Abstract
Purpose
This study evaluated the effect of formalin fixation for near-infrared (NIR) fluorescence imaging of an antibody-dye complex (panitumumab-IRDye800CW) that was intravenously administered to patients with head and neck squamous cell carcinoma (HNSCC) scheduled to undergo surgery of curative intent.
Procedures
HNSCC patients were infused with 25 or 50 mg of panitumumab-IRDye800CW followed by surgery 1–5 days later. Following resection, primary tumor specimens were imaged in a closed-field fluorescence imaging device, before and after formalin fixation. The fluorescence images of formalin-fixed specimens were compared with images prior to formalin fixation. Regions of interest were drawn on the primary tumor and on the adjacent normal tissue on the fluorescence images. The mean fluorescence intensity (MFI) and tumor-to-background ratios (TBRs) of the fresh and formalin-fixed tissues were compared.
Results
Of the 30 enrolled patients, 20 tissue specimens were eligible for this study. Formalin fixation led to an average of 10 % shrinkage in tumor specimen size (p < 0.0001). Tumor MFI in formalin-fixed specimens was on average 10.9 % lower than that in the fresh specimens (p = 0.0002). However, no statistical difference was found between the TBRs of the fresh specimens and those of the formalin-fixed specimens (p = 0.85).
Conclusions
Despite the 11 % decrease in MFI between fresh and formalin-fixed tissue specimens, the relative difference between tumor and normal tissue as measured in TBR remained unchanged. This data suggests that evaluation of formalin-fixed tissue for assessing the accuracy of fluorescence-guided surgery approaches could provide a valid, yet more flexible, alternative to fresh tissue analysis.
Trial Registration
NCT02415881
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgical resection of the primary tumor plays an essential role for cancer treatment, and incomplete surgical resections, including positive margins, are directly correlated with poor prognosis [1,2,3]. Fluorescence-guided surgery has emerged as a novel intraoperative technique that provides surgeons real-time guidance during surgery [4, 5]. Recent clinical introduction and evaluation of fluorescently labeled imaging agents, such as the anti-epidermal growth factor receptor (EGFR) antibodies or the anti-vascular endothelial growth factor (VEGF) antibody, have enabled us to differentiate the tumor regions from the surrounding normal tissues during surgery and demonstrated feasibility of obtaining a tumor-free margin resection in oncologic surgery [4, 6,7,8,9]. Conducted immediately after tumor resection, ex vivo assessments of resected fresh tumor specimens allowed us to predict the closest regions of tumor tissues for both deep and peripheral margins [10, 11]. Moreover, the use of these antibody-dye conjugates allows us to evaluate the delivery and distribution of systemically injected antibodies [12]. Recently, we have reported that antibody-dye conjugate can be used as a surrogate to measure the interpatient and intratumoral heterogeneity of antibody distribution in HNSCC at an unprecedented resolution, resulting in a deeper understanding of the antibody distribution within patient tumors [13].
Recent studies show the structured workflow of the surgical process of the primary tumor and lymph node specimens including an intraoperative setting and pathological process such as formalin fixation [9, 14]. However, there is no clinical data to assess the impact of formalin fixation on the fluorescence signal in resected human tissues during the pathological process. In order to accurately assess the surgical results using a fluorescently labeled tumor-specific imaging agent, it is crucial to understand the changes in the fluorescence intensity of the tumor specimens during the formalin fixation and how this process would affect the differentiation between tumor tissues and adjacent normal tissues. Moreover, to apply the fluorescent-based molecular imaging into common use in the clinical setting, the pathological process for the resected specimens should be optimized and standardized to ensure consistent results. Here, we evaluated the effect of formalin fixation for near-infrared (NIR) fluorescence imaging of an antibody-dye complex (panitumumab-IRDye800CW) that was intravenously administered to patients with head and neck squamous cell carcinoma (HNSCC) scheduled to undergo surgery of curative intent.
