Abstract
Purpose
The objective of the present study is to determine whether uptake of [18F]fluoromethylcholine ([18F]FCH) in comparison with 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) accurately reflects chemotherapy efficacy at the tumor cell level in prostate cancer (PC).
Procedures
The effects of docetaxel and cabazitaxel on viable tumor cell number were explored in four PC cell lines. Cellular uptake of [18F]FDG and [18F]FCH was compared with the effects measured using sulforhodamine B (SRB) assay, cell counting and colony formation assay (CFA), as proximators of viable tumor cell number. Agreement between uptake and cell numbers was assessed by Bland-Altman plots.
Results
[18F]FCH uptake in all PC cell lines significantly correlated to the cell numbers surviving the respective drug concentrations. Bland-Altman analysis showed that [18F]FDG uptake resulted in signal overestimation and higher variability after chemotherapy.
Conclusions
[18F]FCH uptake correlates well with viable tumor cell numbers remaining after docetaxel and cabazitaxel exposure. Radiolabeled choline is a potential response monitoring biomarker after chemotherapy for PC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PC) is the second most common cancer in males worldwide [1]. The disease presents itself mostly in men above the age of 50, and the incidence increases with age. The clinical behavior of PC is very diverse. Some tumors are indolent, do not cause any symptoms, and arise as microscopic, well-differentiated foci that may never become clinically manifest. However, a significant proportion of PC patients presents with or will develop aggressive tumors that lead to morbidity, metastases, and ultimately to death.
The initial systemic treatment in metastatic PC is based on androgen deprivation. Nevertheless, the majority of PC patients will ultimately progress and reach a castration-resistant PC (CRPC) status after starting the anti-hormone treatment. Therapeutic options against CRPC include agents that interfere with androgenic stimulation of tumor growth (e.g., abiraterone, enzalutamide) [2–4], immunotherapy (sipuleucel-T) [5], chemotherapy (docetaxel, cabazitaxel) [6, 7], and bone-seeking radiopharmaceuticals (e.g., Radium-223) [8]. Abiraterone acetate inhibits androgen biosynthesis by irreversibly blocking the CYP17, an essential enzyme in testosterone and estrogen synthesis [2]. When combined with low-dose prednisone, abiraterone improves survival of patients with CRPC [3]. Enzalutamide is an anti-androgen agent with demonstrated potential to inhibit nuclear translocation of the androgen receptor and DNA binding, inducing tumor volume reduction in xenograft models [4]. Sipuleucel-T is an active cellular immunotherapeutic which prolongs survival among men with asymptomatic or minimally symptomatic metastatic CRPC [5]. Docetaxel and cabazitaxel are chemotherapeutic drugs from the taxane class. Their principal mechanism of action is disruption of microtubule function, resulting in cell death [6, 7]. Radium-223 dichloride (Ra-223) is an alpha emitter which selectively binds to areas of increased bone turnover in metastatic lesions. The emitted high-energy alpha particles with short range radiation induce double-stranded DNA breaks, resulting in a highly localized cytotoxic effect in the target areas [8]. However, despite the variety of therapeutic options available, the proper sequencing (e.g., modality, timing) in individual patients is unclear. When chemotherapy is indicated, the initial regimen is docetaxel combined with prednisone [9, 10]. It has been shown that this combination significantly prolongs overall survival, compared to mitoxantrone [6]. For patients progressing after docetaxel, treatment with another taxane, cabazitaxel, is an option. This drug significantly increases overall survival compared with mitoxantrone plus prednisone in men whose disease progressed on docetaxel [7].
However, the actual response to chemotherapeutic regimens in individual patients is variable. It is important to monitor therapeutic (in)efficacy in time, to prevent patients from undergoing futile therapy for too long, since alternative and potentially effective drugs are available. Presently, monitoring of progression is based on a response metrics construct requiring various diagnostic tests, including serum prostate-specific antigen (PSA) measurement and bone scintigraphy [11]. The limitations of the current approach are related to the heterogeneity of metastasized PC (i.e., coexistence of androgen-sensitive and androgen-insensitive components with different impact on e.g., PSA [12]) and to its skeletal predominance (with bone- and computed tomography-scans having difficulties in timely and accurately detecting response). There is thus a clear need for alternative and more accurate response monitoring methods [13–15]. New specific tracers using positron emission tomography (PET) in whole body setting might enable a quantitative assessment of response in metastatic sites (e.g., in lymph nodes and bone), using a single, non-invasive scan procedure.
