Abstract
Background
Being physically active has multiple salutary effects on human health, likely mediated by changes in energy metabolism. Recent reviews have summarized metabolomic responses to acute exercise. However, metabolomic profiles of individuals who exercise regularly are heterogeneous.
Aim of review
We conducted a systematic review to identify metabolites associated with physical activity (PA), fitness, and sedentary time in community-dwelling adults and discussed involved pathways. Twenty-two studies were eligible because they (1) focused on community-dwelling adults from observational studies; (2) assessed PA, fitness, and/or sedentary time, (3) assessed metabolomics in biofluid, and (4) reported on relationships of metabolomics with PA, fitness, and/or sedentary time.
Key scientific concepts of review
Several metabolic pathways were associated with higher PA and fitness and less sedentary time, including tricarboxylic acid cycle, glycolysis, aminoacyl-tRNA biosynthesis, urea cycle, arginine biosynthesis, branch-chain amino acids, and estrogen metabolism. Lipids were strongly associated with PA. Cholesterol low-density lipoproteins and triglycerides were lower with higher PA, while cholesterol high-density lipoproteins were higher. Metabolomic profiles of being physically active and less sedentary indicate active skeletal muscle biosynthesis supported by enhanced oxidative phosphorylation and glycolysis and associated with profound changes in lipid and estrogen metabolism. Future longitudinal studies are needed to understand whether these metabolomic changes account for health benefits associated with PA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Accumulating evidence shows that engaging in physical activity (PA) has multiple salutary effects on human health. Acute exercise exerts profound effects on many biological pathways (Sakaguchi et al., 2019; Schranner et al., 2020). However, metabolic profiles that define physical fitness and/or regular exercise participation are not established.
Metabolomics simultaneously quantifies numerous metabolites with low molecular weights, which are the final products of upstream biological processes and many other physiological functions (Wishart, 2019). It allows for the identification of biological significance underlying changes in metabolism and/or metabolic pathways, a collection of biochemical reactions catalyzed by different enzymes (Hu et al., 2020). Because metabolites are intermediates or products of cellular metabolism, it helps to understand mechanisms underlying the effects of health behaviors on human health and diseases. To this end, metabolomics can provide a comprehensive understanding of metabolic changes associated with chronic adaptations to physical activity (PA). Previous review studies have suggested that the effect of acute exercise may be the opposite of the effect of being physically active over the long term. For example, acute exercise increases oxidative stress and inflammation, while chronic adaptations to regular PA decrease oxidative stress and may also reduce the pro-inflammatory state (Anderson et al., 2016; Mastaloudis et al., 2001; Nikolaidis et al., 2011).
In this systematic review, we aimed to identify metabolites that were associated with PA, fitness, and/or sedentary time in community-dwelling adults, and to identify potential pathways implicated in the benefits of regular PA participation. We hypothesized that metabolite adaptation induced by regular PA participation would share similarities with the effect of acute exercise on TCA cycle intermediates, lipid-related metabolites, and fatty acids. A more advanced understanding of metabolomic profiles of being physically active and fit can provide valuable insights into early prevention and treatment for health conditions that are often age-related, such as cardiovascular and metabolic diseases.
2 Methods
2.1 Literature search and study selection
We followed PRISMA guidelines to conduct this systematic review (Liberati et al., 2009). One author (QT) searched literature that was written in English and published after January 1, 1999, using PubMed and Scopus databases. In the PubMed database, the search terms included (1) "Metabolomics"[Mesh] OR “Metabolite”[tw] OR “Metabolome”[tw], AND (2) "Sedentary Behavior"[Mesh] OR "Cardiorespiratory Fitness"[Mesh] OR "Exercise"[Mesh] OR “Physical activity”[tw] OR “Sedentary time”[tw] OR “Fitness”[tw]. PubMed search retrieved 421 records. In the Scopus database, the same search terms were used; TITLE-ABS-KEY((metabolomics OR metabolite* OR Metabolome) AND (sedentary time* OR fitness OR exercise OR physical activity)). Scopus search retrieved 287 records. Four additional records that were not shown in the PubMed or Scopus search were added. These four records were discovered in relevant literature or based on authors’ knowledge.
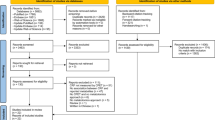
After removing 26 duplicated records, two authors (QT and RM) evaluated the remaining 677 records. Because this systematic review focused on observational studies of community-dwelling adults and biofluid-based metabolomics, we first excluded the following records after screening by title and abstract, including reviews, case reports, intervention studies, studies of acute exercise or other exposures, and studies of unique populations, studies of muscle metabolites, and animal studies (Fig. 1). We further excluded studies that met the following exclusion criteria: (1) biofluid-based metabolomics data were not collected, or only a limited number of metabolites were examined (less than 10), (2) data on physical activity (PA), fitness, and/or sedentary time were not collected, or (3) there were no reported relationships of biofluid-based metabolomics with PA, fitness, and/or sedentary time. A total of 22 studies were eligible and included in this systematic review (Fig. 1).
