Abstract
Background
Frameless neuronavigation allows neurosurgeons to visualize and relate the position of surgical instruments to intracranial pathologies based on preoperative tomographic imaging. However, neuronavigation can often be inaccurate. Multiple factors have been proposed as potential causes, and new technologies are needed to overcome these challenges.
Objective
To evaluate the accuracy of neuronavigation systems compared to near-infrared (NIR) fluorescence imaging using Second Window Indocyanine Green, a novel technique, and to determine factors that lead to neuronavigation errors.
Methods
A retrospective analysis was conducted on 56 patients who underwent primary resections of intracranial tumors. Patients received 5 mg/kg ICG approximately 24 h preoperatively. Intraoperatively, neuronavigation was used to plan craniotomies to place the tumors in the center. After craniotomy, NIR imaging visualized tumor-specific NIR signals. The accuracy of neuronavigation and NIR fluorescence imaging for delineating the tumor boundary prior to durotomy was compared.
Results
The neuronavigation centers and NIR centers were 23.0 ± 7.7 % and 2.6 ± 1.1 % deviated from the tumor centers, respectively, relative to the craniotomy sizes. In 12 cases, significant changes were made to the planned durotomy based on NIR imaging. Patient position was a significant predictor of neuronavigation inaccuracy on both univariate and multivariate analysis, with the prone position having significantly higher inaccuracy (29.2 ± 8.1 %) compared to the supine (16.2 ± 8.1 %, p value < 0.001) or the lateral (17.9 ± 5.1 %, p value = 0.003) positions.
Conclusion
Patient position significantly affects neuronavigation accuracy. Intraoperative NIR fluorescence imaging before durotomy offers an opportunity to readjust the neuronavigation image space to better align with the patient space.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Completeness of surgical resection is a vital factor in the prognosis of intracranial tumors [1,2,3]. In order to achieve maximal safe resection, accurate localization of pathology in the intracranial space intraoperatively is crucial. While magnetic resonance imaging (MRI), computed tomography (CT), and angiography have guided surgical planning for decades, a reliable imaging system to help surgeons intraoperatively in real time has yet to be established. Among the recent adjuncts to neurosurgery, neuronavigation has become increasingly prevalent [4,5,6].
Neuronavigation, or frameless stereotactic navigation, was introduced in 1986 by Roberts and colleagues [7]. In contrast to its immediate, frame-based predecessor, neuronavigation is a “frameless” technique; conferring it increased adaptability and utility [8]. Neuronavigation relies on the spatial registration of anatomical landmarks in the physical “patient space” to the same structures in preoperatively acquired, virtual “image space” [9]. Despite its widespread adoption in neurosurgery, the benefits of neuronavigation remain controversial [10, 11]. One of the most discussed limitations of this technique is the brain shift phenomenon, in which the movement of the soft, pliable brain during surgery invalidates the initial patient-to-image registration. The causes of brain shift are multifold, including physical (i.e., gravity), surgical (i.e., fluid and tissue loss), and biological (i.e., tumor type) factors [4, 12,13,14]. While intraoperative MRI has been trialed to address these issues [15], its use is associated with high cost, low availability, and increased operative length [16, 17].
More recently, fluorescence-guided surgery (FGS) has emerged as a cost-effective alternative to visualize intracranial tumors in real time [18, 19]. In FGS, fluorophores are designed to selectively accumulate in neoplastic tissue, allowing surgeons to better visualize abnormal tissue intraoperatively to increase resection rates. For instance, in a randomized controlled trial, the use of delta-aminolevulinic acid (5-ALA), an FDA-approved visible spectrum fluorescent agent, led to an increased rate of gross total resection and progression-free survival in glioblastoma patients [20]. Our group has been studying indocyanine green (ICG), which fluoresces in the near-infrared (NIR) region (peak emission 805-820 nm), conferring it improved tissue penetration and reduced autofluorescence from the surrounding brain parenchyma compared to visible spectrum agents like 5-ALA [21, 22]. We have demonstrated that intravenous infusions of ICG at 2.5–5 mg/kg into patients 24 h preoperatively allows real-time visualization of gliomas, meningiomas, and brain metastases with high sensitivity (> 85 %) [23,24,25,26,27]. This technique has previously been termed Second Window ICG (SWIG) or TumorGlow™. Since NIR fluorescence penetrates through normal brain and dura (at least 1.5 cm, in prior studies), we have been able to detect SWIG signal from tumors prior to dura opening [23,24,25,26,27]. The ability to accurately visualize the location and boundary of tumors prior to opening the dura allows surgeons to better plan safe and efficient approaches that can improve surgical results and patient outcomes.