2-deoxy-2-[18F]-fluoro-D-glucose ([18F]FDG) is a potential tracer in the monitoring of antimicrotubule therapy effects in PC, but its clinical use thus far has been limited, because [18F]FDG uptake is highly variable and mostly confined to aggressive PC cells [16, 17]. Therefore, other tracers are being evaluated to describe tumor physiology as response to treatment [18–21]. [18F]fluoromethylcholine ([18F]FCH) PET [22] has shown promising results in the localization of locally recurrent or metastatic disease in men with biochemical failure [23–25] as well as in the early detection of bone metastases [26, 27].
Whether [18F]FCH could also be employed in monitoring treatment response in patients receiving docetaxel and cabazitaxel therapy is unclear. Definitive data from clinical studies have not yet become available. Nevertheless, experiments in vitro have shown promising results on the use of PET tracers to monitor anti-androgen treatment or chemotherapy [28]. The objective of the present study is to assess whether changes of [18F]FCH and/or [18F]FDG uptake in PC cells appropriately reflect chemotherapy-induced cytotoxicity.
Materials and Methods
Cell Lines
PC3, DU 145, and LNCaP (−FGC clone) human PC cell lines were originally obtained from the American Type Culture Collection, Rockville, Md., USA (ATCC# CRL 1435; HTB-81; CRL 1740, respectively). R3327-MATLyLu (MLL) rat prostate tumor variant was established in cell culture as described earlier [29, 30] Cell lines were cultured in RPMI-1640 culture medium (Gibco BRL, Life technologies Europe BV, Bleiswijk, The Netherlands), supplemented with 10 % fetal calf serum (Cambrex Fetal Calf Serum EU Standard, #14-801F, Lonza Verviers, Belgium), 100 U/ml penicillin/streptomycin (Gibco BRL), and 1 mM sodium pyruvate and insulin/transferrin/selenite medium supplement (Sigma-Aldrich Chemicals, St. Louis MO, USA) at 37 °C in a humidified atmosphere of 5 % CO2/95 % air. Semi-annual screening demonstrated the cultures to be mycoplasma free.
Drug Incubations
Docetaxel was obtained from Sigma-Aldrich chemicals (Zwijndrecht, the Netherlands) and was dissolved in dimethylsulfoxide to stock concentrations of 10 nM. Cabazitaxel was obtained from Sanofi-Aventis (Aventis Pharma, Antony Cedex, France) and was dissolved in phosphate-buffered salt solution (10 nM). All stock solutions were stored in aliquots at −20 °C until use. For the drug incubations, the respective cell lines were seeded into tissue culture flasks (25 cm, #690160; Greiner Bio-One, Alphen a/d Rijn, The Netherlands) in cell densities of 300.000 (PC3; DU145), 500.000 (LNCaP), or 30.000 (R3327-MATLyLu), respectively. After 24 h, drugs were added in the desired concentrations. After 3 days of drug incubation, the remaining cell numbers were determined using a CASY cell counter (Casy TT, Roche Diagnostics, Almere, The Netherlands) and parallel cultures were either worked up for colony formation assay (CFA) or incubated with 18F-radiolabeled choline and deoxyglucose. All experiments were performed in triplicate and were repeated at least three times.
Sulforhodamine B Assay
Evaluation of drug cytotoxic effects using the sulforhodamine B (SRB)-assay was performed as described earlier [31] In short: 3500 PC3 cells, 3500 DU145 cells, 5000 LNCaP cells, or 1500 MLL cells were seeded in each well of 96-well plates (Cellstar #655180; Greiner BioOne, Frickenhausen, Germany). After 24 h, drugs were added in increasing concentrations and cells were incubated for 3 days. After this incubation time, wells were fixed with trichloroacetic acid (1 h at 4 °C) and stained using SRB solution (0.4 % SRB in 1 % acetic acid). The optical density was measured at 492 nm after reconstitution of the dye in 150 μl 10 mM Tris buffer. The values were normalized to cell density of control cultures (100 %) and were corrected for the optical density values at t = 0 (0 %). Subsequently, inhibitory concentrations were calculated resulting in 10, 50, and 90 % reduction in cell numbers compared to control: IC10, IC50, and IC90, respectively (all from three experiments in triplicate; means ± SEM).