2.2 Analysis
We first evaluated the quality of all 22 studies based on study background and methodology. Three authors scored separately, discussed scores in each study, and reached a consensus on the final assessment (QT, AC, and RM). Criteria and scoring are presented in Supplementary Table 1.
We then summarized key elements of each study, including study cohort, sample size, age, sex, assessment of PA, fitness, and/or sedentary time, sample type, metabolomics technique, number of metabolites examined, and study quality scores. Details of these studies are presented in Table 1, sorted by the number of metabolites examined.
We analyzed metabolites that were associated with PA, fitness, and/or sedentary time (Fig. 2 for Venn diagram) and identified the direction of the associations (Supplementary Figs. 1 and 2 for heatmaps). Of note, when more than one study found the same metabolites, we reported the findings from the study with higher methodology scores. If there was a tie in methodology scores, we reported the findings from the study with higher overall scores.
Venn diagram of metabolites that are upregulated (red) and downregulated (blue) with higher physical activity, higher fitness, and less sedentary time. CA Carboxylic acid-containing metabolites. LPL lysophosphatidylcholines or lysophosphatidylethanolamines. PL phosphatidylcholines, phosphatidylethanolamines, phosphatidylinositol, or phosphoglycerides. *Factor Score
We further conducted the KEGG pathway analysis and SMPDB enrichment analysis via https://www.metaboanalyst.ca/. As various databases exist for metabolomics pathway analysis, we used both KEGG and SMPDB as complementary databases to capture an accurate depiction of the metabolome. Compared to KEGG, SMPDB is focused on small molecules and unique to humans (Frolkis et al., 2010). First, we identified HMDB IDs for metabolites that were associated with PA, fitness, and/or sedentary time. Only metabolites with unique HMDB IDs were considered for pathway analysis and enrichment analysis. We then entered HMDB IDs into the KEGG database for pathway analysis and the SMPDB database for enrichment analysis separately. Pathway analysis parameters were as follows: input type = HMDB ID, visualization method = scatter plot (testing significant features), enrichment method = Hypergeometric Test, Topology analysis = Relative-betweenness Centrality, Reference metabolome = use all compounds in KEGG (homo sapiens) library. Enrichment analysis parameters were as follows: input type = HMDB ID, feature type: metabolites, pathway-based = SMPDB (99 metabolite sets based on normal human metabolic pathways), only use metabolite sets containing at least 2 entries, and use all the compounds in the selected library. Significant pathways were reported at an FDR-adjusted p value < 0.05 or p value < 0.05 as appropriate.
3 Results
3.1 Study design and population
All 22 studies included in this systematic review were cross-sectional. Concerning the quality of the studies, 2 were considered fair, 15 good, and 5 excellent (Supplementary Table 1).
The age of participants ranged from 18 to 96 years. Of the 22 studies, 14 studies included both men and women, 5 examined women only, and 3 examined men only. Study populations were geographically diverse with 10 from Europe, 8 from North America, and 4 from Asia (Table 1).
3.2 Assessment of physical activity, fitness, and sedentary time
PA and fitness are correlated but also distinct in their concepts and assessment methods. In general, PA, fitness, and sedentary time can be quantified by self-report questionnaires or by objective devices or fitness tests. Compared to subjective measures, objective measures of PA can capture the whole spectrum of activity levels, especially low-intensity activities. Of 22 studies, 7 used subjective measures, such as self-report questionnaires with questions regarding the total amount of PA, intensity, duration, frequency, and type of activities; 14 studies used objective measures, such as graded treadmill test, step test, and cycle ergometer test for fitness, and accelerometers or doubly labeled water for PA and sedentary time. One study used both subjective and objective measures to assess PA (Table 1, Supplementary Table 1).
3.3 Metabolomics
We categorized metabolomics techniques as relative/semi-quantitative, mixed, and quantitative which were scored as 1, 2, and 3, respectively. Of 22 studies, 4 used semi-quantitative/relative methods, 8 used mixed methods, and 10 used quantitative methods (Table 1, Supplementary Table 1). Regarding the analytical platform, 17 studies used mass spectrometry, 6 used nuclear magnetic resonance, and 1 used immunoassay. Across 22 studies, the number of metabolites examined ranged from 11 to 337 (Table 1).
3.4 Metabolites associated with PA, fitness, and/or sedentary time
A complete list of metabolites associated with PA, fitness, and/or sedentary time is summarized in a heatmap (Supplementary Figs. 1 and 2). In this section, we specifically focused on metabolites that were consistently associated with at least two parameters (PA, fitness, and/or sedentary time).
Metabolites associated with lower levels of PA/fitness and/or more sedentary time were from classes of carbohydrates (glucose, mannose), carboxylic acid-containing metabolites (lactate, 2-Oxoisopentanoate, 4-Methyl-2-Oxopentanoate), estrogens (2-Methoxyestradiol, 2-Methoxyestrone, 4-Methoxyestradiol, estradiol, estrone, Unconjugated Oestrone), fatty acids (linoleic acid, MUFA, MUFA: total FA ratio, omega 3 FA, omega 6 FA, PUFAs, saturated FA, total FAs), glycoproteins (Alpha-Acid Glycoprotein), ketone bodies (Acetoacetate), proteins/peptides (Gamma-Glutamylvaline), and triglycerides (HDL-TG, IDL-TG, LDL-TG, M-HDL-TG, S-HDL-TG, S-LDL-TG, S-VLDL-TG, total triglycerides, total VLDL-TG, triglyceride, VLDL-TG (M, L, XL), XS-VLDL-TG, XXL-VLDL-TG).
