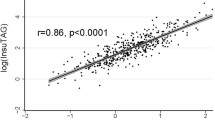
Abstract
Background
The triglyceride–glucose (TyG) index has been considered as insulin resistance (IR) assessment index. The current study aimed to verify the reliability of the TyG index as an IR assessment marker; the study of plasma fatty acids and body fat composition to determine potential metabolic syndrome (MetS) participants with a body mass index (BMI) of between 25.0 and 29.9 kg/m2.
Methods
The study included 378 overweight participants with a body mass index of between 25.0 and 29.9 kg/m2. They were divided into tertiles according to the homeostasis model assessment of IR (HOMA-IR) or the TyG index. The role of the IR assessment index and the relationship with IR-related diseases and the risk factors using gas chromatograph-mass spectrometry, computed tomography, and dual energy X-ray absorptiometry, was investigated.
Results
It was only in the TyG index tertile that the higher TyG index participants showed considerably higher LDL-cholesterol levels. More markedly, a close relationship was observed between the TyG index and the omega-6 polyunsaturated fatty acids compared with the HOMA-IR. Unlike HOMA-IR, with regard to the risks of developing chronic diseases, the MetS, the third tertile of the TyG index, showed an approximately 33.7 times greater odds ratio (OR) of the MetS occurring, compared with the first tertile of the TyG index.
Conclusions
The TyG index may be considered as an IR assessment index. In addition, the TyG index is an advanced tool that reflects the relevance of pro-inflammation levels and the presence of IR-related chronic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Obesity has become a global epidemic and one of the most significant public health problems (Yanni et al., 2018). Obesity has a relationship with insulin resistance (IR), diabetes mellitus (DM), the metabolic syndrome (MetS), and cardiovascular diseases (CVDs) (Genco et al., 2005; Mohamed, 2014). Furthermore, IR is associated with the MetS (Qin et al., 2010). It is crucial to define relationships with the MetS, such as IR and DM, and to continue research in this area. Although IR is the main etiological factor of the MetS, it is not the only connection between IR and the MetS and furthermore, these may have different clinical characteristics. Therefore, research on the relationship between IR and the MetS as well as an understanding of IR is recommended. In recent years, the methods for evaluating IR and insulin sensitivity have been considered in numerous ways (e.g., aspect of financial, convenience, and application scope) (Irace et al., 2013).
The homeostasis model assessment of IR (HOMA-IR) is a widely and commonly used method of measuring IR from fasting glucose and insulin in clinical research (Wallace et al., 2004). However, HOMA-IR requires plasma insulin, meaning that it can place a financial burden on a low-income group (Irace et al., 2013). On the other hand, fasting triglyceride levels can be a more useful measurement than insulin (Simental-Mendía et al., 2008). According to Marceau et al. (Marceau et al., 1999), hepatic triglycerides are the determinant of hepatic IR and hepatic triglycerides affect the intra-myocellular triglycerides of the muscle IR (Phillips et al., 1996). This being so, triglycerides deserve consideration as a determinant factor of IR. Furthermore, the TyG index is also a marker for assessing IR. In addition, unlike HOMA-IR, the TyG index does not need an insulin measurement (Mohammadabadi et al., 2014). Researchers have demonstrated that the TyG index is more expedient and inexpensive than the HOMA-IR as an assessment of the IR index (Mohammadabadi et al., 2014). Furthermore, another study reported that the TyG index is more relevant than weight gain as a diagnosis of type 2 diabetes (Navarro-González et al., 2016). The research of Ebbert et al. (Simental-Mendía et al., 2008) outlined that obesity, and specifically upper body/visceral obesity, is associated with IR. Thus, the TyG index may be considered to be helpful or useful to assess IR in the population. Characteristics, such as the enhanced simplicity and accessibility of the TyG index, can be useful as IR assessment markers (Hosseini, 2017). Nevertheless, the HOMA-IR is still used more than the TyG index in the assessment of IR. These studies have shown that the TyG index may be able to predict the risk of other diseases such as the MetS besides serving as the IR assessment index. Therefore, examining the possibility of the TyG index as a HOMA-IR replacement may lead to an era in which the evaluation of IR becomes easier. Furthermore, the TyG index may increase the prophylactic potential of the MetS in diseases such as DM.
Therefore, this study aimed to determine the correlation between body fat composition, plasma fatty acids (FAs), and the prevalence of the MetS according to the TyG index of overweight Korean adults with a body mass index (BMI) of between 25.0 and 29.9 kg/m2. Furthermore, this study investigated the role of the IR assessment index and the possibility of predicting and preventing these diseases by using the TyG index.
