Abstract
Purpose
Determining the glomerular filtration rate (GFR) is essential for clinical medicine but also for pre-clinical animal studies. Functional imaging using positron emission tomography (PET) allows repetitive almost non-invasive measurements. The aim of the study was the development and evaluation of easily synthesizable PET tracers for GFR measurements in small animals.
Procedures
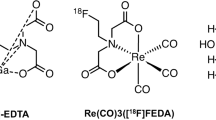
Diethylenetriaminepentaacetic acid (DTPA) and ethylenediaminetetraacetic acid (EDTA) were labeled with Ga-68. The binding to blood cells and plasma proteins was tested in vitro. The distribution of the tracers in rats was analyzed by PET imaging and ex vivo measurements. From the time-activity-curve of the blood compartment (heart) and the total tracer mass excreted by the kidney, the GFR was calculated. These values were compared directly with the inulin clearance in the same animals.
Results
Both tracers did not bind to blood cells. [68Ga]DPTA but not [68Ga]EDTA showed strong binding to plasma proteins. For this reason, [68Ga]DPTA stayed much longer in the blood and only 30 % of the injected dose was eliminated by the kidney within 60 min whereas the excretion of [68Ga]EDTA was 89 ± 1 %. The calculated GFR using [68Ga]EDTA was comparable to the measured inulin clearance in the same animal. Using [68Ga]-DPTA, the measurements led to values which were 80 % below the normal GFR. The results also revealed that definition of the volume of interest for the blood compartment affects the calculation and may lead to a slight overestimation of the GFR.
Conclusions
[68Ga]EDTA is a suitable tracer for GFR calculation from PET imaging in small animals. It is easy to be labeled, and the results are in good accordance with the inulin clearance. [68Ga]DTPA led to a marked underestimation of GFR due to its strong binding to plasma proteins and is therefore not an appropriate tracer for GFR measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Measuring the excretoric kidney function accurately is essential in many pre-clinical studies using small animals such as mice or rats. These measurements should be preferably non-invasive and should allow repetitive studies in the same animal. The “gold standard” for human studies is the inulin clearance [1] during which inulin is constantly infused, and the blood concentration as well as the totally excreted inulin mass is measured. However, in small animals, this technique is difficult to apply because the total urine has to be collected over a longer period for which a bladder catheter is necessary. Alternatively, inulin techniques have been suggested during which fluorescence-labeled inulin is injected as a bolus and several blood samples were taken over time [2, 3]. The glomerular filtration rate (GFR) could be calculated from a biexponential fit of the measured plasma concentration [4]. As an alternative of using inulin, radio-labeled compounds such as [51Cr]EDTA are used in clinical GFR measurements [1, 5, 6]. These tracers can be injected as a bolus, and the GFR is calculated from the decay of the plasma activity. However, the disadvantage is that due to taking multiple blood samples, this technique cannot be applied for repetitive measurements in small animals [2].
For repetitive measurements, non-invasive imaging techniques would be helpful. For this reason, in the clinical setting, radio-labeled compounds preferentially for planar γ-camera imaging have been established. A widely used tracer bases on DTPA (diethylenetriaminepentaacetic acid) which complexes metal ions. This tracer can be easily labeled with Tc-99m and is routinely used in clinical nephrology [5,6,7] but also for larger animals like cats or dogs [8, 9]. The GFR is calculated from the elimination of the tracer from the blood compartment.
Compared to Tc-99m imaging, positron emission tomography (PET) shows a higher sensitivity and better quantifiability. For this reason, several attempts have been undertaken to use PET for GFR measurements. Various tracers have been suggested such as pure [18F]fluoride [10], [18F]fluorodeoxysorbitol [11], or complexed metal ions like Co-55 or Ga-68 [12,13,14,15]. For complexing the metal ions, different carriers such as EDTA, DOTANOC (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-1-Nal3-octreotide) or NOTA (1,4,7-triazacyclononane-triacetic acid) have been tested. However, none of these tracers has been evaluated in a small animal model in direct comparison to the “gold standard” of GFR measurements by using the inulin clearance.
Since DTPA has been successfully used for functional kidney imaging using Tc-99m, the aim of the present study was to analyze the suitability of Ga-68-labeled DTPA for pre-clinical GFR measurements in small animals. Since EDTA labeled with isotopes of metal ions (e.g., Cr-51) has also been used for analyzing kidney function, EDTA was also Ga-68 labeled and the results were compared to those of DTPA.