In classes of amino acids (AA), cholesterol, and lipoprotein particles, some metabolites were upregulated among active and fit individuals (i.e. AA: glutamine, glycine, ornithine, betaine; cholesterol: HDL2-C, HDL-C), while some metabolites were downregulated (AA: alanine, isoleucine, leucine, methionine, phenylalanine, proline, tyrosine, valine, 2-hydroxybutyrate, alpha-hydroxyisovalerate, pyruvate; cholesterol: LDL-C, remnant-C, S-VLDL-C, total cholesterol, VLDL-C, XL-VLDL-C).
3.5 Pathway analysis
The KEGG pathway analysis revealed three pathways that were related to PA, fitness, and sedentary time (FDR p < 0.05), including aminoacyl-tRNA biosynthesis, valine, leucine, and isoleucine biosynthesis, and valine, leucine, and isoleucine degradation (Table 2). The SMPDB enrichment analysis showed several top enrichment pathways related to PA, fitness, and sedentary time, including urea cycle, glucose and alanine cycle, and glycine and serine metabolism (Fig. 3; Supplementary Table 2).
4 Discussion
Overall, multiple metabolites appear to be affected by levels of PA, fitness, and sedentary time. We found that metabolite differences with habitual PA levels share several similarities with those observed after acute exercise or exercise interventions. These similarities include changes in lipids, branch-chain amino acids (BCAAs), and TCA cycle intermediate metabolites, fatty acids, and ketone bodies (Kelly et al., 2020; Sakaguchi et al., 2019; Schranner et al., 2020). While it is difficult to fully understand the role of every metabolite in the metabolic adaptation to PA, the enrichment analysis associated with PA, fitness, and sedentary time identified six specific metabolic pathways; urea cycle, arginine biosynthesis (NO cycle), alanine, aspartate, and glutamate metabolism (TCA cycle), glycine, serine, and threonine metabolism, and valine, leucine, and isoleucine biosynthesis/ degradation.
4.1 Theme 1 Valine, leucine, and isoleucine biosynthesis/degradation
BCAAs are metabolized in muscle tissue by mitochondrial dehydrogenase and branch-chain ketoacid dehydrogenase to produce the branch-chain keto acids (Riddle et al., 2016; Shimomura et al., 2004; Thalacker-Mercer et al., 2020; Valerio et al., 2011). The generated ketoacids are used by the muscle to generate ATP via Kreb’s cycle or transported to the liver for oxidation. BCAA catabolism is an important metabolic process that aids in protein synthesis and energy production. BCAAs are sensed by mTOR as biomarkers of energy and substrate availability (Holecek, 2018). Through this mechanism, BCAAs promote protein synthesis through the S6Kinase and increase mitochondrial biogenesis by stimulating SIRT1 (Nie et al., 2018). In this review, we found that the majority of BCAAs (leucine, isoleucine, valine) were decreasing with higher levels of PA and fitness and less sedentary time, which was consistent with previous findings of exercise training decreasing BCAAs in serum (Pitkanen et al., 2002). Our findings are also in line with recent findings that participants with high muscle quality had lower levels of BCAAs in plasma compared to those with low muscle quality (Moaddel et al., 2016). Notably, in a preliminary study of 18 participants, higher circulating levels of BCAAs were observed in biopsy specimens collected from vastus lateralis of participants with high muscle quality compared to low muscle quality (Gonzalez-Freire et al., 2018). This may suggest that a decrease of BCAAs in circulation may correlate with a corresponding increase in vastus lateralis where it can be used for energy and protein synthesis. It has been hypothesized that PA upregulates the BCAA catabolic pathway, critical in energy production and protein synthesis, and may play an intermediary role between exercise and health benefits (Xiao et al., 2016). Consistent with this hypothesis, fit individuals had lower levels of glutamate, an intermediate of BCAA catabolism. Additionally, the dehydrogenation product of leucine and acetoacetate is ketogenic. The concomitant decrease in branched-chain α-keto acids (BCKAs) metabolites 4-methyl-2-oxopentanoate, 3-methyl-2-oxovalerate and alpha-hydroxyisovalerate is consistent with decreased BCAAs in circulation. Notably, isoleucine and 4-methyl-2-oxopentanoate (BCKA), derived from valine, are also lower with higher levels of PA and fitness, and less sedentary time. BCAAs are converted to BCKAs in the mitochondria and subsequently used in the production of acylcarnitines. BCKAs have been suggested as a predictive biomarker for type 2 diabetes and impaired fasting glucose (Menni et al., 2013).