2 Methods
2.1 Study participants
In this study, the participants were recruited from a prospective cohort study after advertisements had been posted in Seoul, South Korea. The study was conducted using overweight participants. A total of 378 participants were recruited from the Clinical Nutrigenetics/Nutrigenomics Laboratory at Yonsei University between May 1, 2016 and April 27, 2018. The study participants were referred to the Department of Internal Medicine, Yonsei University Severance Hospital. After their health and basic blood parameters, including serum glucose, had been rechecked, individuals who met the study criteria were recommended for study participation. The study participants inclusion criteria were as follows: aged between 20 and 65 years; a BMI of between 25.0 and 29.9 kg/m2 (WHO standards); and no histories/presence of hypertension, DM, or no treatment of CVDs in the last six months. The exclusion criteria were as follows: women who were pregnant or breastfeeding. The study participants were divided into tertiles according to the HOMA-IR and the TyG index: [lower HOMA-IR (H1; < 2.065); middle HOMA-IR (H2; 2.065–2.853), higher HOMA-IR (H3; > 2.853), lower TyG index (T1; < 8.230); middle TyG index (T2; 8.230–8.674) and higher TyG index (T3; > 8.674)]. Those who consented to participate in the program were included in this study. The purpose of the study was explained carefully to all the participants, and their written consent was obtained prior to their participation. The tertile analysis was chosen to maximize the sample size of each group. The Institutional Review Board (IRB) of Yonsei University (IRB No. 7001988-202005-BR-879-01E) and the Severance Hospital approved the study protocol, which complied with the Declaration of Helsinki (1964).
2.2 The MetS diagnosis
In the study, the MetS was diagnosed according to the MetS diagnostic criteria of the American Heart Association and the National Heart, Lung, and Blood Institute (AHA/NHLBI) (Grundy et al., 2005; Organization, 2000). If a patient had three or more out of five risk factors the AHA/NHLBI they were diagnosed as having the MetS. Thus, 0–2 risk factors were classified as non- MetS obesity (n = 309) and 3–5 risk factors were classified as MetS obesity (n = 69). The MetS diagnostic criteria provided by AHA/NHLBI is as follows:
-
1.
≥ 130 mmHg systolic blood pressure or ≥ 85 mmHg diastolic blood pressure.
-
2.
≥ 100 mg/dL fasting blood glucose.
-
3.
≥ 150 mg/dL triglyceride in the blood.
-
4.
HDL-cholesterol in the blood; < 40 mg/dL in men and < 50 mg/dL in women.
-
5.
Waist circumference (≥ 90 cm in men and ≥ 80 cm in women; Western Pacific Region of the WHO; 2000 (Organization, 2000).
2.3 Anthropometric assessments
The body weight and height of the study participants were measured without clothing and shoes in the morning after having an empty stomach for 12 h. The BMI (Inbdy370; Biospace, Cheonan, Korea) was calculated in kilograms per square meter (kg/m2). The waist and hip circumference (measured directly on the skin) was measured to the nearest 0.1 cm, while the study participant was standing with the arms precisely at the atrium level position after a resting period (20 min). The BP was measured twice on the arm using an automatic BP monitor (FT-200S; Jawon Medical, Gyeongsan, Korea); the two or three measurements were than averaged.
2.4 Blood collection and biochemical assessments
The blood samples were collected after an overnight fast of at least 12 h. The venous blood specimens were collected in EDTA-treated whole-blood and serum tubes (BD Vacutainer; Becton, Dickinson and Company, Franklin Lakes, NJ, USA). The drawn blood samples in collection tubes were immediately placed on ice until they arrived at the analytical laboratory (within 1–3 h). The blood samples were centrifuged to obtain the plasma and serum samples, which were then stored at − 80 °C. The levels of triglycerides (TGs), total cholesterol (TC), and high density lipoprotein cholesterol (HDL-cholesterol) were measured. The low-density lipoprotein cholesterol (LDL-cholesterol) levels were calculated using the equation of the Friedewald formula: LDL-cholesterol = total cholesterol − [HDL-cholesterol + (TG/5)]. The obesity-related hormone adiponectin (Human Adiponectin ELISA Kit, Adipogen®, Liestal, Switzerland), was also measured.
2.5 The calculation of HOMA-IR, TyG index, TyG index combined with BMI (TyG-BMI)
The HOMA-IR was calculated as the fasting glucose (mg/dl) × fasting insulin (μIU/ml)/405 (Wallace et al., 2004). The TyG index was calculated as the Ln [fasting glucose (mg/dl) × fasting triglyceride (mg/dl)/2] (Mohd Nor et al., 2016). The TyG-BMI was calculated by multiplying the TyG index with the BMI (Ramírez-Vélez et al., 2019).