Colony Formation Assay
Cells were exposed to IC10, IC50, and IC90 concentrations of either cabazitaxel or docetaxel for 3 days. Subsequently, 200 cells were seeded in each well of 6-well plates for colony formation, as described previously [32]. After 7 to 10 days, the colonies were fixed using 4 % PBS-buffered formaldehyde. The colonies were then stained with Giemsa-solution and counted using a Leica stereomicroscope. All experiments were performed in triplicate and repeated at least three times.
To facilitate the colony formation of LNCaP cells, conditioned medium (RPMI-1640 medium exposed for 24–48 h to growing LNCaP cultures) was added (60:40). This allowed for autocrine stimulation of cell proliferation in this specific cell line. For R3327-MATLyLu cells, a modification of the colony formation assay (CFA) technique was used in order to account for the low efficient adherence of these cells to culture flask surfaces, as described earlier [29] Briefly, after drug exposure, MLL cells were seeded in 0.25 % agar (in PBS) which was layered on top of semisolid agar (0.375 %).
Choline and Deoxyglucose Labeling
[18F]FCH and [18F]FDG were prepared in the radiochemistry unit of the department of Radiology and Nuclear Medicine of the VU University Medical Center, Amsterdam, The Netherlands. Addition of the label (around 2 MBq) was performed 1 h after replenishment of the growing cultures with fresh medium (in the case of [18F]FDG labeling, glucose-free RPMI 1640 medium was used for this replenishment). After incubation with the radiopharmaceutical for 1 h, the cultures were washed twice using sterile phosphate-buffered salt solution and the uptake of the radiotracer in cells after trypsinization was measured using a gamma counter. The activity was calculated in percentage of total counts added and corrected for the cell number. All experiments were performed in triplicate and repeated at least three times.
Western Blotting
Western blotting was performed as described previously [31]. In brief, cells were exposed to either cabazitaxel or docetaxel for 3 days, washed with PBS, and scraped in lysis buffer (Cell Signalling Technology Inc., supplemented with 0.04 % protease inhibitor cocktail). Protein amounts in the supernatants were determined by the Bio-Rad assay (Bio-Rad Laboratories, Veenendaal, the Netherlands); 40 μg of protein was separated on a 10 % SDS-PAGE and electroblotted onto polyvinylidenedifluoride (PVDF) membranes (Millipore ImmobilonTM –FL PVDF, 0.45 μm). Subsequently, the membranes were blocked and incubated overnight at 4 °C with the primary antibody anti-GLUT-1 (polyclonal #ab15309; dilution 1:1000; Abcam, Cambridge, UK) or alternatively with anti-β actin (#A5441; 1:10,000; Sigma-Aldrich Chemicals, Deisenhofer, Germany) as loading control. Subsequently, the membranes were incubated with the secondary antibody against mouse (goat-α-mouse-IRDye (680; #926-32220), Westburg, Leusden, The Netherlands). The bands were scanned using an Odyssey Infrared Imager (Westburg) at high quality, and expression levels were quantified with the Odyssey software program LI-COR Biosciences.
Cell Volume Determinations
Using CASY TT electronic cell counter, the effects of drug incubations of docetaxel and cabazitaxel (at IC90 concentration level) on average cell volume were determined after 3 days of incubation for all cell lines. Average volume is reported in the results in femtoliter (fl).
Analysis and Statistics
Agreement between uptake of radiolabeled [18F]FCH and [18F]FDG in the four PC cell lines and cell number was assessed by means of Bland-Altman plots, in which the differences between uptake of the tracer (%) and cell number (%) were plotted against the averages of these values [33, 34].
Results
Effect of Cabazitaxel and Docetaxel on Prostate Cancer Cell Viability
The drug induced effects on the four PC cell lines after an incubation of 3 days were assessed using the SRB assay. In Table 1, the IC10, IC50, and IC90 values were taken to delineate a representative and differential range of drug effect levels to be used to compare [18F]FCH/[18F]FDG uptake with measures of viable cell numbers after drug incubation. The sensitivity of the various cell lines was reflected in their differential pattern of sensitivity towards docetaxel and cabazitaxel.