Materials and Methods
Study Design
The protocol for this clinical study was approved by the Stanford University Institutional Review Board (IRB 35064) and the FDA and is listed on clinicaltrials.gov as NCT02415881. Informed consent was obtained from all individual participants included in the study. The research was conducted in full accordance with FDA’s ICH-GCP guidelines, the Helsinki Declaration of 1975 and its amendments, and the laws and regulations of the USA.
Between August 2017 and February 2019, 30 patients were enrolled in the study and infused with 25 or 50 mg of panitumumab-IRDye800CW 1–5 days prior to surgery. Following resection, primary tumors were imaged with a near-infrared fluorescence imager on the day of the surgery and following formalin fixation.
Ex Vivo Fluorescence Image Analysis of the Primary Tumor
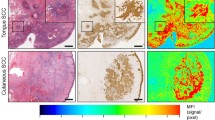
The workflow for the analysis of fluorescence imaging of the whole specimen is illustrated in Fig. 1. In brief, following excision and surgeon’s gross examination of the tissue specimen, the specimen was imaged with the closed-field fluorescence imaging device (Pearl Triology; or IGP-ELVIS; LI-COR Biosciences Inc., Lincoln, NE, USA), which enables the measurement of the fluorescence intensity and tissue area in the specimens and allows for imaging in a controlled environment, including elimination of ambient light [10, 15]. Thereafter, specimens were transferred to pathology for formalin fixation overnight or over the weekend (Friday to Monday) per standard of care. Specimens were fixed in 10 % neutral buffered formalin for 1–4 days at ambient/room temperature in sealed, opaque plastic containers with limited light exposure. Specimens were fixed in a volume of formalin equal to or greater than the volume of tissue in the sample. Directly before grossing of the primary tumor specimen, the formalin-fixed tissue specimen was re-imaged in the closed-field fluorescence imaging device.
Workflow of the primary tumor analysis. Following excision of the tissue specimen, the specimen was imaged in a closed-field fluorescence imaging device. Hereafter, the tissue specimen was transferred to pathology for formalin fixation and re-imaged in the fluorescence imaging device. To determine the mean fluorescence intensity (MFI) and the tumor-to-background ratio (TBR), multiple regions of interest (ROIs) were drawn on the primary tumor and the adjacent normal tissue using the fluorescence imaging device–integrated software. Tissue MFIs were then calculated by dividing the sum of the measured fluorescence intensities by the sum of the areas. The TBR was calculated by dividing the primary tumor MFI by the adjacent normal tissue MFI.
The fluorescence images of formalin-fixed specimen were compared with images prior to formalin fixation. Regions of interest (ROIs) were drawn on the primary tumor and in the adjacent normal tissue using a fluorescence imaging device–integrated software (Image Studio; LI-COR Biosciences, Inc.) as described previously by us [16]. Briefly, mean fluorescent intensities (MFI) were determined as follows:
Tumor-to-background ratios (TBRs) were calculated as follows:
Statistical Analysis
Descriptive statistics and figures were obtained using GraphPad Prism (Version 6.0c, GraphPad Software, La Jolla, CA, USA). MFI and TBR of the primary tumor between fresh and formalin-fixed conditions were analyzed with a paired t test. Differences of change rate of MFI and TBR between the overnight fixation group and the over the weekend fixation group were analyzed with a Mann–Whitney U test. The variance in signal was defined as the coefficient of variance (CV), which is the standard deviation divided by mean signal intensity and describes the heterogeneity of the signals [17]. Pearson correlation analysis among fluorescence signals was used to explore the relationship between the fresh condition and the formalin-fixed condition. All data are presented as means or means ± standard deviation (SD), and a two-sided p value of 0.05 or less was considered statistically significant.