In this retrospective study, we compare the accuracy of the surgeons’ interpretation of neuronavigation systems to NIR fluorescence through the unopened dura. We then investigate factors that lead to neuronavigation errors. Based on prior experience, we hypothesized that patient positioning would significantly affect the accuracy of neuronavigation, with the prone position leading to greater inaccuracies. Furthermore, we hypothesized that the accuracy of NIR fluorescence imaging would not be affected by patient positioning or other intraoperative factors.
Methods
Study Population
All adult patients (> 18 years) undergoing primary resection of central nervous system tumors were enrolled between October 2014 and January 2019 in a registered clinical trial that was approved by the institutional review board. Exclusion criteria were pregnancy and allergy to contrast dye, iodide, or shellfish. All patients gave informed consent for the research trial. For this retrospective analysis, additional criteria were designed to exclude patients in whom the craniotomy could not be centered over the tumor or those in whom NIR imaging would not be reliable: non-enhancing tumors, prior craniotomies, parasagittal tumors, tumors > 10 mm below the cortex, tumor diameter > 5 cm, irregular tumor shape, skull base tumors, surgery performed endoscopically, and any other surgeries in which the craniotomy was intentionally placed off-center for anatomic reasons. Thus, in the included patients, all craniotomies should have been centered over the tumor.
Near-Infrared Imaging
All patients were infused intravenously with either 2.5 mg/kg (after April 2018) or 5 mg/kg (before April 2018) ICG (C43H47N2O6S2.Na; Akorn Pharmaceuticals, IL, USA) approximately 24 h preoperatively at an outpatient infusion center. All cases were imaged using the FDA-approved, NIR-capable VisionSense Iridium™ exoscope system (VisionSense, Philadelphia, PA) [25].
Stereotactic Neuronavigation Registration
All patients underwent preoperative 1 mm-slice MRI of the brain with intravenous gadolinium. In the operating room, “surface matching” was used to register the preoperative MRI to the stereotactic coordinate space by tracing several points on the face with a focus on surfaces that are difficult to deform (e.g., nasal bridge, orbital ridge). Registration accuracy was confirmed by assessing whether the navigation system correctly localized external landmarks (e.g., external auditory canal, cranial sutures). For each case, one of the following commercially available neuronavigation devices was used: Stryker, Medtronic StealthStation, BrainLab, and BrainLab Curve. The registration was performed by residents prior to the surgery and confirmed by the senior surgeons prior to incision.
Study Procedure
After skin incision and skull exposure, neuronavigation was again used to outline the tumor. Craniotomy boundaries were planned to center the tumor within the craniotomy. Upon craniectomy, NIR signal was documented over the intact dura. In cases when neuronavigation and NIR imaging differed in tumor localization, NIR imaging was used to plan the dura opening.
Determination of Tumor Center, NIR Center, and Neuronavigation Center
Intraoperative videos were recorded and analyzed postoperatively by independent reviewers. By carefully visualizing the tumor boundary upon exposure and then resection of the mass, the tumor boundaries were determined (since these tumors were completely resected, the tumor boundaries were determined post-resection). These images were then transposed on the view over the intact dura to determine the tumor boundary on the dura. Then, the resulting white light image and the NIR images over the dura were exported into ImageJ (National Institute of Health, Bethesda, MD), and the centroid function (Analyze= > Set Measurements) was used to find the center of mass of tumor outlines using white light (tumor center) and NIR (NIR center) (Figs. 1 and 2). For the NIR imaging, areas of signal-to-background > 2 were used as the tumor boundary, based on our prior studies. Similarly, the center of mass and areas of the craniotomies were calculated (Neuronavigation Center). Then, the distance between the tumor centers and each of the NIR center and neuronavigation center was calculated and divided by the square root of the area of the craniotomy in order to arrive at the percent deviation factor, allowing each deviation to be assessed relative to the size of the craniotomy and controlling for differences in imaging distance. This was necessary, as actual size measurements were not performed at the time of surgery.