Subsequently, the chosen concentrations (IC10, IC50, and IC90) of docetaxel and cabazitaxel, aimed at reflecting low, medium, and highly effective treatment results, respectively, were used in a 3-day incubation scheme with cells from the four PC lines to delineate efficacy on colony forming capacity. The colony forming ability at the IC10 and IC90 dose levels of both drugs decreased in a dose-dependent manner and analogously to the SRB-derived survival values, for all cell lines (Fig. 1).
CFA-determined treatment effects of docetaxel and cabazitaxel on proliferation of prostate cancer cells correlated to SRB-determined toxicity levels (IC10, IC50, and IC90 are drug concentrations resulting in 10, 50, and 90 % reduction in cell numbers compared to control value, respectively). Surviving fractions are given in percentage compared to control ± standard deviation, carried out in triplicate using prostate cancer cell lines PC3, DU145, LNCaP, and MLL. a effect of docetaxel. b effect of cabazitaxel. Left bar IC10, middle bar IC50, right bar IC90.
Effect of Treatment on [18F]FCH and [18F]FDG Signal
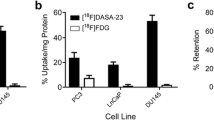
To evaluate the effect of docetaxel and cabazitaxel on [18F]FCH and [18F]FDG uptake, cells were exposed for 3 days to IC10, IC50, and IC90 concentrations of the drugs, after which the cellular uptake of the radiotracer was measured. In all four cell lines, a clear relation was observed between actual cell numbers counted after treatment and level of [18F]FCH and [18F]FDG uptake. Using Bland-Altman plots, we analyzed the degree to which the [18F]FCH/[18F]FDG uptake co-varied with viable cell numbers, in response to drug concentration in cells from the respective cell lines (Fig. 2). Average difference between tracer uptake and cell number was closer to 0 for [18F]FCH compared to [18F]FDG with the exception of the DU145 cell line after treatment with cabazitaxel. [18F]FDG uptake overestimated the numbers of cells remaining after docetaxel and cabazitaxel treatment in PC3 and MLL cell lines (Table 2). Furthermore, agreement between uptake and cell numbers was generally worse for [18F]FDG when compared to [18F]FCH as can be seen from the increased limits of agreement (determined as 1.96 times standard deviation of the difference between tracer uptake and cell number) for the Bland-Altman analyses (Table 2).
Bland-Altman plots for cellular [18F]FCH uptake in four prostate tumor cell lines (a PC3, c DU145, e LNCaP, g MLL) and [18F]FDG uptake (b PC3, d DU145, f LNCaP, h MLL), after docetaxel and cabazitaxel treatment. The x-axis shows the average between cell number percent and uptake percent and the y-axis shows the absolute difference between cell number percent and uptake percent. The straight lines indicate the average uptake after incubation with docetaxel (black) and cabazitaxel (red). The dotted lines indicate the limits of agreement for uptake after incubation with docetaxel (black) and cabazitaxel (red).
To explain the increased [18F]FDG uptake by PC cells after incubation with docetaxel and cabazitaxel, we measured the expression of glut-1 glucose transporters using Western blot after incubation at IC90 drug concentrations. The glut-1 transporter protein expression appeared not to be upregulated by treatment with either docetaxel or cabazitaxel (Fig. 3).
a Western blot displaying stained antibody incubations using anti-glut-1 antibody on cell homogenates of four prostate cancer cell lines PC3, MLL, DU145, and LNCaP, after treatment with IC90 concentrations of docetaxel and cabazitaxel. Anti-β actin was used as a loading control. b Expression levels were quantified with the Odyssey software.
Since taxane exposure may lead to increase in cell volume through stabilization of microtubules and inadequate cell division, an effect on radiotracer uptake on a per cell basis may result. Therefore, the mean volume of PC cells from the four different cell lines was determined after 3 days of incubation with IC90 concentrations of docetaxel or cabazitaxel. The mean cell volume increased with a factor about 2 or 3 times for all four cell lines after incubation with each of the two chemotherapeutic drugs, most notably for PC3 and MLL (Fig. 4).