4.2 Theme 2. Aminoacyl-tRNA biosynthesis
Transfer RNAs (tRNA) are central nucleic acid molecules, and their interaction with aminoacyl-tRNA synthetase protein enzymes defines the specificities of proteins for the genetic code (Borchers & Pieler, 2010). Aminoacyl-tRNA synthetases (ARSs) are ubiquitously expressed enzymes essential for the first step in protein synthesis and can also play non-catalytic roles in diverse biological processes (Boczonadi et al., 2018; Lee et al., 2004; Ognjenovic & Simonovic, 2018; PouralijanAmiri et al., 2020). In this review, ARSs are identified as a significant pathway associated with higher PA and fitness, and less sedentary time, with 6 common significant metabolites (Glutamine, Methionine, Valine, Alanine, Isoleucine, Leucine). This pattern is consistent with the role of aminoacyl-tRNA synthetase in protein synthesis. Further, the ARSs pathway has been identified in several studies as a significant pathway for high-intensity interval training (HIIT) (Castro et al., 2019; Robinson et al., 2017). Another study of a combined 8-week continuous endurance training and HIIT program identified ARSs and carbohydrate and amino acid metabolism as the most common pathways associated with baseline serum metabolite levels (Castro et al., 2019). These findings are also consistent with recent studies demonstrating that essential amino acids and resistance exercise are stimulators of skeletal muscle protein synthesis in human and animal models (Drummond et al., 2009).
4.3 Theme 3. Arginine biosynthesis (urea cycle, NO cycle)
Several metabolites in arginine metabolism, including glutamine, arginine, citrulline, and ornithine, tended to be higher with higher PA, whereas glutamate levels were lower. The increase in arginine, citrulline and ornithine suggests a role in both the nitric oxide (NO) cycle and urea cycle. The concomitant increase in arginine and citrulline suggests an increase in circulating NO levels with higher levels of PA and fitness, consistent with NO’s role as a powerful vasodilatory. NO is a signaling molecule involved in many physiological and pathological processes. Previous studies have also shown that exercise training stimulates NO synthesis in endothelial cells (Nosarev et al., 2014), stimulates the release of NO (Lewis et al., 1999; Nosarev et al., 2014; Tsukiyama et al., 2017), and increases NO in skeletal muscle in response to exercise (Dyakova et al., 2015). While electron paramagnetic resonance spectroscopy is used for the direct measurement of NO, indirect methods, such as measurement of NOS activities and/or activation of molecular targets, or products of reactions of NO, are predominantly used (Csonka et al., 2015). Recently, the global arginine bioavailability ratio (arginine to [ornithine + citrulline] ratio) has been identified as a proxy measure of NO synthetic capacity in vivo (Baranyi et al., 2015, Ali-Sisto et al. 2018, Moaddel et al., 2018). Future studies are needed to explore ratio measures, which may offer a window to new therapeutic targets. The concomitant increase in arginine, ornithine, and creatinine also suggests increasing levels of urea via the urea cycle. This is consistent with previous findings reporting an increase in circulating urea levels after prolonged exercise and suggesting new protein synthesis is paralleled by high rates of protein degradation, possibly due to the chronic microdamage accumulation during muscle contraction (Haralambie & Berg, 1976).
4.4 Theme 4. Glycolysis and TCA cycle (Alanine, aspartate, and glutamate metabolism)
Glucose levels were lower with higher levels of PA and fitness, and/or less sedentary time. This finding is consistent with previous studies showing decreased glucose after acute exercise (Sakaguchi et al., 2019; Schranner et al., 2020) and chronic improvements in insulin sensitivity and superior baseline glycemic control with regular exercise (Bird & Hawley, 2016). These findings further support the notion that increasing fitness is beneficial in the prevention and control of diabetes, in addition to reducing the risk of cardiovascular mortality (Riddell & Perkins, 2009). The end-products of glycolysis, pyruvate, and lactate, were also decreasing with higher PA (Gray et al., 2014), consistent with previous findings that glycolytic pathway metabolites decreased after exercise training (Schranner et al., 2020). It is important to highlight that since there is an upregulation of aerobic metabolism, there may be less need to access the extra energy required for glycolysis. Pyruvate drives several major biosynthetic pathways intersecting the citric acid cycle (Gray et al., 2014). It is worth noting that the lactate/pyruvate ratio is an indicator of mitochondrial function (Parikh et al., 2015). Whether this ratio is related to PA or exercise requires further investigation.
Several studies have shown that citrate synthase activity in human muscles increased after exercise training and acute exercise (Bruce et al., 2004; Carter et al., 2001; Coggan et al., 1993; Howarth et al., 2004; Leek et al., 2001). Interestingly, while glucose, pyruvate, lactate, and alanine were found lower with higher PA, citrate was not significantly associated with PA, fitness, or sedentary time in three out of four studies reporting citrate (Kujala et al., 2019; Pang et al., 2019; Xiao et al., 2016). Only one study reported a modest increase in serum levels with higher PA (Kujala et al., 2013). This is consistent with recent reports that prolonged exercise training attenuates the exercise-induced increase in TCA cycle intermediates in vastus lateralis (Howarth et al., 2004). It is also possible that these changes could be detected in muscles but may not be detected in circulation because of the integrity of mitochondria. These data suggest that TCA cycle intermediate metabolites increase at the start of training but then stabilize when mitochondrial biogenesis has coped with the increased demand for regular PA.