In ideal, tracer of the GFR should stay unbound in the plasma compartment and should be fully excreted by filtration in the glomerulus. Therefore, the binding of the tracers to blood cells and plasma proteins was tested. To validate the suitability of the tracer to reflect the GFR, the in vivo results were compared to inulin measurements in the same animals.
Material and Methods
Tracer Synthesis
Ga-68 was eluted from a Ge-68/Ga-68 generator (Eckert and Ziegler Eurotope, Berlin, Germany) as described previously [16]. Ga3+-68 of the generator eluate was trapped on a shortened cation exchanger cartridge (Agilent, 100 mg, 1 ml, 40 μm) and then eluted with 5.5 M NaCl/0.1 M HCl solution directly into a sodium acetate-buffered solution containing DTPA (5 mg; 12.7 μmol) or EDTA (5 mg; 17.1 μmol). The solution was heated to 95 °C for 10 min. After labeling, neutralization, sterile filtration, and quality control (pH, instant thin-layer chromatography (iTLC), HPLC), the radiopharmaceutical can be directly administered to animals, without determination of organic solvents.
RBC and Plasma Protein Binding
For RBC-binding measurements, heparinized human blood samples of healthy donors were incubated with the respective tracer (dose, 2.0 ± 0.3 MBq) for 10 or 30 min. Afterwards, the blood was centrifugated, washed with PBS, the activity in the cell pellet was measured in a borehole and normalized to the activity of the whole blood sample. The protein-binding measurement follows a protocol described previously [17]. In brief, plasma samples (centrifugated from fresh heparinized blood) or an equal volume of PBS were incubated with the respective tracer (dose, 2.0 ± 0.3 MBq) for 10 or 30 min. Afterwards, the samples were filtered through a 3-kDa Millipore filter. The protein bound activity was calculated from the measured activity of the filtrate and the filter membrane taking into account the unspecific binding to the membrane as determined from the PBS samples (≈ 5 % of the total activity).
Animal Experiments
Animal experiments were performed in male Copenhagen rats (body weight 190 to 305 g) housed in the animal care facility of the University of Halle. Animals were allowed access to food and water ad libitum before the investigation. All experiments had previously been approved by the regional animal ethics committee and were conducted in accordance with the German Law for Animal Protection. For the experiments, animals were anesthetized with pentobarbital (40 mg/kg, i.p., Narcoren, Merial, Hallbergmoos, Germany). A small cannula was inserted into the tail vein for application of the tracer.
For ex vivo biodistribution measurements, the tracer was injected i.v. as a bolus in the anesthetized rats. After 2 or 60 min, respectively, the animals were euthanized and different organs (blood, urine, heart, lung, liver, adrenal gland, kidney, prostate, testis, spleen, thymus, gut, brain) were taken. After weighing the samples, the activity was determined in a γ-counter.
PET Imaging
For PET imaging, the rats were anesthetized with pentobarbital, placed in supine position in the scanner. The body temperature was monitored through a rectal probe. PET imaging was performed on a nanoScan PC PET/x-ray computed tomography (CT) (Mediso GmbH, Münster, Germany). The radiotracers with an activity of 18–22 MBq were administered as a bolus injection via the tail vein catheter. Dynamic PET studies were acquired in 3D mode for 60 min followed by an anatomical CT scan. Afterwards, the PET listmode data were histogrammed into 54 frames and reconstructed using OSEM algorithm. Volumes of interests (VOIs) were defined for the heart, lung, liver, kidney, and bladder. For the analysis of the optimal VOI for the blood compartment, VOIs were set also over the blood-filled ventricle and over the abdominal aorta.
GFR Measurements with Inulin
In a second experimental series, the PET measurements were directly compared with invasive analyses of the blood concentration of the tracer and with simultaneous determination of the inulin concentration which serve as “gold standard” of GFR measurements in the same animal. For direct comparison of both techniques in the same animal, the radio-labeled tracer was injected simultaneously with fluorescent-labeled inulin (inulin-FITC, Sigma-Aldrich GmbH, Munich, Germany) dissolved in saline. The inulin dose was 8.6–10.0 mg per animal. At distinct time intervals (2, 4, 7, 10, 15, 20, 40, and 60 min after injection), small blood samples (200 μl) were taken and the tracer activity in 90 μl blood plasma was measured in a γ-counter and the inulin concentration of the same plasma sample with a fluorescence multi-well reader.