2.6 Gas chromatograph–mass spectrometry analysis
The fatty acid methyl esters of all the samples were separated through a capillary column (VF-WAXms 30 m × 0.32 mm; film thickness, 0.25 μm; Agilent Technologies, Middelburg, Netherlands) and analyzed using an Agilent Technologies 7890 N gas chromatograph coupled to an Agilent Technologies 5977A quadrupole mass selective spectrometer with a triple-axis detector (Agilent, Palo Alto, CA, USA). Helium was used as the carrier gas with a flow rate of 1.0 mL/min. The temperature of the injector was 240 °C. The injection volume of the sample was 1 μL in a split less mode. The temperature program was as follows: the initial temperature started at 50 °C for 2.3 min (Warensjö et al., 2006); and was raised to 175 °C in units of 50 °C/min (Karelis, 2008), and then to 230 °C in units of 2 °C/min (Livingstone et al., 2013). The quadrupole mass spectrometer was operated in electron ionization mode and full scan monitoring mode (m/z 50–800). The solvent delay time was three minutes. The temperatures of the transfer line and quadrupole were set at 230 °C and 150 °C, respectively. The source temperature was 230 °C with the electron energy at 70 eV.
The relative retention time and mass spectrum were compared to the FAME standard to identify the methyl ester peaks of all the samples. To quantify, methyl ester peaks on all samples were calculated by comparing peak areas with the internal standard (ISTD) compound. The values that were determined by taking a relative measurement of the plasma fatty acid peak area to the peak area of the ISTD were used as the relative peak area. After collecting all the samples for assessment to minimize errors caused by changes in the instrument condition, the plasma FAs were analyzed under the same examination conditions. Additionally, the values of n-6 and n-3 were calculated as the sum of the average peak area of each of the FAs that corresponded to the polyunsaturated FAs (PUFAs) omega-6 and omega-3, respectively.
2.7 Body fat composition measurements
The abdominal fat distribution was measured at the first lumbar vertebra (L1) and the fourth lumbar vertebra (L4) using computed tomography (CT). The scanning parameters were a slice thickness of 1 mm at 200 mA and 120 kVp, with a 48-cm field of view. The abdominal adipose tissue was determined using an attenuation range of − 150 to − 50 Hounsfield units in the CT images: (e.g. whole fat area; WFA (cm2), visceral fat area; VFA (cm2), subcuaneous fat area; SFA (cm2), and visceral/subcuraneous fat ratio; VSR). The body composition of the study participants was measured via dual energy X-ray absorptiometry (DEXA) to determine the fat percentage (%), fat mass (g), and lean body mass (g).
2.8 Statistical analysis
The statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 24.0 software (IBM/SPSS, Chicago, IL, USA). The skewed variables were transformed logarithmically. The one-way analysis of variance (ANOVA) and the Bonferroni method were conducted to compare how continuous variables differed between the tertiles. The analysis of covariance (ANCOVA) was used to adjust for confounding factors such as age. Chi-square tests were conducted to compare the categorical variables. Multivariate analyses were conducted to investigate the HOMA-IR and the TyG index as independent predictors for the ORs and the presence of the MetS obesity. The results are expressed as the means ± standard errors (SE). A two tailed P-values < 0.05 were considered to be statistically significant.
3 Results
3.1 Participants
A total of 378 overweight participants between the ages of 20 and 65 (years; 40.1 ± 0.57) and both sexes (male; n = 122 and female; n = 256) participated in the study (data not shown).
3.2 Anthropometric parameters and characteristics of each IR assessment index tertile
The anthropometric parameters and characteristics of the TyG index are shown in Table 1. These participants were divided into three groups in tertiles (n = 126; respectively). The age was statistically significantly different between the three groups (P = 0.001). The weight and waist-hip ratio adjusted by age variable were statistically significantly different between the three groups (P = 0.006, P = 0.010, and P = 0.001, respectively). The systolic blood pressure (SBP) and diastolic blood pressure (DBP) were statistically significantly different between the three groups (P ≤ 0.001). These participants were divided into three groups according to HOMA-IR tertiles (n = 126). The weight, BMI, waist-hip ratio, percentage of body fat, SBP, and DBP were statistically significantly different between the three groups (P = 0.013, P ≤ 0.001, P = 0.035, P ≤ 0.001, P = 0.034, and P = 0.001, respectively).