Results
Patient Characteristics
Of the 30 enrolled patients, tissue specimens of 10 patients had to be excluded due to difficulty of matching the pre- and post-fixation whole specimens caused by the different imaging angles or by their complicated three-dimensional tumor anatomy. Characteristics of the remainder 20 patients are shown in Table 1. The mean time difference between infusion of drug and the start of surgery was 43 h (range 17–120) and the average formalin fixation time of the tissue specimens was 29 h (range 17–94). The most common primary tumor site was the tongue (45 %), followed by the buccal mucosa (20 %) and retromolar trigone (15 %).
Tissue Shrinkage of the Primary Tumor After Formalin Fixation
We assessed the areas of the primary tumor tissue specimens directly after resection and following formalin fixation to determine using the fluorescence imaging device–integrated software if tissue shrinkage had occurred (Fig. 2a). On average, following formalin fixation, the tissue decreased 9.9 % (range 0–17) in size (p < 0.0001, Fig. 2b).
Changes of Tumor MFI and TBR After Formalin Fixation
Figure 3 shows the primary tumor MFI and TBR for all 20 evaluated tissue specimens. A high-interpatient variability for MFIs was found between fresh and formalin-fixed tissue samples (CV = 0.66 and 0.68 for fresh and formalin-fixed specimens, respectively). Low interpatient variability was found for TBRs in fresh (CV = 0.31) and formalin-fixed (CV = 0.35) tissue specimens with an average TBR of 4.4 (range 2.3–8.5). Comparing fresh tissue MFI to the MFI of the formalin-fixed tumor tissue specimens resulted in a strong correlation (Supplemental Figure 1; R2 = 0.93). Findings were similar for TBR (Supplemental Figure 1, R2 = 0.97).
Figure 4a demonstrates a representative case in which a decrease in primary tumor MFI was seen following the formalin fixation process. The MFI of tumor in formalin-fixed specimens was on average 10.9 % lower than that of the fresh specimens (p = 0.0002, 0.27 ± 0.18 a.u. vs. 0.24 ± 0.17 a.u.). No statistically significant difference was found for TBRs when comparing fresh and formalin specimens (p = 0.85, 4.4 ± 1.4 vs. 4.4 ± 1.6).
Before and after the plot of changes in tumor MFI and TBR. a A representative case in which a decrease in primary tumor MFI was seen following the formalin fixation process. b The MFI of tumor in formalin-fixed specimens was on average 10.9 % lower than the fresh specimens (p = 0.0002). However, no statistically significant difference was found for TBRs when comparing fresh and formalin-fixed specimens (p = 0.85).
To evaluate if the fluorescence signal remains stable over the number of scans, we did additional experiments by imaging the same piece of tissue 10 times. As shown in Supplemental Figure 2, we measured the signal decrease of the tissue compared to the first time of imaging and only found up to 6 % decrease over the 10 times, suggesting that repetitive imaging might only contribute to a small part of the fluorescence decrease for the resected tissue specimens.
Time Effect of Formalin Fixation for Tumor MFI and TBR
To evaluate the effect of the time of formalin fixation on tumor MFI and TBR, we compared the overnight fixation group (n = 16, mean; 20 h (range 17–22)) to the specimens that were formalin fixed over the weekend (n = 4, mean; 67 h (range 40–94)) with no significant differences in tumor MFI and TBR found (Fig. 5; p = 0.75 and p = 0.38, respectively).
Discussion
There is a lack of clinical data regarding the impact of formalin fixation on the changes of fluorescence signals in resected tissue specimens during the pathological process. With the rise of clinical trials investigating novel and/or repurposed fluorescently labeled imaging agents (some of which are already in phase 2/3), this is of considerable importance as a majority of tissue analyses are currently being performed on formalin-fixed tissue specimens [4, 18,19,20]. The current study demonstrates that after systemic administration of a fluorescently labeled antibody, an average 11 % decrease of fluorescence intensity was found in the resected primary tumor specimens after the formalin fixation. However, no significant differences in TBR were seen between the fresh and formalin-fixed specimens. Moreover, the fluorescence signals in the resected tumor specimens remained stable within 1–4 days after formalin fixation. These results suggest that although after the formalin fixation a decreased MFI is seen, it does not affect their relative differences between the tumor and the surrounding normal tissue as measured by TBR, demonstrating the rigidness of the model.