GFR Calculation
For the calculation of the tracer clearance (CLTracer) as a measure of the GFR accurate, methods have been described which base on the time-activity-curves of the blood and the bladder compartment [10, 18]. Based on this model, the following equation has been used for calculating the GFR from PET data:
with A urine (60 min) = total bladder activity at t = 60 min, A blood (t) = TAC of the blood compartment.
In experiments where the measurement of the total urine amount was not feasible (e.g., for inulin measurements), calculations from multiple plasma measurements applying a biexponential fit of the blood compartment curve were used resulting in a reliable measure of the tracer clearance [19, 20].
Statistical Analysis
Results are expressed as means ± SEM. Differences between groups were assessed by the two-tailed t test for paired or unpaired samples. The significance level was set at α = 5 % for all comparisons.
Results
Chemistry
The radiochemical yields of the labeling were > 99 %, and the purity of > 97 % was routinely achieved as determined by radio HPLC and instant thin-layer chromatography (iTLC). The product solution was stable for at least 3 h.
RBC and Plasma Protein Binding
Incubating whole blood with either of the tracers showed only minor binding to cellular components (Fig. 1a). Incubation for 10 min resulted in approximately 2 % binding to the cells which only slightly increased after 30 min. The binding to plasma proteins, however, differed significantly between [68Ga]EDTA and [68Ga]DTPA (Fig. 1b). EDTA binding to plasma proteins was in the same range as the unspecific binding to the filter membrane (< 5.0 %). However, DTPA showed more than 60 % binding after 10 min and almost 90 % after 30 min.
Subsequently, the tracers not bound to proteins which passed the filter were analyzed by thin-layer chromatography. These measurements showed that both tracers stayed completely intact after incubation with human plasma.
In Vivo Tracer Distribution
In accordance with the pronounced differences in plasma protein binding (Fig. 1), the in vivo distribution of [68Ga]EDTA and [68Ga]DTPA measured by PET imaging was also markedly different. For the blood compartment, a VOI was located over the whole heart. Both tracers showed a rapid decrease in heart activity immediately after injection followed by slight fluctuations during the first 3–4 min which were probably the result of the initial redistribution between the different body compartments. [68Ga]EDTA concentration in the blood compartment decreased continuously over the whole observation period resulting in an almost non-detectable level (0.14 ± 0.06 %ID/ml) after 60 min (Fig. 2a). However, [68Ga]DTPA reached a concentration of 1.7 %ID/ml after 5 min and stayed almost constant until the end of the observation period which is probably the result of the high-protein binding of this tracer. Since only free (unbound) tracer can be filtered in the glomerulus, the renal excretion was also different for both tracers. The unbound [68Ga]EDTA was eliminated to 89.4 ± 1.0 % whereas [68Ga]DTPA was eliminated only by 34.4 ± 1.5 %ID after 60 min (Fig. 2b).
The kidney concentration of the tracers was not significantly different (Fig. 3a) even though [68Ga]EDTA showed a tendency to higher values probably due to the higher filtration of this tracer as indicated by a significantly higher bladder (urine) concentration. After 20 min, the mean activity in the kidney increased temporarily in the EDTA group. However, this rise was just an artifact due to a movement of one animal during imaging. This artifact not only affected the kidney activity measurement but led also to a slight temporary decline in the bladder activity (Fig. 2b). The liver concentration of both tracers was also almost identical until 20 min after injection (Fig. 3b). After this, the [68Ga]EDTA decreased continuously indicating that the liver VOI reflects the concentration in the blood compartment for this tracer. The [68Ga]DTPA level in the liver remained on an elevated level (Fig. 3b) which could be either the result of the higher blood concentration (Fig. 2a) or could indicate phagocytosis of plasma proteins together with bound [68Ga]DTPA by the reticuloendothelial system of the liver. This accumulation due to protein degradation may also explain the slight (but not statistically significant) increase of the liver activity at 30 to 60 min after injection. After 60 min, the liver concentrations of the both tracers were significantly different. Other organs (e.g., lung) showed no marked differences between the two tracers in the PET imaging.