3.3 Obesity-related hormone and biochemical analysis
The obesity-related hormone and lipid profiles of the TyG index are shown in Table 1. The TC, triglyceride, LDL-cholesterol, HDL-cholesterol, and adiponectin were statistically significantly different between the tertiles (P ≤ 0.001). The TC, triglyceride, HDL-cholesterol, and adiponectin were statistically significantly different between the HOMA-IR tertiles (P = 0.037, P ≤ 0.001, P = 0.015, and P = 0.003, respectively).
3.4 Laboratory measurements and inflammatory markers
The laboratory measurements of the TyG index are shown in Table 2. The white blood cell (WBC), aspartate aminotransferase (AST), and high-sensitivity C-reactive protein (hs-CRP) were statistically significantly different between the three groups (P = 0.001, P = 0.010, and P = 0.030, respectively). The alanine aminotransferase (ALT), glucose, insulin, and C-peptide were statistically significantly different between the three groups (P ≤ 0.001). The WBC was statistically significantly different between the three groups of the HOMA-IR tertiles (P = 0.001). The platelet (Plt), ALT, glucose, insulin, and C-peptide were statistically significantly different between the three groups of the HOMA-IR tertiles (P ≤ 0.001).
3.5 Plasma FAs levels assessment of each IR assessment index tertile
Table 3 presents an assessment of the plasma FAs levels of omega-6 PUFAs (n-6 PUFAs; μg/mL, ppm) and omega-3 PUFAs (n-3 PUFAs; μg/mL, ppm), n-6, n-3, and a ratio of n-6/n-3 of the TyG index. The linoleic acid (LA), gamma-LA, eicosanoic acid, and dihomo-gamma-linolenic acid of n-6 PUFAs were statistically significantly different between each of the tertile groups of the TyG index (P ≤ 0.001). The alpha-linolenic acid (ALA) and eicosatetraenoic (DPA) of n-3 PUFAs were statistically significantly different between each of the tertile groups of the TyG index (P ≤ 0.001 and P = 0.027, respectively). The n-6, n-3, and the ratio n-6/n-3 were statistically significantly different between each of the tertile groups of the TyG index (P ≤ 0.001, P ≤ 0.001, and P = 0.031, respectively). The ratios of n-6/n-3 of all the tertile groups of the TyG index were above 4.00 to < 5.00. However, arachidonic acid and dicosatetranoic acid of n-6 PUFAs and eicosapentaenoic acid and docosahexaenoic acid (DHA) of n-3 PUFAs were not statistically significantly different between each tertile group of the TyG index. The DPA and DHA of n-3 PUFAs were statistically significantly different between each tertile group of the HOMA-IR (P = 0.006 and P = 0.002, respectively). The remaining variables were not significantly different between the tertile groups.
Table S1 presents an assessment of the plasma FAs levels of saturated fatty acids (SFAs; μg/mL, ppm) and monounsaturated fatty acid (MUFAs; μg/mL, ppm) of each IR assessment index tertile. Lauric acid, myristic acid, palmitic acid, and stearic acid of SFAs were statistically significantly different between each tertile group of the TyG index (P ≤ 0.001). Myristic acid, pentacyclic acid, palmitic acid, and stearic acid of SFAs were statistically significantly different between each tertile group of the HOMA-IR (P = 0.003, P = 0.001, P = 0.010, and P = 0.009, respectively). Palmitoleic acid, cis-10-heptadecenoic acid, oleic acid, and eicosanoic acid of the MUFAs were statistically significantly different between each tertile group of the TyG index (P ≤ 0.001). In particular, all the FAs belonging to the MUFAs, n-6 PUFAs, n-6, n-3, and the ratio n-6/n-3 were found not to be statistically significantly different between each tertile group of HOMA-IR.