4.5 Theme 5. Lipids
Several lipids were downregulated in individuals with high levels of PA and fitness, including specific lipoprotein particles, cholesterol and triglycerides, and fatty acids. Several LDL particles were found lower with higher PA and fitness, including cholesterol from LDL and VLDL particles. Correspondingly, with higher PA and fitness and less sedentary time, we observed a concomitant decrease in cholesterol, and triglyceride-containing particles (Lu et al., 2011). Conversely, higher PA and fitness and less sedentary time were associated with higher levels of cholesterol from HDL particles, which was consistent with previous reports that ApoA1 was upregulated with higher PA, fitness, and less sedentary time (Gu et al., 2018; Kujala et al., 2013, 2019; Pang et al., 2019). ApoA1, a major apolipoprotein in HDL, has both antioxidant and anti-inflammatory effects and can stimulate endothelial production of nitric oxide (Lu et al., 2011). The increase in HDL and ApoA1 with higher PA and fitness is further supported by the increase in cholesterol esters, phosphatidylcholines, and sphingomyelin because these lipids are present in HDL. There is evidence that HDL particles contribute to the efflux of cholesterol from the arterial endothelium which prevents the initial phase of the atherosclerotic process. The findings of this review of the literature are consistent with the results of exercise intervention studies showing that aerobic exercise improves the lipid profile, particularly increasing HDL-C (Mann et al., 2014; Wang & Xu, 2017). A recent aerobic exercise intervention study reported a small but statistically significant reduction in total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglycerides ranging in a span of 0.08 mmol/L to 0.10 mmol/L, as well as an increase in HDL-C about 0.05 mmol/L (Patel et al., 2017).
4.6 Theme 6. Estrogen
In our review, six studies examined estrogen metabolites in women. Parent estrogens, including estrone, conjugated estrone, unconjugated estrone, estradiol, and testosterone, were downregulated in physically active and fit women. This pattern was detected in both premenopausal and postmenopausal women and findings were consistent in urine and serum samples. Most estrogen metabolites in three estrogen metabolism pathways (2-, 4-, and 16-Hydroxylation) were consistently downregulated, such as 2-Hydroxyestrone-3-methyl-ether, 2-methoxyestrone, 2-methoxyestrone, 4-hydroxyestrone, 4-methoxyestrone, 16-Epiestriol, and 17-Epiestriol. Relative to parent estrogen, increased hydroxylation at 2-, 4-, and 6-sites was associated with higher PA and fitness or less sedentary time. Collectively, these data suggest that high levels of PA may increase estrogen hydroxylation and metabolism and subsequently lower urinary or serum estrogen levels. Notably, the 2-Hydroxyestrone/16α-Hydroxyestrone ratio, lower levels of which predict breast cancer risk, is found higher in women with higher levels of PA and fitness, and less sedentary time. Findings on the effect of long-term exercise training on estrogen metabolites are somewhat mixed. Some studies reported that a 12-month exercise intervention increased the 2-Hydroxyestrone/16α-Hydroxyestrone ratio in healthy premenopausal women (Smith et al., 2013), and increased fitness by exercise intervention was associated with decreased levels of 2-pathway and 2-Hydroxyestrone concentrations in postmenopausal women (Matthews et al., 2018). Others did not find a significant effect of 12-month exercise training on 2-Hydroxyestrone, 16α-Hydroxyestrone, or 2-Hydroxyestrone/16α-Hydroxyestrone ratio in either postmenopausal women (Atkinson et al., 2004) or premenopausal women (Campbell et al., 2007). These discrepancies may be due to variations in sample characteristics, such as menopause status and hormone treatment, exercise intervention components, or measurements of estrogen metabolites. Future studies are needed to understand whether being physically active and fit affects the estrogen metabolomic profile in a meaningful direction in both men and women and whether menopause status or aging affects such profile.
As hypothesized, the metabolomic profiles of high levels of PA and fitness share some consistencies with metabolite changes to acute exercise but also differ in several aspects. First, while there is a strong signature of increased mitochondrial biogenesis and enhancement of energy metabolism immediately after acute exercise, blood metabolites that convey these signatures become less evident as individuals assume an active lifestyle. Second, while there is an overall increase in TCA intermediates, lactate, and pyruvate during and immediately after acute exercise, the TCA cycle appears to be more efficient in individuals with regular PA participation.
This study has several strengths. First, to address the diversity of metabolomics and other measures, we evaluated the quality of each study and summarized metabolite findings based on scoring. Second, to comprehensively capture the effect of PA on metabolites, we considered three measures, including PA, fitness, and sedentary time. Third, we conducted both pathway analyses and enrichment pathway analyses to gain an understanding of the related pathways involved. Limitations also exist. First, due to limited data on the relationship between metabolomics and sedentary time, it was difficult to compare patterns across three measures. Second, this review only focused on metabolites measured in biofluids.