Discussion
In the present study, we compared the reduction in cellular uptake of radioactive [18F]FCH in prostate tumor cells in vitro under different burdens of docetaxel and cabazitaxel, with parameters of cell viability. The radiotracer uptake was proportional to the number of cells counted after therapy, and these cell numbers correlated to clonogenic capacity as an additional sign of (reproductive) viability of the cells surviving the treatment. From this comparison, we conclude [18F]FCH to be an adequate read-out parameter for response to treatment. [18F]FDG, on the other hand, showed in the case of PC3 and MLL an overestimation of the viable cell numbers remaining after therapy. This could lead to misinterpretation of the treatment outcome.
Non-invasive and timely therapy monitoring using PET would enable to quickly evaluate treatment efficacy in individual cancer patients thereby tailoring therapy, improving healthcare and quality of life, while offering economic benefits. Therefore, we examined the possibility to use [18F]FCH as a novel read-out for chemotherapy response monitoring in PC cells after treatment with either docetaxel or cabazitaxel, which are presently the drugs of choice in clinical PC chemotherapy.
A panel of different prostatic tumor cell lines was used, comprising both androgen-sensitive (LNCaP) as well as androgen-insensitive cell lines (PC3, DU145, and R3327 MATLyLu). An influence of prostate cancer differentiation in patients on the level of [18F]FDG uptake has been described by Schwarzenböck et al. [35]. It has been shown that the grade of differentiation of PC cells is inversely proportional to the level of [18F]FDG uptake. In our experiments, the relative sensitivity of treatment effects as evidenced by [18F]FCH and [18F]FDG uptake was investigated. The observed differential sensitivity of the various cell lines for the respective drugs is thought to reflect differences in expression of drug transporters like ABCC4 [30, 36]. All four cell lines investigated were [18F]FCH and [18F]FDG avid, and the relative changes of tracer uptake and viable cell numbers upon exposure to docetaxel and cabazitaxel were closely related. Whether the efficacy of chemotherapy in PC in vivo will also be reflected by such a proportional decrease of the [18F]FCH signal, remains to be determined. However, in animal experiments employing PC xenografts, a reduced uptake of [11C]choline compared to muscle tissue was shown within a week after docetaxel therapy and thereby confirms our present conclusions [37, 38]. In the present comparative study, we have explored four different PC cell lines, two radiotracers (18F-radiolabeled choline and FDG), and two chemotherapeutic drugs in a range of three concentrations.
Although we did not measure [18F]FCH and [18F]FDG levels before and after drug treatment, the radiotracers were supplied in excess and are therefore not supposed to be a limiting factor.
Docetaxel/cabazitaxel may contribute to an effect on the cellular uptake of [18F]FCH signal through a modulation of choline metabolism [39]. Choline transporters mediate the cellular uptake and the transport is the rate limiting step for the synthesis of phosphocholine which is enhanced in malignant transformation. Choline transporters of high and low affinity have been found in normal prostate tissue and are increased in prostate cancer [40]. Expression of choline transporters—and therefore choline uptake rate—has been described to be influenced by anticancer drugs [28, 41]. Such an effect could become visible in a decreased choline uptake per cell. Nevertheless, in the present study, the choline uptake signal was correlated closely to the surviving cell numbers after treatment with docetaxel and cabazitaxel. Therefore, we conclude that interference with choline metabolism is not a confounder in the present observations.