3.6 Percentage, fat mass and lean body mass by DEXA evaluation and abdominal fat areas by CT evaluation of each IR assessment index tertile
The percentage, fat mass and lean body mass obtained using DEXA evaluations and abdominal fat areas using CT evaluations of each IR assessment index tertile are shown in Table 4. The fat percentage (%) and lean fat mass (g) by DEXA were statistically significantly different between the TyG index tertiles (P = 0.047 and P = 0.002, respectively). The abdominal fat areas were obtained using CT evaluations of the first and fourth lumbar vertebra (L1 and L4): (i.e. WFA (cm2), VFA (cm2), SFA (cm2), and VSR). The WFA, VFA, and VSR of the L1 were statistically significantly different between the three groups (P ≤ 0.001). The VFA and VSR of the L4 were statistically significantly different between the three groups (P ≤ 0.001). The fat percentage (%) and fat mass (g) were statistically significantly different between the HOMA-IR tertiles (P = 0.003 and P ≤ 0.001, respectively). The fat mass (g) obtained using DEXA tended to be higher in the H3 tertile than in the H1 and H2 tertiles. The abdominal fat areas were obtained using CT evaluations of first and fourth lumbar vertebra (L1 and L4). The WFA, VFA, and SFA of the L1 were statistically significantly different between the three groups (P ≤ 0.001, P = 0.001, and P ≤ 0.001, respectively). The WFA, VFA, and VSR of the L4 were statistically significantly different between the three groups (P ≤ 0.001, P ≤ 0.001, and P = 0.014, respectively).
3.7 The TyG index and the HOMA-IR of the MetS obesity presence
The HOMA-IR and the TyG indexes are shown in Table 5. The mean of the HOMA-IR and the TyG indexes were statistically significantly different between non-MetS obesity and MetS obesity of the two groups (P ≤ 0.001).
3.8 Predictors of the MetS obesity prevalence ORs
The ORs of the MetS obesity according to the tertiles of the HOMA-IR are shown in Table 5. The OR of the MetS obesity obtained using a logistic regression analysis showed that the prevalence OR of the T3 [OR 33.708; 95% confidence interval (CI) 7.003–162.250; P < 0.001] was 33.7 times as high as the T1. On the other hand, the prevalence OR of the H1 and H3 [OR 2.729; 95% CI 0.890–8.364; P = 0.079] were not statistically significantly different between the tertiles.
4 Discussion
This current study investigated the reliability of the TyG index in the assessment of IR markers through the comparison and analysis of each TyG index and the HOMA-IR value. In addition, the potential of the of TyG index as an advanced tool, was identified. To investigate this effectively, this study compared the TyG index with the HOMA-IR tertile. The current study’s results showed that there were significant differences between the TyG index and HOMA-IR, such as in the anthropometric parameters, characteristics, and body fat compositions and that the TyG index may play a role as an IR assessment index. Additionally, the current study’s results showed that the TyG index was associated with FA concentrations. Thus, the TyG index may an advanced tool that reflects the relevance of FAs-related clinical symptoms and the presence of IR-related chronic disease such as the MetS.
The significant differences between the TyG index and HOMA-IR for lipid profiles and adiponectin were similar. However, only the TyG index tertile and the LDL-cholesterol were significantly different. In addition, the higher TyG index tertile showed significantly increased LDL-cholesterol levels. The results of other studies also suggested that only the TyG index and the LDL-cholesterol were significantly different (Irace et al., 2013). This result may indicate that the TyG index and LDL-cholesterol had a deep relationship because the TyG index was calculated using triglyceride levels. However, it should be noted that this result demonstrated the relationship between the lipid profile in blood and the TyG index rather than with the HOMA-IR. That is to say that the TyG index had a stronger relationship with the lipid profile in the blood than the HOMA-IR.
The C-peptide was significantly different in the TyG index and HOMA-IR. In addition, the hs-CRP was significantly different in the TyG index, compared to the HOMA-IR. These laboratory measurement results were within the normal range. The C-peptide has anti-inflammatory properties at the level of the vascular endothelia and vascular smooth muscle cells that are exposed to varieties of stimulation (Haidet et al., 2009; Luppi et al., 2013). Therefore, C-peptide is utilized as an evaluation factor for the insulin secretion ability (Cauter et al., 1992). A level of hs-CRP in the human blood is a well-known inflammatory marker such as interleukin-12 and interleukin-6. (Can et al., 2011; Snel et al., 2011). Therefore, the TyG index can also play a role in an IR assessment index like the HOMA-IR. Furthermore, the TyG index may be related more to related-inflammatory markers than HOMA-IR.