5 Conclusion
The metabolomic profiles of physically active, fit, and less sedentary individuals may indicate a favorable biosynthesis of macromolecules, likely in skeletal muscle. This is supported by enhanced oxidative phosphorylation and glycolysis and improved lipid and estrogen metabolism, arginine biosynthesis, and an efficient TCA cycle. Notably, these findings are based on observational studies and only some of the findings reported here are confirmed in intervention studies. Future research on intervention studies is needed to confirm these findings. Whether metabolic changes are in the causal pathways to the benefits of being physically active and fit on health and longevity warrants future investigations.
References
Anderson, C., Milne, G. L., Sandler, D. P., & Nichols, H. B. (2016). Oxidative stress in relation to diet and physical activity among premenopausal women. British Journal of Nutrition, 116(8), 1416–1424. https://doi.org/10.1017/S0007114516003226
Assi, N., Gunter, M. J., Thomas, D. C., Leitzmann, M., Stepien, M., Chajes, V., Philip, T., Vineis, P., Bamia, C., Boutron-Ruault, M. C., Sandanger, T. M., Molinuevo, A., Boshuizen, H., Sundkvist, A., Kuhn, T., Travis, R., Overvad, K., Riboli, E., Scalbert… Ferrari, P. (2018) Metabolic signature of healthy lifestyle and its relation with risk of hepatocellular carcinoma in a large European cohort.American Journal of Clinical Nutrition108(1), 117-126. https://doi.org/10.1093/ajcn/nqy074
Atkinson, C., Lampe, J. W., Tworoger, S. S., Ulrich, C. M., Bowen, D., Irwin, M. L., Schwartz, R. S., Rajan, B. K., Yasui, Y., Potter, J. D., & McTiernan, A. (2004). Effects of a moderate intensity exercise intervention on estrogen metabolism in postmenopausal women. Cancer Epidemiology and Prevention Biomarkers, 13(5), 868–874.
Bird, S. R., & Hawley, J. A. (2016). Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport & Exercise Medicine, 2(1), e000143. https://doi.org/10.1136/bmjsem-2016-000143
Boczonadi, V., Jennings, M. J., & Horvath, R. (2018). The role of tRNA synthetases in neurological and neuromuscular disorders. FEBS Letters, 592(5), 703–717. https://doi.org/10.1002/1873-3468.12962
Borchers, A., & Pieler, T. (2010). Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes (basel), 1(3), 413–426. https://doi.org/10.3390/genes1030413
Bruce, C. R., Kriketos, A. D., Cooney, G. J., & Hawley, J. A. (2004). Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with Type 2 diabetes. Diabetologia, 47(1), 23–30. https://doi.org/10.1007/s00125-003-1265-7
Campbell, K. L., Westerlind, K. C., Harber, V. J., Bell, G. J., Mackey, J. R., & Courneya, K. S. (2007). Effects of aerobic exercise training on estrogen metabolism in premenopausal women: A randomized controlled trial. Cancer Epidemiology, Biomarkers & Prevention, 16(4), 731–739. https://doi.org/10.1158/1055-9965.EPI-06-0784
Carter, S. L., Rennie, C. D., Hamilton, S. J., & Tarnopolsky. . (2001). Changes in skeletal muscle in males and females following endurance training. Canadian Journal of Physiology and Pharmacology, 79(5), 386–392.
Castro, A., Duft, R. G., Ferreira, M. L. V., Andrade, A. L. L., Gaspari, A. F., Silva, L. M., Oliveira-Nunes, S. G., Cavaglieri, C. R., Ghosh, S., Bouchard, C., & Chacon-Mikahil, M. P. T. (2019). Association of skeletal muscle and serum metabolites with maximum power output gains in response to continuous endurance or high-intensity interval training programs: The TIMES study - A randomized controlled trial. PLoS ONE, 14(2), e0212115. https://doi.org/10.1371/journal.pone.0212115
Chorell, E., Svensson, M. B., Moritz, T., & Antti, H. (2012). Physical fitness level is reflected by alterations in the human plasma metabolome. Molecular BioSystems, 8(4), 1187–1196. https://doi.org/10.1039/c2mb05428k
Coggan, A. R., Abduljalil, A. M., Swanson, S. C., Earle, M. S., Farris, J. W., Mendenhall, L. A., & Robitaille, P. M. (1993). Muscle metabolism during exercise in young and older untrained and endurance-trained men. Journal of Applied Physiology, 75(5), 2125–2133. https://doi.org/10.1152/jappl.1993.75.5.2125
Csonka, C., Pali, T., Bencsik, P., Gorbe, A., Ferdinandy, P., & Csont, T. (2015). Measurement of NO in biological samples. British Journal of Pharmacology, 172(6), 1620–1632. https://doi.org/10.1111/bph.12832