Since only 30 % of the injected [68Ga]DTPA was eliminated through the kidney (Fig. 2b), it was unclear in which organ the tracer remained. For this reason, ex vivo measurements of the biodistribution in tissue samples were performed which confirmed the data obtained by PET imaging (Table 1). Most of the [68Ga]DTPA was found in the blood compartment (1.3 ± 0.1 %ID/g tissue) and did not extravasate into other organs.
Calculated Glomerular Filtration Rate
Using Eq. (1) [10, 18], the GFR was calculated from the integral of the time-activity curve of the blood compartment (Fig. 2a) and the totally excreted tracer mass (bladder; Fig. 2b) after 60 min. Using these values, the GFR was 1.61 ± 0.16 ml/min/100 g body weight for [68Ga]EDTA and 0.19 ± 0.03 ml/min/100 g body weight for [68Ga]DTPA (Fig. 4, black bars). The GFR calculated from [68Ga]DTPA was much lower than that described for normal rats [21] which is the result of the high-protein binding of the tracer and the resulting reduced renal excretion (Fig. 2).
Tracer clearance of [68Ga]DTPA and [68Ga]EDTA calculated from the time course of the tracer activity in the bladder and the blood compartment. For the blood compartment, VOIs were located either over the whole heart or the blood-filled right ventricle of the heart or over the abdominal aorta. n = 3.
The value calculated from the [68Ga]EDTA tracer is slightly above the value of normal rats. Here, it has been taken into account that for the blood compartment, a VOI over the whole heart has been used. However, this VOI not only contains the blood compartment but also the myocardium. Therefore, the impact of other VOIs on the calculated GFR was analyzed. Besides a VOI covering the whole heart, VOIs were set only in the blood-filled volume of the right ventricle and over part of the abdominal aorta. The results of the GFR using these VOIs are shown in Fig. 4.
Using the VOI located only over the ventricle led to 10–20 % lower GFR values compared to whole heart VOI. This difference results from the cardiomyocytes in the whole heart VOI leading to a lower tracer level as compared to pure blood resulting in a higher GFR value. For this reason, the ventricle VOI seems to result in more reliable values. However, in animals smaller than rats (e.g., mice) this VOI becomes very small and difficult to define. Using the aorta, VOI resulted in higher GFR values because this structure is even in rats rather small so that the signal measured in the VOI is always overlaid by other structures resulting in a lower GFR value. The GFR differences using the different VOIs were not statistically significant for both tracers.
Comparison with the Inulin Clearance
To test the reliability of the new tracers for GFR measurements, the results of in vivo PET imaging and ex vivo blood samples were compared with inulin measurements as a “gold standard” of GFR analysis in the same animal. For this purpose, the tracer and fluorescence-labeled inulin were injected simultaneously and blood samples were taken during PET imaging.
Figure 5a, b shows the time course of the Ga-68 tracer concentration measured in the blood samples (dots) and in PET images (squares) as well as the inulin concentration in the blood samples (triangles). All values were normalized to the total injected dose of the tracer or the inulin, respectively. For both tracers, the imaging values correspond well with the tracer concentration in the blood samples. For [68Ga]DTPA, the tracer concentration was significantly higher presumably as a result of the much higher protein binding of this compound. For [68Ga]EDTA, the tracer concentration (in the blood sample and the PET images) followed in parallel the inulin level showing similar kinetics.
Blood concentration of Ga-68-labeled tracers measured directly in blood samples or by PET (heart VOI) over 60 min after injection. For comparison, the time course of the plasma concentration of fluorescence-labeled inulin in the same animals is shown. a [68Ga]DTPA (n = 4). b [68Ga]EDTA (n = 6). *p < 0.05, **p < 0.01 Ga-68 tracer in the blood sample vs. FITC-inulin.