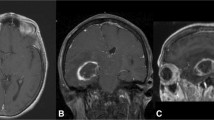
Example of a well-centered craniotomy using neuronavigation in a supine case. (A–C) preoperatively, T1 MRI was performed with and without gadolinium contrast agent at 1 mm resolution. The axial (A), sagittal (B), and coronal (C) images demonstrate a superficial, well-circumscribed, contrast-enhancing mass measuring 22 mm × 23 mm × 15 mm extending to the cortex, consistent with a metastasis. (D–G) Intraoperatively, the craniotomy borders were planned on the exposed skull using neuronavigation to center the tumor. After craniotomy (black, solid line), white- light imaging (D) does not visualize the tumor. However, based on white light imaging during and after resection, the tumor boundary was transposed onto the dura (white, solid line) and the tumor center is shown (black triangle). With near-infrared fluorescence imaging (E), the fluorescence from the tumor is clearly visible (black, dotted line) and the NIR center (black, dotted circle) closely approximates the tumor center (1.2 %). The neuronavigation center (black square) also aligns well with the tumor center, with 4.3 % deviation. The tumor is visible with white light after durotomy (F), and the location of near-infrared fluorescence is confirmed to be coming from the tumor (G).
Example of a poorly centered craniotomy using neuronavigation in a supine case. (A-C) Preoperatively, T1 MRI was performed with and without gadolinium contrast agent at 1 mm resolution. The axial (a), sagittal (B), and coronal (C) images demonstrate a superficial, spherical mass measuring 19 mm × 15 mm × 20 mm in the right frontal lobe, consistent with a metastasis. (D–G) Intraoperatively, the craniotomy borders were planned on the exposed skull using neuronavigation to center the tumor. After craniotomy (black, solid line), white light imaging (D) does not visualize the tumor. However, based on white light imaging during and after resection, the tumor boundary was transposed onto the dura (white, solid line) and the tumor center is shown (black triangle). With near-infrared fluorescence imaging (E), the fluorescence from the tumor is clearly visible (black, dotted line) and the NIR center (black, dotted circle) closely approximates the tumor center (1.9 %). The neuronavigation center (black square), in contrast, aligns poorly with the tumor center, with 27.1 % deviation. The tumor is visible with white light after durotomy (F), and the location of near-infrared fluorescence is confirmed to be coming from the tumor (G). Prior to durotomy, the surgeon felt more confident in the fluorescence imaging results than in the neuronavigation, so durotomy was performed over the site of fluorescence rather than over the neuronavigation center.
Clinical Data
For each patient, the following data were collected retrospectively from the electronic medical records: age, gender, ethnicity, post-graduate training year of the residents (both as number of years and < 4 (junior) or ≥ 4 (senior)), pathological diagnosis, location and side of the tumor, tumor depth (distance from cortex to the most superficial aspect of the tumor), neuronavigation device, and patient head position (supine, lateral, or prone).
Statistical Analysis
Statistical analyses were performed using STATA 10™ (StataCorp LLC, College Station, TX). The Mann–Whitney test was used when comparing values. Each clinical variable was examined to assess their predictive capabilities for percent deviation using linear regression (age, tumor depth), analysis of variance (gender, ethnicity, pathology, tumor location, neuronavigation device, patient position), or Mann–Whitney test (gender, resident year). For the initial screening univariate analysis, a p value cutoff of 0.2 was used to determine variables for the multivariate analysis. An analysis of covariance was performed on the screened variables to assess for potential factors that could affect neuronavigation accuracy. A p value cutoff of < 0.05 was used to determine statistical significance.
Results
Patient Recruitment
Between October 2014 and January 2019, 215 patients underwent craniotomy under the SWIG protocol for intracranial tumors. For this retrospective study, the following cases were excluded according to our exclusion criteria: 37 repeat craniotomies; 25 tumor-depth > 10 mm; 15 with low NIR signal; 57 parasagittal tumors (craniotomy could not be centered over the tumor with neuronavigation); and 25 poor visualization of craniotomy borders with NIR imaging (camera field of view too narrow). Fifty-six patients were included in the final analysis (22 metastases, 21 high-grade gliomas, 11 meningiomas, and 2 Other). Thirty-one patients were supine for the surgery, 12 were lateral decubitus, and 13 were prone. The clinical characteristics are summarized in Table 1.