Dallal, C. M., Brinton, L. A., Matthews, C. E., Pfeiffer, R. M., Hartman, T. J., Lissowska, J., Falk, R. T., Garcia-Closas, M., Xu, X., Veenstra, T. D., & Gierach, G. L. (2016). Association of active and sedentary behaviors with postmenopausal estrogen metabolism. Medicine and Science in Sports and Exercise, 48(3), 439–448. https://doi.org/10.1249/MSS.0000000000000790
Ding, M., Zeleznik, O. A., Guasch-Ferre, M., Hu, J., Lasky-Su, J., Lee, I. M., Jackson, R. D., Shadyab, A. H., LaMonte, M. J., Clish, C., Eliassen, A. H., Sacks, F., Willett, W. C., Hu, F. B., Rexrode, K. M., & Kraft, P. (2019). Metabolome-wide association study of the relationship between habitual physical activity and plasma metabolite levels. American Journal of Epidemiology, 188(11), 1932–1943. https://doi.org/10.1093/aje/kwz171
Drummond, M. J., Dreyer, H. C., Fry, C. S., Glynn, E. L., & Rasmussen, B. B. (2009). Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. Journal of Applied Physiology, 106(4), 1374–1384. https://doi.org/10.1152/japplphysiol.91397.2008
Dyakova, E. Y., Kapilevich, L. V., Shylko, V. G., Popov, S. V., & Anfinogenova, Y. (2015). Physical exercise associated with NO production: Signaling pathways and significance in health and disease. Frontiers in Cell and Developmental Biology. https://doi.org/10.3389/fcell.2015.00019
Fabbri, E., Yang, A., Simonsick, E. M., Chia, C. W., Zoli, M., Haughey, N. J., Mielke, M. M., Ferrucci, L., & Coen, P. M. (2016). Circulating ceramides are inversely associated with cardiorespiratory fitness in participants aged 54–96 years from the Baltimore Longitudinal Study of Aging. Aging Cell, 15(5), 825–831. https://doi.org/10.1111/acel.12491
Floegel, A., Wientzek, A., Bachlechner, U., Jacobs, S., Drogan, D., Prehn, C., Adamski, J., Krumsiek, J., Schulze, M. B., Pischon, T., & Boeing, H. (2014). Linking diet, physical activity, cardiorespiratory fitness and obesity to serum metabolite networks: Findings from a population-based study. International Journal of Obesity , 38(11), 1388–1396. https://doi.org/10.1038/ijo.2014.39
Frolkis, A., Knox, C., Lim, E., Jewison, T., Law, V., Hau, D. D., Liu, P., Gautam, B., Ly, S., Guo, A. C., Xia, J., Liang, Y., Shrivastava, S., & Wishart, D. S. (2010). SMPDB: The Small Molecule Pathway Database. Nucleic Acids Research, 38, D480-487. https://doi.org/10.1093/nar/gkp1002
Fukai, K., Harada, S., Iida, M., Kurihara, A., Takeuchi, A., Kuwabara, K., Sugiyama, D., Okamura, T., Akiyama, M., Nishiwaki, Y., Oguma, Y., Suzuki, A., Suzuki, C., Hirayama, A., Sugimoto, M., Soga, T., Tomita, M., & Takebayashi, T. (2016). metabolic profiling of total physical activity and sedentary behavior in community-dwelling men. PLoS ONE, 11(10), e0164877. https://doi.org/10.1371/journal.pone.0164877
Gonzalez-Freire, M., Adelnia, F., Moaddel, R., & Ferrucci, L. (2018). Searching for a mitochondrial root to the decline in muscle function with ageing. Journal of Cachexia, Sarcopenia and Muscle, 9(3), 435–440. https://doi.org/10.1002/jcsm.12313
Gray, L. R., Tompkins, S. C., & Taylor, E. B. (2014). Regulation of pyruvate metabolism and human disease. Cellular and Molecular Life Sciences, 71(14), 2577–2604. https://doi.org/10.1007/s00018-013-1539-2
Gu, Q., Spinelli, J. J., Dummer, T. B. J., McDonald, T. E., Moore, S. C., & Murphy, R. A. (2018). Metabolic profiling of adherence to diet, physical activity and body size recommendations for cancer prevention. Science and Reports, 8(1), 16293. https://doi.org/10.1038/s41598-018-34662-7
Haralambie, G., & Berg, A. (1976). Serum urea and amino nitrogen changes with exercise duration. European Journal of Applied Physiology and Occupational Physiology, 36(1), 39–48. https://doi.org/10.1007/BF00421632
Holecek, M. (2018). Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutrition & Metabolism (london), 15, 33. https://doi.org/10.1186/s12986-018-0271-1
Howarth, K. R., LeBlanc, P. J., Heigenhauser, G. J., & Gibala, M. J. (2004). Effect of endurance training on muscle TCA cycle metabolism during exercise in humans. Journal of Applied Physiology, 97(2), 579–584. https://doi.org/10.1152/japplphysiol.01344.2003