Since it was not possible to collect the complete urine for the measurement of the excrete inulin mass necessary for GFR calculation with Eq. (1), the GFR was calculated from a biexponential fit of the blood concentration [19, 20]. Using this method, the inulin clearance (= GFR) was calculated to be 0.91 ± 0.09 ml/min/100 g body weight. A direct comparison of the inulin-based GFR with the PET-based GFR (Fig. 6a) showed that with [68Ga]DTPA, the PET-based GFR was significantly lower approximately by the factor of 10. With [68Ga]EDTA, the PET-based GFR was slightly but not statistically significantly higher compared to the inulin clearance (Fig. 6a; 0.90 ± 0.13 vs. 0.74 ± 0.13 ml/min/100 g body weight). The GFR calculated from the [68Ga]EDTA concentration in blood samples resulted in lower but statistically not significant values. When directly comparing the inulin- and [68Ga]tracer-PET-based GFR calculations in the same animals (Fig. 6b), a good correlation was found for [68Ga] EDTA (r 2 = 0.75) but not for [68Ga]DTPA (r 2 = 0.09). On average using [68Ga]EDTA-PET led to GFR measurements which were 29 ± 11 % higher than with inulin (Fig. 6b). This difference results from the fact that in the PET analysis the blood concentration was calculated from a VOI over the whole heart which includes not only the blood-filled ventricle but also myocardium. This leads to an underestimation of the activity in the blood compartment. Since this value is used as the denominator in Eq. (1), the calculated GFR is higher. This difference can be counterbalanced by a using correction factor.
a Glomerular filtration rate (GFR) calculated from the blood concentration of [68Ga]EDTA and [68Ga]DTPA measured directly in blood samples or by PET (heart VOI) over 60 min after injection. For comparison, the GFR calculated from inulin measurements in the same animals is shown. DTPA n = 4, EDTA n = 6. *p < 0.05. b Direct correlation between inulin- and Ga-68 tracer-PET-based GFR calculations in the same animals.
Discussion
The present study clearly indicates that stable metal chelates are filtrated through the glomerular barrier as long as the compound is not bound to larger structure like plasma proteins. In the in vitro studies [68Ga]EDTA showed almost no protein binding resulting in a renal elimination of almost 90 % of the injected dose within 60 min. In contrast, [68Ga]DTPA was excreted by only 30 % as a result of binding to molecules which could be retained by a filter with a cutoff at 3 kDa which is similar to the pore size of the glomerular barrier.
In the present study, the protein binding of the DTPA-based tracer was found to be very high (> 80 % after 30 min). High-protein binding of DTPA has also described previously. Rehling et al. [22] found the binding of [99mTc]DTPA to be more than 10 %. The difference to the findings of the present study could be explained since both studies used filters with different pore sizes. Rehling et al. used filters with a cutoff at 10 kDa whereas in the present study 3 kDa was used. Since only molecules up to 5 kDa are filtered freely in the glomerulus, the use of 10 kDa membrane filters may underestimate the relevance of DTPA protein binding. Another explanation of the differences in protein binding might be that using different metals (Tc-99m or Ga-68) may affect the protein binding. The latter mechanism could explain why [99mTc]DTPA is a suitable tracer for GFR measurements in clinical routine. In previous studies, it has been discussed that the protein binding of [99mTc]DTPA is (at least partially) reversible [23]. The authors postulate that reversible binding might be of less importance for long-term filtration measurements. However, the present study clearly shows that up to 30 min DTPA binds strongly to plasma proteins. Since Ga-68 has only a short half-life (68 min), the measurements have to be carried out within 1 h during which even the reversible binding of DTPA is relevant. Anyhow, all studies show that the high-protein binding of DTPA cannot be ignored because it may underestimate the filtration. DTPA is obviously not freely filterable, and therefore, the elimination through the kidney is limited. When calculating the GFR from the DTPA measurements using Eq. (1), the resulting GFR is much too low.
Rehling et al. [22] also described the protein binding of [51Cr]EDTA to be 13 %. This could not be confirmed in the present study. [68Ga]EDTA binding was close to the background signal corresponding to a value < 5 %. These results could also provide an indication that the kind of metal ion chelated affects the tracer-protein interaction.
As a result of the protein binding [68Ga]DTPA stayed constantly in the blood compartment as determined by PET imaging or activity measurements of blood samples (Fig. 5a). Since the protein-bound tracer cannot be filtered through the glomerular barrier (cutoff molecular weight ≈ 5.5 kDa), only 30 % of the tracer was excreted by the urine. Due to the limited glomerular filtration of the tracer, the calculation of the GFR led to a strong underestimation independently whether Eq. (1) was used (taking the eliminated mass in the urine into account) [10, 18] or calculated from the decay of the tracer level in the blood [19, 20] (Fig. 5a). For this reason, [68Ga]DTPA seems not to be a suitable tracer for filtration measurements in small animals as long as the GFR is calculated from the TAC of the blood compartment. In contrast, almost 90 % of the injected [68Ga]EDTA was found in the urine indicating that this tracer fulfills much better the requirement of a suitable GFR-tracer to be freely filterable.