Formalin, a formaldehyde solution buffered to a neutral pH, is the most prevalent tissue fixative used for morphological preservation as a standard pathological process, and it forms cross-linkages between peptides and forms hydroxymethyl groups on reactive amino acid side chains [21]. Human specimens are usually fixed overnight with fixation times varying from 12 to 48 h and are influenced by factors such as tissue components, specimen size, and temperature [22, 23]. Tissue specimen shrinkage of about 10 % was found between fresh and formalin-fixed tissues consistent with previous literature [24, 25], with no difference found if the specimen was fixed overnight or over the weekend. The soaking of the tissues in formalin could result in clearance of remaining fluorescence imaging agents from circulation (i.e., blood vessels) and the exposure to ambient light may also reduce the fluorescence signal over time [26]. Our study demonstrated an average 11 % decrease in MFI between the fresh and formalin-fixed specimens, but no effect on TBR was found suggesting that the signal decrease is similar for tumor tissue as well as the surrounding normal tissue.
By using the closed-field fluorescence imaging devices, quantitative and objective evaluation of the tissue specimens was achieved due to the controlled imaging environment, which eliminated ambient light and fixed camera-tissue distance [15]. Back-table fluorescence assessment on fresh tissue specimens enables surgeons to predict the closest deep and peripheral margins and to detect secondary primary tumor during surgery [10, 11, 17]. However, despite fluorescence imaging being near-real-time when using such a device, specimen assessments for factors such as sensitivity and/or specificity, and negative and positive predictive values of the imaging agent used are generally better calculated on formalin-fixed tissue due to logistical challenges and constraints when wanting to perform such analysis in the operating room. With the current study, it was demonstrated ex vivo formalin-fixed specimen analysis can be used as a valid alternative to fresh tissue specimen analysis. By fixating the tissue specimens in a state as close to the original as possible, we can connect intraoperative surgical situation (in vivo) and pathological evaluation after resection (ex vivo) and apply the near-infrared fluorescence imaging with an antibody-dye conjugate into a clinical use in surgical oncology.
Conclusion
Despite the 11 % decrease in MFI between fresh and formalin-fixed tissue specimens, the relative difference between tumor and normal tissue as measured in TBR remained unchanged. This data suggests that evaluation of formalin-fixed tissue for assessing the accuracy of fluorescence-guided surgery approaches could provide a valid, yet more flexible, alternative to fresh tissue analysis.
References
Orosco RK, Tapia VJ, Califano JA, Clary B, Cohen EEW, Kane C et al (2018) Positive surgical margins in the 10 most common solid cancers. Sci Rep 8(1):5686
Eldeeb H, Macmillan C, Elwell C, Hammod A (2012) The effect of the surgical margins on the outcome of patients with head and neck squamous cell carcinoma: single institution experience. Cancer Biol Med 9(1):29–33
Ettl T, El-Gindi A, Hautmann M, Gosau M, Weber F, Rohrmeier C et al (2016) Positive frozen section margins predict local recurrence in R0-resected squamous cell carcinoma of the head and neck. Oral Oncol 55:17–23