At present, it is unknown how the increased [18F]FDG uptake after treatment in some cell lines is brought about. Especially, the overestimation of viable cell numbers by the [18F]FDG signal after chemotherapy deserves attention as it may result in a false negative interpretation of treatment efficacy. Possible explanations for an observed disjointed relationship of [18F]FDG response from viable cell numbers after chemotherapy may relate to differential effects on hexokinase and glut-1 expression [42], but mostly these effects result in decreased [18F]FDG uptake rather than the opposite. Moreover, in our present in vitro study, we did not observe changes of glut-1 expression to explain increased [18F]FDG uptake after drug treatment. However, a very different cause for this effect in our experiments may have been found in the observed increase of cell volume after docetaxel or cabazitaxel treatment. The increased volume of such swollen cells after therapy may result in increased [18F]FDG signal per cell. The absence of these effects in the case of [18F]FCH may be related to different metabolic pathways, but this aspect has to be further investigated. Interestingly, other authors have also found increased levels of radiotracer uptake after therapy [28, 43]. Cellular stress resulting in increased cellular metabolism or otherwise increased cell permeability has been given as an explanation for a flare at 10 min after start of treatment [28]. A transient increase in FDG uptake has been described by Bjurberg et al. [43] followed by a rapid decrease. In general, timing of the monitoring seems to be important. In our analyses, uptake of the PET tracers was measured after 3 days of drug incubation, thereby bypassing any eventual, initial flare phenomena. The observed cell number-independent increase in [18F]FDG signal, coupled to other known drawbacks of [18F]FDG PET in PC (general low uptake, disturbing sensitivity to inflammatory processes, scar tissue, and radiotherapy mediated effects) together with the proportional, observed treatment effects in [18F]FCH uptake, contribute to a preferential role rising up for [18F]FCH PET compared to [18F]FDG PET as a candidate drug response monitoring tool in PC patients.
Conclusions
Our in vitro data demonstrate that the cellular [18F]FCH uptake correlates well with viable tumor cell number after docetaxel and cabazitaxel for all PC cell lines, while [18F]FDG at times overestimated the cell number after drug exposure. This suggests that [18F]FCH is more accurate and therefore more suitable than [18F]FDG as a response monitoring PET tracer in chemotherapy of prostate cancer using docetaxel and cabazitaxel.
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Fizazi K, Scher HI, Molina A et al (2012) Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 13:983–992
Ryan CJ, Smith MR, de Bono JS et al (2013) Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138–148
Scher HI, Fizazi K, Saad F et al (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187–1197
Kantoff PW, Higano CS, Shore ND et al (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–422
Berthold DR, Pond GR, Soban F et al (2008) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 26:242–245
De Bono JS, Oudard S, Ozguroglu M et al (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376:1147–1154
Parker C, Nilsson S, Heinrich D et al (2013) Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369:213–223
Basch EM, Somerfield MR, Beer TM et al (2007) American Society of Clinical Oncology endorsement of the Cancer Care Ontario Practice Guideline on nonhormonal therapy for men with metastatic hormone-refractory (castration-resistant) prostate cancer. J Clin Oncol 25:5313–5318
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 65:467–479
Scher HI, Morris MJ, Basch E, Heller G (2011) End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. J Clin Oncol 29:3695–3704
Lilja H, Ulmert D, Vickers AJ (2008) Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer 8:268–278
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Costelloe CM, Chuang HH, Madewell JE, Ueno NT (2010) Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J Cancer 1:80–92
Wallace TJ, Torre T, Grob M et al (2014) Current approaches, challenges and future directions for monitoring treatment response in prostate cancer. J Cancer 5:3–24
Price DT, Coleman RE, Liao RP et al (2002) Comparison of [18F]fluorocholine and [18F]fluorodeoxyglucose for positron emission tomography of androgen dependent and androgen independent prostate cancer. J Urol 168:273–280
Morris MJ, Akhurst T, Larson SM et al (2005) Fluorodeoxyglucose positron emission tomography as an outcome measure for castrate metastatic prostate cancer treated with antimicrotubule chemotherapy. Clin Cancer Res 11:3210–3216