Early studies already used the metal ion chelator EDTA labeled with positron emitters (Co-55) for measuring the GFR [12]. Recently, Hofman et al. [13, 24] suggested to use Ga-68 labeled EDTA to obtain the GFR in a clinical study and compared these results with the [51Cr]EDTA. For calculation of the GFR, they used the method of renography in which a VOI is located over the kidney. However, the temporal change of the whole kidney activity is the consequence of different mechanisms. On the one hand, the activity in tubuli results from the filtration of the tracer in the glomeruli. On the other hand, the activity depends on the tracer concentration in the blood compartment of the kidney. For this reason, the overall TAC of the kidney reflects the filtration rate in the glomeruli but also the kidney perfusion [25]. Therefore, it is not possible to calculate the GFR directly from a renogram. The authors described an empirical function (obtained from a correlation analysis with blood sample measurements) of the patients’ GFR and parts of the renogram [13, 24]. For the clinical setting, this procedure is possible because it limits the time of the data acquisition (in this case to 20 min). However, in animal experiments, the analysis using Eq. (1) is preferable.
The GFR values obtained in the present study by [68Ga]EDTA-PET measurements conform well with the inulin data revealing the suitability of the new tracer for functional kidney imaging. However, as shown in Fig. 6a, the GFR calculated from the inulin-based measurements was significantly higher than the values based of the tracer measurements in the blood samples. This has also been described in the literature. A direct comparison of inulin and [51Cr]EDTA showed 10 % lower GFR values for the EDTA [5, 26]. However, the underlying mechanism is still unclear. Maybe a negative partial charge of [51Cr]EDTA [5] could hinder filtration through the glomerular barrier.
In conclusion, the present study demonstrates that [68Ga]EDTA is a suitable tracer for GFR calculation from PET imaging in small animals. The tracer can be easily labeled (due to the availability of Ge-68/Ga-68 generators), and the results in rats are in good accordance with the “gold standard” measured by the inulin clearance. The results also illustrate that the VOI for the blood compartment should be set as precisely as possible and to avoid the incorporation of other tissues into this VOI. In the heart, the VOI should be restricted to the blood-filled ventricle. In smaller animals than rats (e.g., mice), this is probably not possible so that the VOI has to be defined over the whole heart resulting in a slight overestimation of the GFR. [68Ga]DTPA leads to a marked underestimation of GFR due to its strong binding to plasma proteins and is therefore not an appropriate tracer for GFR measurements. Further experiments are needed to evaluate the new tracer under pathological conditions, e.g., in animals with kidney damage.
Abbreviations
- DTPA:
-
Diethylenetriaminepentaacetic acid
- EDTA:
-
Ethylenediaminetetraacetic acid
- GFR:
-
Glomerular filtration rate
- PBS:
-
Phosphate buffered saline
- %ID:
-
Percent of injected dose
- RBC:
-
Red blood cells (erythrocytes)
- TAC:
-
Time-activity curve
- VOI:
-
Volume of interest
References
Beierwaltes WH, Harrison-Bernard LM, Sullivan JC, Mattson DL (2013) Assessment of renal function; clearance, the renal microcirculation, renal blood flow, and metabolic balance. Compr Physiol 3:165–200
Schock-Kusch D, Geraci S, Ermeling E et al (2013) Reliability of transcutaneous measurement of renal function in various strains of conscious mice. PLoS One 8:e71519
Von Hendy-Willson VE, Pressler BM (2011) An overview of glomerular filtration rate testing in dogs and cats. Vet J 188:156–165
Qi Z, Whitt I, Mehta A et al (2004) Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286:F590–F596