Rosenthal EL, Warram JM, de Boer E, Chung TK, Korb ML, Brandwein-Gensler M, Strong TV, Schmalbach CE, Morlandt AB, Agarwal G, Hartman YE, Carroll WR, Richman JS, Clemons LK, Nabell LM, Zinn KR (2015) Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin Cancer Res 21(16):3658–3666
Zhang RR, Schroeder AB, Grudzinski JJ, Rosenthal EL, Warram JM, Pinchuk AN, Eliceiri KW, Kuo JS, Weichert JP (2017) Beyond the margins: real-time detection of cancer using targeted fluorophores. Nat Rev Clin Oncol 14(6):347–364
Gao RW, Teraphongphom NT, van den Berg NS, Martin BA, Oberhelman NJ, Divi V et al (2018) Determination of tumor margins with surgical specimen mapping using near-infrared fluorescence. Cancer Res 78(17):5144–5154
Lu G, van den Berg NS, Martin BA, Nishio N, Hart ZP, van Keulen S, Fakurnejad S, Chirita SU, Raymundo RC, Yi G, Zhou Q, Fisher GA, Rosenthal EL, Poultsides GA (2020) Tumour-specific fluorescence-guided surgery for pancreatic cancer using panitumumab-IRDye800CW: a phase 1 single-centre, open-label, single-arm, dose-escalation study. Lancet Gastroenterol Hepatol 5:753–764
Lamberts LE, Koch M, de Jong JS, Adams ALL, Glatz J, Kranendonk MEG, Terwisscha van Scheltinga AGT, Jansen L, de Vries J, Lub-de Hooge MN, Schröder CP, Jorritsma-Smit A, Linssen MD, de Boer E, van der Vegt B, Nagengast WB, Elias SG, Oliveira S, Witkamp AJ, Mali WPTM, van der Wall E, van Diest PJ, de Vries EGE, Ntziachristos V, van Dam GM (2017) Tumor-specific uptake of fluorescent bevacizumab-IRDye800CW microdosing in patients with primary breast cancer: a phase I feasibility study. Clin Cancer Res 23(11):2730–2741
Koller M, Qiu S-Q, Linssen MD, Jansen L, Kelder W, de Vries J et al (2018) Implementation and benchmarking of a novel analytical framework to clinically evaluate tumor-specific fluorescent tracers. Nat Commun 9(1):3739
van Keulen S, Nishio N, Birkeland A, Fakurnejad S, Martin B, Forouzanfar T et al (2019) The sentinel margin: intraoperative ex vivo specimen mapping using relative fluorescence intensity. Clin Cancer Res 25(15):4656–4662
Fakurnejad S, Krishnan G, van Keulen S, Nishio N, Birkeland AC, Baik FM et al (2019) Intraoperative molecular imaging for ex vivo assessment of peripheral margins in oral squamous cell carcinoma. Front Oncol 9:1476
de Boer E, Warram JM, Tucker MD, Hartman YE, Moore LS, de Jong JS, Chung TK, Korb ML, Zinn KR, van Dam GM, Rosenthal EL, Brandwein-Gensler MS (2015) In vivo fluorescence immunohistochemistry: localization of fluorescently labeled cetuximab in squamous cell carcinomas. Sci Rep 5:10169
Lu G, Fakurnejad S, Martin BA, van den Berg NS, van Keulen S, Nishio N, Zhu AJ, Chirita SU, Zhou Q, Gao RW, Kong CS, Fischbein N, Penta M, Colevas AD, Rosenthal EL (2020) Predicting therapeutic antibody delivery into human head and neck cancers. Clin Cancer Res 26(11):2582–2594
Nishio N, van den Berg NS, van Keulen S, Martin BA, Fakurnejad S, Teraphongphom N et al (2019) Optical molecular imaging can differentiate metastatic from benign lymph nodes in head and neck cancer. Nat Commun 10(1):5044
van Keulen S, van den Berg NS, Nishio N, Birkeland A, Zhou Q, Lu G, Wang HW, Middendorf L, Forouzanfar T, Martin BA, Colevas AD, Rosenthal EL (2019) Rapid, non-invasive fluorescence margin assessment: optical specimen mapping in oral squamous cell carcinoma. Oral Oncol 88:58–65
Nishio N, van den Berg NS, van Keulen S, Martin BA, Fakurnejad S, Zhou Q, Lu G, Chirita SU, Kaplan MJ, Divi V, Colevas AD, Rosenthal EL (2020) Optimal dosing strategy for fluorescence-guided surgery with panitumumab-IRDye800CW in head and neck cancer. Mol Imaging Biol 22(1):156–164