The n-6 PUFAs have a relationship with pro-inflammation (Wood et al., 2014). In one research study, concerns were raised that an increase in n-6 PUFAs intake could have negative impacts on metabolic health (Ailhaud et al., 2006). This suggestion was based on the fact that n-6 PUFAs increased the risk of pro-inflammation (Innes & Calder, 2018). In this study, although both the IR assessment indexes were divided into tertiles of overweight participants, the TyG index showed a close relationship with the n-6 PUFAs, compared with the HOMA-IR. Some researchers suggested that IR had the same tendency to cause inflammation in humans (Tinius et al., 2020). Furthermore, the several n-3 PUFAs and MUFAs in this study also indicated a significant increase in the TyG index tertiles. Therefore, the findings of the current study indicated an association between the TyG index and FA concentrations, rather than the HOMA-IR. Based on related n-3 PUFAs with protecting endothelial cells and cardiomyocytes (Mollace et al., 2013) and reducing cytokine expression (Calder, 2015), the n-3 PUFAs are considered to have a positive influence as an anti-inflammatories (Giacobbe et al., 2020). According to the body fat composition investigation result (Table 4) obtained using CT and DEXA, in both the TyG index and HOMA-IR, the L1 and L4 of the CT evaluation and the body fat composition of the DEXA evaluation increased significantly. Some studies have suggested that there was relationships between body fat or fat accumulation and the circulating free fatty acids (Ebbert & Jensen, 2013). In this regard, it is natural that both n-6 and n-3 PUFAs levels for overweight participants showed an increase. Nevertheless, this study’s results showed the relationship between the TyG index and n-6 PUFAs compared with the HOMA-IR, more clearly. Furthermore, as the TyG index tertile increased, the n-6 and n-3 were increased significantly and the ratio n-6/n-3 was decreased significantly. In other words, this was indicated that the n-3 increased to a greater degree than the n-6 in the TyG index tertile. In this regard, both the ALA and DPA corresponding to the n-3 increased significantly in the TyG index. Whereas, in the HOMA-IR tertile, the DPA was increased significantly and the DHA decreased significantly. Therefore, the TyG index had a deep relationship between the plasma FAs compared with the HOMA-IR. However, the current study being a cross-sectional study, may have had limitations in explaining a causal relationship between each IR assessment index and the plasma fatty acids. Thus, further study will be needed with longer study periods to investigate the causal relationship between each index and the plasma fatty acids. Some research has suggested that plasma FAs profiles are reflected by the FAs composition of dietary fat and the endogenous FAs synthesis (Katan et al., 1997). In addition, plasma FAs were reflected by the low and homogeneous intake of n-3 PUFAs in the present participants; the plasma FAs levels in the blood sample are at the levels of homeostasis and the low-end of the numerical range observed in humans (Ishihara et al., 2019). However, the current study did not investigate dietary FAs or examine the plasma FAs over longer periods. Thus, further studies of the TyG index will be needed to investigate the relationship between dietary FAs and plasma FAs over longer examination periods.
The results of the TyG index and the HOMA-IR for the presence of MetS obesity were significantly different. The TyG index and ORs of the MetS obese were also significantly different. Navarro-González et al. (2016) suggested that in the case of MetS and unhealthy obese participants, the TyG index and the HOMA-IR were shown to be significantly different. However, in this study, the ORs of the MetS obesity and the HOMA-IR were not significantly different. Some research studies have reported that HOMA-IR and the abdominal obese or metabolic unhealthy obese were significantly different (Berezina et al., 2015), whereas, the T3 had a 33.7 times greater probability of occurring in MetS obesity than in T1. Unlike in the HOMA- IR, these results were supported by references to the deep relationship between the TyG index and the occurrence of MetS obesity. Therefore, the TyG index may be a useful tool that reflects the relevance of the presence of IR-related chronic diseases such as the MetS.
The current study included only the Korean population. Thus, definitions of the general TyG index using plasma fatty acids and body fat compositions may apply only to the Korean or East Asian populations. Application of general TyG index features obtained from the current study may be limited in other populations such as those in the West and in Europe. Moreover, the findings of this study may not have an adequate impact due to the lower number of study participants. Due to the limitations of the current study as a cross-sectional study causal relationships could not be established. Although the results of this study have shown that several factors were significantly different, it was difficult to explain any causal relationships.
In addition, a further limitation of the current study was that it was conducted with limited specific participants, all of whom were overweight with a BMIs of between 25.0 and 29.9 kg/m2 and during a short examination period study. Accordingly, further studies of the TyG index will need a continuous investigation with more subdivisions and a larger number of participants with more details (e.g., criteria of sex, age, and BMI) and exact results.
Nevertheless, based on the results in the current study, the TyG index should be considered reliable as an IR assessment index. Furthermore, the TyG index will be an advanced tool that reflects the relevance of FAs-related clinical symptoms and the presence of IR-related chronic disease such as the MetS. However, it should be noted that the TyG index had shorter application periods than the HOMA-IR (Mohammadabadi et al., 2014).
5 Conclusions
In conclusion, the TyG index has a reliable value as an alternative to the IR assessment index. In addition, the TyG index is an advanced tool that reflects the relevance of the pro-inflammation levels and the presence of IR-related chronic diseases.