Oyama N, Hasegawa Y, Kiyono Y et al (2011) Early response assessment in prostate carcinoma by 18F-fluorothymidine following anticancer therapy with docetaxel using preclinical tumour models. Eur J Nucl Med Mol Imaging 38:81–89
Yu EY, Muzi M, Hackenbracht JA et al (2011) C11-acetate and F-18 FDG PET for men with prostate cancer bone metastases: relative findings and response to therapy. Clin Nucl Med 36:192–198
Witney TH, Fortt RR, Aboagye EO (2014) Preclinical assessment of carboplatin treatment efficacy in lung cancer by 18F-ICMT-11-positron emission tomography. PLoS One 9:e91694
Afshar-Oromieh A, Zechmann CM, Malcher A et al (2014) Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 41:11–20
DeGrado TR, Baldwin SW, Wang S et al (2001) Synthesis and evaluation of (18)F-labeled choline analogs as oncologic PET tracers. J Nucl Med 42:1805–1814
Evangelista L, Zattoni F, Guttilla A et al (2013) Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med 38:305–314
Cimitan M, Bortolus R, Morassut S et al (2006) [18F]fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur J Nucl Med Mol Imaging 33:1387–1398
Umbehr MH, Müntener M, Hany T et al (2013) The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol 64:106–117
Beheshti M, Vali R, Waldenberger P et al (2009) The use of F-18 choline PET in the assessment of bone metastases in prostate cancer: correlation with morphological changes on CT. Mol Imaging Biol 11:446–454
Beheshti M, Vali R, Waldenberger P et al (2008) Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET-CT: a comparative study. Eur J Nucl Med Mol Imaging 35:1766–1774
Müller SA, Holzapfel K, Seidl C et al (2009) Characterization of choline uptake in prostate cancer cells following bicalutamide and docetaxel treatment. Eur J Nucl Med Mol Imaging 36:1434–1442
Geldof AA, Rao BR, de Voogt HJ (1986) Direct effects of chemotherapeutic agents on rat prostate tumor clonogenic cells. Anticancer Res 6:837–840
Oprea-Lager DE, Bijnsdorp IV, Van Moorselaar RJA et al (2013) ABCC4 Decreases docetaxel and not cabazitaxel efficacy in prostate cancer cells in vitro. Anticancer Res 33:387–391
Bijnsdorp IV, Kruyt FA, Gokoel S et al (2008) Synergistic interaction between trifluorothymidine and docetaxel is sequence dependent. Cancer Sci 99:2302–2308
Mastbergen SC, Duivenvoorden I, Versteegh RT, Geldof AA (2000) Cell cycle arrest and clonogenic tumor cell kill by divergent chemotherapeutic drugs. Anticancer Res 20:1833–1838
Altman DG, Bland JM (1983) Measurement in medicine: the analysis of method comparison studies. Statisics 32:307–317
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Schwarzenböck S, Souvatzoglou M, Krause BJ (2012) Choline PET and PET/CT in primary diagnosis and staging of prostate cancer. Theranostics 2:318–330
Vrignaud P, Sémiond D, Lejeune P et al (2013) Preclinical antitumor activity of cabazitaxel, a semisynthetic taxane active in taxane-resistant tumors. Clin Cancer Res 19:2973–2983
Schwarzenböck S, Sachs D, Souvatzoglou M et al (2013) [11C]choline as a pharmacodynamic marker for docetaxel therapy. Response assessment in a LNCaP prostate cancer xenograft mouse model. Nuklearmedizin 52:141–147
Krause BJ, Souvatzoglou M, Herrmann K et al (2010) [11C]Choline as pharmacodynamic marker for therapy response assessment in a prostate cancer xenograft model. Eur J Nucl Med Mol Imaging 37:1861–1868
Jensen LR, Huuse EM, Bathen TF et al (2010) Assessment of early docetaxel response in an experimental model of human breast cancer using DCE-MRI, ex vivo HR MAS, and in vivo 1H MRS. NMR Biomed 23:56–65
Awwad HM, Geisel J, Obeid R (2012) The role of choline in prostate cancer. Clin Biochem 45:1548–1553
Taguchi C, Inazu M, Saiki I et al (2014) Functional analysis of [methyl-(3)H]choline uptake in glioblastoma cells: influence of anti-cancer and central nervous system drugs. Biochem Pharmacol 88:303–312
Engles JM, Quarless SA, Mambo E et al (2006) Stunning and its effect on 3H-FDG uptake and key gene expression in breast cancer cells undergoing chemotherapy. J Nucl Med 47:603–608
Bjurberg M, Henriksson E, Brun E et al (2009) Early changes in 2-deoxy-2-[18F]fluoro-D-glucose metabolism in squamous-cell carcinoma during chemotherapy in vivo and in vitro. Cancer Biother Radiopharm 24:327–332
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oprea-Lager, D.E., van Kanten, M.P., van Moorselaar, R.J.A. et al. [18F]Fluoromethylcholine as a Chemotherapy Response Read-Out in Prostate Cancer Cells. Mol Imaging Biol 17, 319–327 (2015). https://doi.org/10.1007/s11307-014-0803-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0803-7