van Keulen S, Nishio N, Fakurnejad S, Birkeland A, Martin BA, Lu G, Zhou Q, Chirita SU, Forouzanfar T, Colevas AD, van den Berg NS, Rosenthal EL (2019) The clinical application of fluorescence-guided surgery in head and neck cancer. J Nucl Med 60(6):758–763
van Dam GM, Themelis G, Crane LMA, Harlaar NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJ, van der Zee A, Bart J, Low PS, Ntziachristos V (2011) Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med 17(10):1315–1319
Boogerd LSF, Hoogstins CES, Schaap DP, Kusters M, Handgraaf HJM, van der Valk MJM, Hilling DE, Holman FA, Peeters KCMJ, Mieog JSD, van de Velde CJH, Farina-Sarasqueta A, van Lijnschoten I, Framery B, Pèlegrin A, Gutowski M, Nienhuijs SW, de Hingh IHJT, Nieuwenhuijzen GAP, Rutten HJT, Cailler F, Burggraaf J, Vahrmeijer AL (2018) Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: a dose-escalation pilot study. Lancet Gastroenterol Hepatol. 3(3):181–191
Predina JD, Newton AD, Connolly C, Dunbar A, Baldassari M, Deshpande C et al (2018) Identification of a folate receptor-targeted near-infrared molecular contrast agent to localize pulmonary adenocarcinomas. Mol Ther 26(2):390–403
Frankel A (2012) Formalin fixation in the “-omics” era: a primer for the surgeon-scientist. ANZ J Surg 82(6):395–402
Hewitt SM, Lewis FA, Cao Y, Conrad RC, Cronin M, Danenberg KD, Goralski TJ, Langmore JP, Raja RG, Williams PM, Palma JF, Warrington JA (2008) Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med 132(12):1929–1935
Werner M, Chott A, Fabiano A, Battifora H (2000) Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol 24(7):1016–1019
Chen C-H, Hsu M-Y, Jiang R-S, Wu S-H, Chen F-J, Liu S-A (2012) Shrinkage of head and neck cancer specimens after formalin fixation. J Chin Med Assoc 75(3):109–113
Tran T, Sundaram CP, Bahler CD, Eble JN, Grignon DJ, Monn MF, Simper NB, Cheng L (2015) Correcting the shrinkage effects of formalin fixation and tissue processing for renal tumors: toward standardization of pathological reporting of tumor size. J Cancer 6(8):759–766
Otali D, Stockard CR, Oelschlager DK, Wan W, Manne U, Watts SA, Grizzle WE (2009) Combined effects of formalin fixation and tissue processing on immunorecognition. Biotech Histochem 84(5):223–247
Funding
This project was supported partly by the Stanford Comprehensive Cancer Center, the Netherlands Organization for Scientific Research (Rubicon; 019.171LW.022), the National Institutes of Health and the National Cancer Institute (R01CA190306), the Stanford Molecular Imaging Scholars (SMIS) program (T32CA118681), and a scientific research grant of YOKOYAMA Foundation for Clinical Pharmacology (YRY-1702). Institutional equipment loans were received from LI-COR Biosciences, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
ELR acts as a consultant for LICOR Biosciences, which manufactures IRDye800, and has equipment loans from this company. All other authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplemental Figure 1
Correlations of tumor MFI and TBR in fresh and formalin-fixed tissue. Tumor MFIs and TBR in the formalin-fixed specimens were found to strongly correlate with those in the fresh specimens. (R2 = 0.93 and 0.97, respectively). (PNG 90 kb)
Supplemental Figure 2
Decrease of fluorescence signals in the same piece of tissue over the 10 times. (PNG 202 kb)
Rights and permissions
About this article
Cite this article
Kapoor, S., Lu, G., van den Berg, N.S. et al. Effect of Formalin Fixation for Near-Infrared Fluorescence Imaging with an Antibody-Dye Conjugate in Head and Neck Cancer Patients. Mol Imaging Biol 23, 270–276 (2021). https://doi.org/10.1007/s11307-020-01553-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01553-1