References
Ailhaud, G., Massiera, F., Weill, P., Legrand, P., Alessandri, J.-M., & Guesnet, P. (2006). Temporal changes in dietary fats: Role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Progress in Lipid Research, 45, 203–236.
Berezina, A., Belyaeva, O., Berkovich, O., Baranova, E., Karonova, T., Bazhenova, E., Brovin, D., Grineva, E., & Shlyakhto, E. (2015). Prevalence, risk factors, and genetic traits in metabolically healthy and unhealthy obese individuals. BioMed Research International. https://doi.org/10.1155/2015/548734
Calder, P. C. (2015). Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1851, 469–484.
Can, M., Sancar, E., Harma, M., Guven, B., Mungan, G., & Acikgoz, S. (2011). Inflammatory markers in preeclamptic patients. Clinical Chemistry and Laboratory Medicine, 49, 1469–1472.
Ebbert, J. O., & Jensen, M. D. (2013). Fat depots, free fatty acids, and dyslipidemia. Nutrients, 5, 498–508.
Genco, R. J., Grossi, S. G., Ho, A., Nishimura, F., & Murayama, Y. (2005). A proposed model linking inflammation to obesity, diabetes, and periodontal infections. Journal of Periodontology, 76, 2075–2084.
Giacobbe, J., Benoiton, B., Zunszain, P., Pariante, C. M., & Borsini, A. (2020). The anti-inflammatory role of omega-3 polyunsaturated fatty acids metabolites in pre-clinical models of psychiatric, neurodegenerative, and neurological disorders. Frontiers in Psychiatry, 11, 122.
Grundy, S. M., Cleeman, J. I., Daniels, S. R., Donato, K. A., Eckel, R. H., Franklin, B. A., Gordon, D. J., Krauss, R. M., Savage, P. J., & Smith, S. C., Jr. (2005). Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation, 112, 2735–2752.
Haidet, J., Cifarelli, V., Trucco, M., & Luppi, P. (2009). Anti-inflammatory properties of C-peptide. The Review of Diabetic Studies: RDS, 6, 168.
Hosseini, S. M. (2017). Triglyceride–glucose index simulation. Journal of Clinical and Basic Research, 1, 11–16.
Innes, J. K., & Calder, P. C. (2018). Omega-6 fatty acids and inflammation. Prostaglandins, Leukotrienes and Essential Fatty Acids, 132, 41–48.
Irace, C., Carallo, C., Scavelli, F., De Franceschi, M., Esposito, T., Tripolino, C., & Gnasso, A. (2013). Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. International Journal of Clinical Practice, 67, 665–672.
Ishihara, T., Yoshida, M., & Arita, M. (2019). Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. International Immunology, 31, 559–567.
Karelis, A. D. (2008). Metabolically healthy but obese individuals. The Lancet, 372, 1281–1283.
Katan, M., Deslypere, J., Van Birgelen, A., Penders, M., & Zegwaard, M. (1997). Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: An 18-month controlled study. Journal of Lipid Research, 38, 2012–2022.
Livingstone, K., Givens, D., Cockcroft, J., Pickering, J., & Lovegrove, J. (2013). Is fatty acid intake a predictor of arterial stiffness and blood pressure in men? Evidence from the Caerphilly Prospective Study. Nutrition, Metabolism and Cardiovascular Diseases, 23, 1079–1085.
Luppi, P., Kallas, Å., & Wahren, J. (2013). Can C-peptide mediated anti-inflammatory effects retard the development of microvascular complications of type 1 diabetes? Diabetes/Metabolism Research and Reviews, 29, 357–362.
Marceau, P., Biron, S., Hould, F.-S., Marceau, S., Simard, S., Thung, S., & Kral, J. (1999). Liver pathology and the metabolic syndrome X in severe obesity. The Journal of Clinical Endocrinology & Metabolism, 84, 1513–1517.
Mohamed, S. (2014). Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovasular disease. Trends in Food Science & Technology, 35, 114–128.
Mohammadabadi, F., Vafaiyan, Z., Hosseini, S. M., Aryaie, M., & Eshghinia, S. (2014). Assessment of insulin resistance with two methods: HOMA-IR and TyG index in Iranian obese women. Iranian Journal of Diabetes and Obesity, 6, 23–27.
Mohd Nor, N. S., Lee, S., Bacha, F., Tfayli, H., & Arslanian, S. (2016). Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: Comparison with the hyperinsulinemic–euglycemic clamp. Pediatric Diabetes, 17, 458–465.