Transdural Near-Infrared Fluorescence Imaging of Gross Tumor
In all 56 patients, the NIR fluorescence properly delineated the tumor boundary prior to opening the dura, with minimal deviations between the tumor center and the NIR center (mean 2.6 ± 1.1 % relative to craniotomy size; range 1.2–4.4 %) regardless of patient positioning or tumor type (Figs. 1 and 2).
Transdural Neuronavigation of Gross Tumor
The Stryker neuronavigation system was used in 21 cases, followed by BrainLab (n = 17), BrainLab Curve (n = 14), and Medtronic Stealth (n = 4). Senior neurosurgery residents (postgraduate year 4 or greater) performed 44/56 registrations, while junior residents performed 12/56. In order to assess neuronavigation reliability in this retrospective study, the accuracy of craniotomy placement was used as a surrogate. Compared to the tumor center, the Neuronavigation Center was, on average, 23.0 ± 7.7 % (range 4.3–47.6 %) deviated relative to the size of the craniotomy. Deviations < 15 % (Fig. 1) were not largely noticeable intraoperatively; conversely, deviations > 15 % (Fig. 2) were easily recognized as such. In 12 cases of severe neuronavigation inaccuracies, dura opening was significantly altered based on NIR fluorescence.
Predictive Factors for Neuronavigation Deviation
The following potential factors that could contribute to neuronavigation inaccuracies were collected from patient charts: age, gender, ethnicity, tumor type, tumor location, tumor depth, patient position intraoperatively, neuronavigation device, and seniority of the resident performing the registration. From univariate analysis, tumor depth (p value = 0.17) and patient position (p value = 0.0004) were selected for multivariate analysis (Table 2). These two variables were analyzed using analysis of covariance, which revealed only patient position to be significant (p value = 0.0083). The prone position was associated with significantly higher inaccuracies (29.2 ± 8.1 %) compared to the supine (16.2 ± 8.1 %, p value < 0.001) or the lateral (17.9 ± 5.1 %, p value = 0.003) positions (Fig. 3). Furthermore, using 15% deviation as the cutoff for clinically significant deviation, 12/13 (92.3 %) of prone cases were significantly deviated, versus 16/31 (52 %) for supine and 4/8 (50 %) for lateral cases (chi-2 p value = 0.036).
Distribution of neuronavigation deviation by patient position. Multivariate analysis revealed patient position to be the only significant factor affecting neuronavigation accuracy in our patient cohort. The supine and lateral positions demonstrated similar levels of deviations (16.2 ± 8.1 %, 17.9 ± 5.1 %; p value = 0.99). The prone position, in contrast, demonstrated a significantly higher level of deviation (29.2 ± 8.1 %) compared to the supine (p value < 0.001) and lateral (p value = 0.003) positions.
Discussion
Accurate localization within the intracranial space is vital to the safe and effective surgical treatment of patients with intracranial lesions. Thus, neurosurgeons have strived to enhance the precision of anatomical and functional localization within the cranium through various imaging modalities, such as CT and MRI. Stereotactic coordination using headframes further increased the accuracy for surgical targets [28]. More recently, frameless navigation systems have become widely adopted and have demonstrated potential for improving patient outcomes in tumor resections. However, it is well recognized that neuronavigation accuracy is affected by numerous factors. Wang and Song, in their review, classify potential causes of neuronavigation errors into two groups, one caused by actual differences between the images and patients and the other caused by human errors in translating surgical tool position into the image space [13]. While some of these causes are easily addressable, such as properly positioning the tracking device or mitigating errors in fiducial registration, others, such as brain deformations during surgery, can be very difficult to correct. Due to these difficulties, the benefits of neuronavigation in increasing the extent of resection and prolonging patient survival have not been clearly defined [9,10,11].
In 2015, we began enrolling our first patients in our clinical trial to investigate the utility of intraoperative NIR imaging with SWIG in real-time visualization of neoplastic tissue in intracranial tumors. Since NIR fluorescence penetrates through > 1 cm of brain parenchyma and dura, we observed NIR fluorescence signals from tumors immediately after craniotomy, prior to durotomy. During these cases, it was observed that neuronavigation was often inconsistent with the location of the NIR fluorescence and that the margin of error seemed to be exacerbated when the patients were prone. In order to better examine this trend, we designed this retrospective study to compare the accuracy of neuronavigation to NIR fluorescence and to determine the factors that affected neuronavigation accuracy. Due to the limited amount of neuronavigation data available retrospectively, we used the accuracy of neuronavigation-guided craniotomy placement as a surrogate for neuronavigation accuracy. Hence, we were able to compare the accuracy of neuronavigation and NIR fluorescence imaging in accurately delineating the tumor center. In an analysis of 9 potential variables, the patient position significantly affected neuronavigation accuracy, while NIR fluorescence imaging was not affected by any of the variables. This was consistent with a prior study by Asano et al., which demonstrated that the prone position led to larger inaccuracies using an optical neuronavigation system [29].