Henriksen UL, Henriksen JH (2015) The clearance concept with special reference to determination of glomerular filtration rate in patients with fluid retention. Clin Physiol Funct Imaging 35:7–16
Santos J, Martins LS (2015) Estimating glomerular filtration rate in kidney transplantation: still searching for the best marker. World J Nephrol 4:345–353
Haufe SE, Riedmuller K, Haberkorn U (2006) Nuclear medicine procedures for the diagnosis of acute and chronic renal failure. Nephron Clin Pract 103:c77–c84
Debruyn K, Vandermeulen E, Saunders JH et al (2013) Effect of background region of interest and time-interval selection on glomerular filtration ratio estimation by percentage dose uptake of 99mTc-DTPA in comparison with 51Cr-EDTA clearance in healthy cats. J Feline Med Surg 15:698–705
Hecht S, Lawson SM, Lane IF et al (2010) 99mTc-DTPA diuretic renal scintigraphy in dogs with nephroureterolithiasis. Can Vet J 51:1360–1366
Schnöckel U, Reuter S, Stegger L et al (2008) Dynamic 18F-fluoride small animal PET to noninvasively assess renal function in rats. Eur J Nucl Med Mol Imaging 35:2267–2274
Wakabayashi H, Werner RA, Hayakawa N et al (2016) Initial preclinical evaluation of 18F-fluorodeoxysorbitol PET as a novel functional renal imaging agent. J Nucl Med 57:1625–1628
Goethals P, Volkaert A, Vandewielle C et al (2000) 55Co-EDTA for renal imaging using positron emission tomography (PET): a feasibility study. Nucl Med Biol 27:77–81
Hofman MS, Hicks RJ (2016) Gallium-68 EDTA PET/CT for renal imaging. Semin Nucl Med 46:448–461
Kaewput C, Vinjamuri S (2016) Comparison of renal uptake of 68Ga-DOTANOC PET/CT and estimated glomerular filtration rate before and after peptide receptor radionuclide therapy in patients with metastatic neuroendocrine tumours. Nucl Med Commun 37:1325–1332
Lee JY, Jeong JM, Kim YJ et al (2014) Preparation of Ga-68-NOTA as a renal PET agent and feasibility tests in mice. Nucl Med Biol 41:210–215
Mueller D, Breeman WA, Klette I et al (2016) Radiolabeling of DOTA-like conjugated peptides with generator-produced 68Ga and using NaCl-based cationic elution method. Nat Protoc 11:1057–1066
Basken NE, Mathias CJ, Lipka AE, Green MA (2008) Species dependence of [64Cu]Cu-Bis(thiosemicarbazone) radiopharmaceutical binding to serum albumins. Nucl Med Biol 35:281–286
Daniel GB, Mitchell SK, Mawby D et al (1999) Renal nuclear medicine: a review. Vet Radiol Ultrasound 40:572–587
Fleming JS, Zivanovic MA, Blake GM, Burniston M, Cosgriff PS, British Nuclear Medicine S (2004) Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun 25:759–769
Wanasundara SN, Wesolowski MJ, Barnfield MC et al (2016) Accurate and precise plasma clearance measurement using four 99mTc-DTPA plasma samples over 4 h. Nucl Med Commun 37:79–86
Jobin J, Bonjour JP (1985) Measurement of glomerular filtration rate in conscious unrestrained rats with inulin infused by implanted osmotic pumps. Am J Phys 248:F734–F738
Rehling M, Nielsen LE, Marqversen J (2001) Protein binding of 99Tcm-DTPA compared with other GFR tracers. Nucl Med Commun 22:617–623
Russell CD, Bischoff PG, Rowell KL et al (1988) Estimation of extracellular fluid volume from plasma clearance on technetium-99m DTPA by a single-injection, two-sample method. J Nucl Med 29:255–258
Hofman M, Binns D, Johnston V et al (2015) 68Ga-EDTA PET/CT imaging and plasma clearance for glomerular filtration rate quantification: comparison to conventional 51Cr-EDTA. J Nucl Med 56:405–409
Pedersen EB (2000) New tools in diagnosing renal artery stenosis. Kidney Int 57:2657–2677
Brøchner-Mortensen J (1985) Current status on assessment and measurement of glomerular filtration rate. Clin Physiol 5:1–17
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gündel, D., Pohle, U., Prell, E. et al. Assessing Glomerular Filtration in Small Animals Using [68Ga]DTPA and [68Ga]EDTA with PET Imaging. Mol Imaging Biol 20, 457–464 (2018). https://doi.org/10.1007/s11307-017-1135-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-017-1135-1