Mollace, V., Gliozzi, M., Carresi, C., Musolino, V., & Oppedisano, F. (2013). Re-assessing the mechanism of action of n-3 PUFAs. International Journal of Cardiology, 170, S8–S11.
Navarro-González, D., Sánchez-Íñigo, L., Fernández-Montero, A., Pastrana-Delgado, J., & Martinez, J. A. (2016). TyG index change is more determinant for forecasting type 2 diabetes onset than weight gain. Medicine, 95, e3646.
World Health Organization. (2000) International association for the study of obesity, International Obesity Taskforce. The Asia-Pacific perspective: redefining obesity and its treatment, 15–21.
Phillips, D., Caddy, S., Ilic, V., Fielding, B., Frayn, K., Borthwick, A., & Taylor, R. (1996). Intramuscular triglyceride and muscle insulin sensitivity: Evidence for a relationship in nondiabetic subjects. Metabolism, 45, 947–950.
Qin, B., Panickar, K. S., & Anderson, R. A. (2010). Cinnamon: Potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. Journal of Diabetes Science and Technology, 4, 685–693.
Ramírez-Vélez, R., Pérez-Sousa, M. Á., González-Ruíz, K., Cano-Gutierrez, C. A., Schmidt-RioValle, J., Correa-Rodríguez, M., Izquierdo, M., Romero-García, J. A., Campos-Rodríguez, A. Y., & Triana-Reina, H. R. (2019). Obesity-and lipid-related parameters in the identification of older adults with a high risk of prediabetes according to the American diabetes association: An analysis of the 2015 health, well-being, and aging study. Nutrients, 11, 2654.
Simental-Mendía, L. E., Rodríguez-Morán, M., & Guerrero-Romero, F. (2008). The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metabolic Syndrome and Related Disorders, 6, 299–304.
Snel, M., van Diepen, J. A., Stijnen, T., Pijl, H., Romijn, J. A., Meinders, A., Voshol, P., & Jazet, I. M. (2011). Immediate and long-term effects of addition of exercise to a 16-week very low calorie diet on low-grade inflammation in obese, insulin-dependent type 2 diabetic patients. Food and Chemical Toxicology, 49, 3104–3111.
Tinius, R. A., Blankenship, M. M., Furgal, K. E., Cade, W. T., Pearson, K. J., Rowland, N. S., Pearson, R. C., Hoover, D. L., & Maples, J. M. (2020). Metabolic flexibility is impaired in women who are pregnant and overweight/obese and related to insulin resistance and inflammation. Metabolism, 104, 154142.
Van Cauter, E., Mestrez, F., Sturis, J., & Polonsky, K. S. (1992). Estimation of insulin secretion rates from C-peptide levels: Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes, 41, 368–377.
Wallace, T. M., Levy, J. C., & Matthews, D. R. (2004). Use and abuse of HOMA modeling. Diabetes Care, 27, 1487–1495.
Warensjö, E., Öhrvall, M., & Vessby, B. (2006). Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutrition, Metabolism and Cardiovascular Diseases, 16, 128–136.
Wood, K., Lau, A., Mantzioris, E., Gibson, R., Ramsden, C., & Muhlhausler, B. (2014). A low omega-6 polyunsaturated fatty acid (n-6 PUFA) diet increases omega-3 (n-3) long chain PUFA status in plasma phospholipids in humans. Prostaglandins, Leukotrienes and Essential Fatty Acids, 90, 133–138.
Yanni, A. E., Stamataki, N. S., Konstantopoulos, P., Stoupaki, M., Abeliatis, A., Nikolakea, I., Perrea, D., Karathanos, V. T., & Tentolouris, N. (2018). Controlling type-2 diabetes by inclusion of Cr-enriched yeast bread in the daily dietary pattern: A randomized clinical trial. European Journal of Nutrition, 57, 259–267.
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
No funding was provided.
Author information
Authors and Affiliations
Contributions
SRJ analyzed and visualized the formal data, wrote the original draft, and reviewed and edited the manuscript, conceptualization, data curating, investigation, acquisition, and analyzing of the data. Also, contributed to the conceptualization, project administration, and supervision. JHL contributed to the conceptualization, data curation, interpretation of the data, project administration and supervision. Both authors critically revised the manuscript, read and approved the final manuscript, and agreed to be held fully accountable for the integrity and accuracy of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jeong, S., Lee, J.H. The verification of the reliability of a triglyceride–glucose index and its availability as an advanced tool. Metabolomics 17, 97 (2021). https://doi.org/10.1007/s11306-021-01837-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-021-01837-9