The inaccuracies associated with the prone position likely stems from multiple factors. Most current neuronavigation systems (including those used at our institution) rely on surface registration to align the image space to the patient space. When this registration is performed on the anterior surface of the face for prone cases, poor access to facial surface features can limit accurate registration. In addition, small errors from anterior registrations may propagate into larger errors over the increased distance between the registration surface and the posterior operative surface. Furthermore, preoperative images are performed with the patient supine, but when patients are placed prone, the skin and overall head shape changes due to gravity, which exacerbates the discrepancy between the image space and patient space. Finally, because the brain is relatively mobile compared to the skull, the prone position likely affects the degree and direction of brain shift differently than in the supine position. Although some neurosurgeons use point-to-point fiducial registration, skull screws, or intraoperative CT to mitigate some of these factors and improve image-to-patient registration, there remains a need for more accurate intraoperative, real-time navigation, especially in the prone position.
Even if perfect registration is achieved, neuronavigation loses accuracy intraoperatively. Opening the dura and draining cerebrospinal fluid, removal of tissue, and retracting the brain are all examples of unavoidable steps in surgery that change the intraoperative patient space that would not be reflected in the pre-acquired image space, no matter how well-registered. A potential approach to rectifying this issue is to readjust the image space to reflect changes in the patient space, which could be accomplished, for instance, by acquiring a new MRI scan intraoperatively. This could be done after the patient has been positioned to calibrate the neuronavigation system and could even be performed at the end of the case to assess for margins that could then be localized using neuronavigation [15, 30,31,32]. However, intraoperative MRI is expensive, time-consuming, cumbersome, and not widely available; furthermore, this approach still cannot account for intraoperative brain deformations that occur between scans. Repeatedly performing intraoperative MRI would prohibitively interfere with the surgical workflow and increase both surgical cost and time.
Alternatively, we propose FGS with NIR fluorescence as an adjunctive technique that may work synergistically with neuronavigation to help surgeons approach tumors more safely and to evaluate margins more accurately. In FGS, fluorophores are designed to accumulate in neoplastic tissue, such as through passive permeation (Second Window ICG), enzymatic conversion (5-ALA), or receptor targeting (ABY-029, OTL38) [20, 24, 33,34,35,36,37]. Since the fluorescence is directly detected from neoplastic areas, fluorescence imaging can account for changes in real time, unlike neuronavigation. Furthermore, unlike visible spectrum fluorescence, NIR fluorescence can be detected through > 1 cm of normal tissue, allowing surgeons to detect neoplastic tissue that may be normally obscured from view. Since the dura is < 0.5 mm thick, NIR fluorescence imaging offers superb visualization of tissue that have accumulated fluorophores, before any opening is made in the dura. Based on our observations in this study, we believe that intraoperative NIR fluorescence imaging offers an opportunity to readjust the neuronavigation image space to better align with the patient space, improving neuronavigation accuracy. For instance, prior to durotomy, when the tumor is clearly visualized with NIR fluorescence, neuronavigation could be realigned using NIR fluorescence as the marker of tumor location. Unlike current techniques for updating registration data using known landmarks (bone or vasculature) that are indirectly related to the tumor location, using NIR fluorescence would allow direct registration to the tumor. With such readjustments at multiple intervals throughout the surgery, neuronavigation accuracy could improve even when the patient is prone or when tumor resection distorts the anatomy, since NIR fluorescence accurately reflects any changes in real time.
Furthermore, the addition of neuronavigation to FGS with SWIG has one major advantage over FGS-alone. We have previously demonstrated that SWIG leads to NIR fluorescence in gadolinium-enhancing tissue; conversely, non-enhancing tumors or non-enhancing portions of heterogeneously enhancing tumors do not demonstrate significant NIR fluorescence with SWIG, which is a major limitation of SWIG. In contrast, neuronavigation can visualize non-enhancing tissues, as well as T2 abnormalities and other signal changes. Thus, by combining SWIG and neuronavigation in resections of heterogeneously enhancing tumors, neurosurgeons could synergistically apply both technologies to enhance the detection of neoplastic tissue.
