E, Baxi S, Boyle J, Morris LGT, Ganly I, Wong R, Humm J (2016) Strategy of using intratreatment hypoxia imaging to selectively and safely guide radiation dose de-escalation concurrent with chemotherapy for locoregionally advanced human papillomavirus-related oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys 96:9–17
Kubota K, Itoh M, Ozaki K, Ono S, Tashiro M, Yamaguchi K, Akaizawa T, Yamada K, Fukuda H (2001) Advantage of delayed whole body FDG-PET imaging for tumour detection. Eur J Nucl Med 28:696–703
Funding
This work was supported by the Grant-in-Aid for Scientific Research A (grant number JP25253078) and Grant-in-Aid for Scientific Research C (grant number 18K07751) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (Approval number C1053-1) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Consent for Publication
The authors affirm that human research participants provided informed consent for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawamura, M., Yoshimura, M., Shimizu, Y. et al. Evaluation of Optimal Post-Injection Timing of Hypoxic Imaging with 18F-Fluoromisonidazole-PET/CT. Mol Imaging Biol 23, 597–603 (2021). https://doi.org/10.1007/s11307-021-01580-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-021-01580-6