Finally, novel techniques of visualizing fluorophores are currently under investigation that may offer superior visualization compared to simple optical imaging, which is the current standard in the operating room. Optical fluorescence imaging is limited by the 2-dimensional nature of the acquired images; although deep fluorescence can be detected as aforementioned, one cannot easily distinguish superficial from deep signals, since both would appear to be on the surface. In contrast, photoacoustic imaging and diffuse optical tomography both theoretically offer tomographic imaging in real time and have demonstrated the use of ICG as a contrast agent [38,39,40]. Such advances in fluorescence imaging can only improve the utility and applicability of FGS in the future.
A limitation of this study is that, as a retrospective study, we did not have direct data from the neuronavigation systems to compare to the fluorescence imaging. Instead, we used craniotomy placement as a surrogate, since our institutional policy is to plan the craniotomy using neuronavigation in such a way as to place the tumor in the center. However, we carefully designed our inclusion and exclusion criteria for the analysis to limit our study to tumors in which this craniotomy placement was feasible; thus, in this population, the craniotomy centers should not have deviated significantly from the tumor centers. Furthermore, at the time of surgery, we did not directly measure the size of the craniotomy, making it difficult to exactly quantify the extent of deviation; thus, we had to use the degree of deviation relative to the size of the craniotomy as a proxy. Even with this limited retrospective study, we were able to demonstrate that patient positioning significantly affects neuronavigation accuracy, and we offer an interesting, novel way to improve neuronavigation accuracy intraoperatively. Further studies to compare the two techniques during the resection and at the margins would be informative.
Conclusion
Accurate navigation within the intracranial space is crucial for neurosurgeons to better achieve safe maximal resection of intracranial tumors. Frameless stereotactic navigation, a ubiquitous technology now, relies on the registration of preoperatively acquired image space to the patient space in order to provide 3-dimensional guidance. However, it has been well documented that neuronavigation suffers from inaccuracies due to multiple factors that cannot easily be corrected. In this study, we used intraoperative near-infrared fluorescence imaging with Second Window ICG, a novel technique for fluorescence visualization of neoplastic tissue, to assess the accuracy of neuronavigation in 56 patients with intracranial tumors. Overall, we found that patient positioning in the operating room significantly affects neuronavigation accuracy, with the prone position being the significantly less accurate than the supine or lateral positions, while near-infrared imaging was not affected by positioning. Thus, we propose using near-infrared fluorescence imaging to provide real-time, intraoperative adjustments to neuronavigation to improve the accuracy of intracranial navigation.
References
Bucci MK, Maity A, Ph D et al (2004) Near complete surgical resection predicts a favorable outcome in pediatric patients with nonbrainstem , malignant gliomas results from a single center in the magnetic resonance imaging era. Cancer. https://doi.org/10.1002/cncr.20422
Anderson TMD (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. Cancer 95:190–198
Tumor B, Francisco S (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8. https://doi.org/10.3171/2011.2.JNS10998
Thomas NWD, Sinclair J (2019) Image-guided neurosurgery : history and current clinical applications. J Med Imaging Radiat Sci 46(3):331–342. https://doi.org/10.1016/j.jmir.2015.06.003
Ferrant M, Nabavi A, Macq B et al (2002) Serial registration of intraoperative MR images of the brain. Med Image Anal 6:337–359
Resection BC (2017) Clinical Application of Multimodal Neuronavigation System in Neuroendoscope-Assisted Skull. J Craniofac Surg 28(6):554–557. https://doi.org/10.1097/SCS.0000000000003859
Murray W, Enberger HK (1986) A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg 65:545–549
Enchev Y (2009) Neuronavigation: geneology, reality, and prospects. Neurosurg Focus 27(3):E11. https://doi.org/10.3171/2009.6.focus09109
Orringer DA, Golby A, Jolesz F (2012) Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices 9(5):491–500. https://doi.org/10.1586/erd.12.42
Rainer Wirtz WS, Albert FK, Schwaderer M et al (2016) The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol Res. https://doi.org/10.1080/01616412.2000.11740684
Willems PWA, Taphoorn MJB, Burger H, van der Sprenkel JWB, Tulleken CAF (2008) Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg 104(3):360–368. https://doi.org/10.3171/jns.2006.104.3.360
Gerard IJ, Kersten-Oertel M, Petrecca K, Sirhan D, Hall JA, Collins DL (2017) Brain shift in neuronavigation of brain tumors: a review. Med Image Anal. https://doi.org/10.1016/j.media.2016.08.007
Wang MN, Song ZJ (2011) Classification and analysis of the errors in neuronavigation. Neurosurgery. https://doi.org/10.1227/NEU.0b013e318209cc45
Hartkens T, Hill DLG, Castellano-Smith AD et al (2003) Measurement and analysis of brain deformation during neurosurgery. IEEE Trans Med Imaging. https://doi.org/10.1109/TMI.2002.806596
Nimsky C, Ganslandt O, Kober H, Ph D, Buchfelder M, Fahlbusch R (2001) Intraoperative magnetic resonance imaging combined with neuronavigation : a new concept. Neurosurgery 48(5):1082–1089
Shah MN, Leonard JR, Inder G et al (2012) Intraoperative magnetic resonance imaging to reduce the rate of early reoperation for lesion resection in pediatric neurosurgery. J Neurosurg Pediatr. https://doi.org/10.3171/2011.12.peds11227
Senft C, Franz K, Ulrich CT et al (2010) Low field intraoperative MRI-guided surgery of gliomas: a single center experience. Clin Neurol Neurosurg. https://doi.org/10.1016/j.clineuro.2009.12.003
Bettag C (2019) Endoscopic fluorescence-guided resection increases radicality in glioblastoma surgery. Oper Neurosurg (Hagerstown):1–6. https://doi.org/10.1093/ons/opz082
Zhao S, Wu J, Wang C et al (2013) Intraoperative fluorescence-guided resection of high- grade malignant gliomas using 5-aminolevulinic acid – induced porphyrins : a systematic review and meta- analysis of prospective studies. PLoS One 8(5). https://doi.org/10.1371/journal.-pone.0063682
Stummer W, Pichlmeier U, Meinel T et al (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(06)70665-9
Cherrick GR, Leevy CM, Charles S, Davidson CS (1960) Indocyanine green : observations on its physical properties , plasma decay, and hepatic extraction Find the latest version. J Clin Invest 39(4):592–600
Madajewski B, Judy BF, Mouchli A, Kapoor V, Holt D, Wang MD (2012) Intraoperative near-infrared imaging of surgical wounds after tumor resections can detect residual disease. Clin Cancer Res:5741–5752. https://doi.org/10.1158/1078-0432.CCR-12-1188
Lee JYK, Pierce JT, Zeh R, Cho SS, Salinas R, Nie S, Singhal S (2017) Intraoperative near-infrared optical contrast can localize brain metastases. World Neurosurg 106:120–130. https://doi.org/10.1016/j.wneu.2017.06.128
Cho SS, Salinas R, Lee JYK (2019) Indocyanine-green for fluorescence-guided surgery of brain tumors : evidence, techniques, and practical experience. Front Surg 6:1–13. https://doi.org/10.3389/fsurg.2019.00011
Cho SS, Zeh R, Pierce JT, Salinas R, Singhal S, Lee JYK (2018) Comparison of near-infrared imaging camera systems for intracranial tumor detection. Mol Imaging Biol 20:213–220. https://doi.org/10.1007/s11307-017-1107-5
Lee JYK, Thawani JP, Pierce J, Zeh R, Martinez-Lage M, Chanin M, Venegas O, Nims S, Learned K, Keating J, Singhal S (2016) Intraoperative near-infrared optical imaging can localize gadolinium-enhancing Gliomas during surgery. Neurosurgery. 79(6):856–871. https://doi.org/10.1227/NEU.0000000000001450
Lee JYK, Pierce JT, Thawani JP et al (2018) Near-infrared fluorescent image-guided surgery for intracranial meningioma. J Neurosurg 128:380–390. https://doi.org/10.3171/2016.10.JNS161636.380
Leksell L, Lindquist C, Adler JR, Leksell D, Jernberg B, Steiner L (1987) A new fixation device for the Leksell stereotaxic system. J Neurosurg 66(4):626–629. https://doi.org/10.3171/jns.1987.66.4.0626
Asano K, Katayama K, Kakuta K, Oyama K, Ohkuma H (2017) Assessment of the accuracy and errors of head-up display by an optical neuronavigation system in brain tumor surgery. Oper Neurosurg 13(1):23–35. https://doi.org/10.1093/ons/opw001
Li P, Qian R, Niu C, Fu X (2017) Impact of intraoperative MRI-guided resection on resection and survival in patient with gliomas: a meta-analysis. Curr Med Res Opin. https://doi.org/10.1080/03007995.2016.1275935
Kubben PL, ter Meulen KJ, Schijns OEMG, ter Laak-Poort M, van Overbeeke J, van Santbrink H (2011) Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol 12(11):1062–1070. https://doi.org/10.1016/S1470-2045(11)70130-9
Kubben PL, Scholtes F, Schijns OEMG, ter Laak-Poort M, Teernstra OP, Kessels AG, van Overbeeke J, Martin DH, van Santbrink H (2014) Intraoperative magnetic resonance imaging versus standard neuronavigation for the neurosurgical treatment of glioblastoma: a randomized controlled trial. Surg Neurol Int 5(1):70. https://doi.org/10.4103/2152-7806.132572
Lee JYK, Cho SS, Zeh R et al (2018) Folate receptor overexpression can be visualized in real time during pituitary adenoma endoscopic transsphenoidal surgery with near-infrared imaging. J Neurosurg 129(2):390–403. https://doi.org/10.3171/2017.2.JNS163191
Cho SS, Zeh R, Pierce JT et al (2019) Folate receptor near-infrared optical imaging provides sensitive and specific intraoperative visualization of nonfunctional pituitary adenomas. Oper Neurosurg. 16(1):59–70. https://doi.org/10.1093/ons/opy034
Cho SS, Jeon J, Buch L, et al. (2018) Intraoperative near-infrared imaging with receptor-specific versus passive delivery of fluorescent agents in pituitary adenomas :1–11. doi:https://doi.org/10.3171/2018.7.JNS181642
Elliott JT, Marra K, Evans LT, Davis SC, Samkoe KS, Feldwisch J, Paulsen KD, Roberts DW, Pogue BW (2017) Simultaneous In Vivo fluorescent markers for perfusion, Protoporphyrin metabolism, and EGFR expression for optically guided identification of orthotopic glioma. Clin Cancer Res 23(9):2203–2212. https://doi.org/10.1158/1078-0432.CCR-16-1400
de Souza ALR, Marra K, Gunn J, Samkoe KS, Hoopes PJ, Feldwisch J, Paulsen KD, Pogue BW (2017) Fluorescent Affibody molecule administered in vivo at a microdose level labels EGFR expressing Glioma Tumor regions. Mol Imaging Biol 19(1):41–48. https://doi.org/10.1007/s11307-016-0980-7
Zanganeh S, Hamby CV, Backer MV, Backer JM Enhanced fluorescence diffuse optical tomography with indocyanine green- encapsulating liposomes targeted to receptors for vascular endothelial growth factor in tumor vasculature with indocyanine green-encapsulating liposomes. J Neurosurg. https://doi.org/10.1117/1.JBO.18.12.126014
Xu C, Kumavor PD, Zhu Q Indocyanine green enhanced co- registered diffuse optical tomography and photoacoustic tomography tomography and photoacoustic tomography. J Biomed Opt. https://doi.org/10.1117/1.JBO.18.12.126006
Sano K, Ohashi M, Kanazaki K et al (2017) Indocyanine Green-Labeled Polysarcosine for in vivo photoacoustic tumor imaging. Bioconjug Chem. https://doi.org/10.1021/acs.bioconjchem.6b00715
Funding
Supported in part by the Institute for Translational Medicine and Therapeutics of the Perelman School of Medicine at the University of Pennsylvania (JYKL). In addition, research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000003 (JKYL). In addition, research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001880 (SSC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclosures
None, except as stated above in Funding.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cho, S.S., Teng, C.W., Ramayya, A. et al. Surface-Registration Frameless Stereotactic Navigation Is Less Accurate During Prone Surgeries: Intraoperative Near-Infrared Visualization Using Second Window Indocyanine Green Offers an Adjunct. Mol Imaging Biol 22, 1572–1580 (2020). https://doi.org/10.1007/s11307-020-01495-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01495-8