Hu, L., Liu, J., Zhang, W., Wang, T., Zhang, N., Lee, Y. H., & Lu, H. (2020). Functional metabolomics decipher biochemical functions and associated mechanisms underlie small-molecule metabolism. Mass Spectrometry Reviews, 39(5–6), 417–433. https://doi.org/10.1002/mas.21611
Kelly, R. S., Kelly, M. P., & Kelly, P. (2020). Metabolomics, physical activity, exercise and health: A review of the current evidence. Biochimica Et Biophysica Acta, Molecular Basis of Disease, 1866(12), 165936. https://doi.org/10.1016/j.bbadis.2020.165936
Koh, A. S., Gao, F., Tan, R. S., Zhong, L., Leng, S., Zhao, X., Fridianto, K. T., Ching, J., Lee, S. Y., Keng, B. M. H., Yeo, T. J., Tan, S. Y., Tan, H. C., Lim, C. T., Koh, W. P., & Kovalik, J. P. (2018). Metabolomic correlates of aerobic capacity among elderly adults. Clinical Cardiology, 41(10), 1300–1307. https://doi.org/10.1002/clc.23016
Kujala, U. M., Makinen, V. P., Heinonen, I., Soininen, P., Kangas, A. J., Leskinen, T. H., Rahkila, P., Wurtz, P., Kovanen, V., Cheng, S., Sipila, S., Hirvensalo, M., Telama, R., Tammelin, T., Savolainen, M. J., Pouta, A., O'Reilly, P. F., Mantyselka, P., ViikariAla-Korpela, M. (2013)Long-term leisure-time physical activity and serum metabolome. Circulation, 127(3), 340-348. https://doi.org/10.1161/CIRCULATIONAHA.112.105551
Kujala, U. M., Vaara, J. P., Kainulainen, H., Vasankari, T., Vaara, E., & Kyrolainen, H. (2019). Associations of aerobic fitness and maximal muscular strength with metabolites in young men. JAMA Network Open, 2(8), e198265. https://doi.org/10.1001/jamanetworkopen.2019.8265
Lee, S. W., Cho, B. H., Park, S. G., & Kim, S. (2004). Aminoacyl-tRNA synthetase complexes: Beyond translation. Journal of Cell Science, 117(Pt 17), 3725–3734. https://doi.org/10.1242/jcs.01342
Leek, B. T., Mudaliar, S. R., Henry, R., Mathieu-Costello, O., & Richardson, R. S. (2001). Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 280(2), R441-447. https://doi.org/10.1152/ajpregu.2001.280.2.R441
Lewis, T. V., Dart, A. M., Chin-Dusting, J. P., & Kingwell, B. A. (1999). Exercise training increases basal nitric oxide production from the forearm in hypercholesterolemic patients. Arteriosclerosis, Thrombosis, and Vascular Biology, 19(11), 2782–2787. https://doi.org/10.1161/01.atv.19.11.2782
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P., Clarke, M., Devereaux, P. J., Kleijnen, J., & Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ, 339, b2700. https://doi.org/10.1136/bmj.b2700
Lu, M., Lu, Q., Zhang, Y., & Tian, G. (2011). ApoB/apoA1 is an effective predictor of coronary heart disease risk in overweight and obesity. Journal of Biomedical Research, 25(4), 266–273. https://doi.org/10.1016/S1674-8301(11)60036-5
Lustgarten, M. S., Price, L. L., Logvinenko, T., Hatzis, C., Padukone, N., Reo, N. V., Phillips, E. M., Kirn, D., Mills, J., & Fielding, R. A. (2013). Identification of serum analytes and metabolites associated with aerobic capacity. European Journal of Applied Physiology, 113(5), 1311–1320. https://doi.org/10.1007/s00421-012-2555-x
Mann, S., Beedie, C., & Jimenez, A. (2014). Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Medicine (auckland, n. z.), 44(2), 211–221. https://doi.org/10.1007/s40279-013-0110-5
Mastaloudis, A., Leonard, S. W., & Traber, M. G. (2001). Oxidative stress in athletes during extreme endurance exercise. Free Radical Biology & Medicine, 31(7), 911–922. https://doi.org/10.1016/s0891-5849(01)00667-0
Matthews, C. E., Fortner, R. T., Xu, X., Hankinson, S. E., Eliassen, A. H., & Ziegler, R. G. (2012). Association between physical activity and urinary estrogens and estrogen metabolites in premenopausal women. Journal of Clinical Endocrinology and Metabolism, 97(10), 3724–3733. https://doi.org/10.1210/jc.2012-1732
Matthews, C. E., Sampson, J. N., Brenner, D. R., Moore, S. C., Courneya, K. S., Ziegler, R. G., & Friedenreich, C. M. (2018). Effects of exercise and cardiorespiratory fitness on estrogen metabolism in postmenopausal women. Cancer Epidemiology, Biomarkers & Prevention, 27(12), 1480–1482. https://doi.org/10.1158/1055-9965.EPI-17-0900
Menni, C., Fauman, E., Erte, I., Perry, J. R., Kastenmuller, G., Shin, S. Y., Petersen, A. K., Hyde, C., Psatha, M., Ward, K. J., Yuan, W., Milburn, M., Palmer, C. N., Frayling, T. M., Trimmer, J., Bell, J. T., Gieger, C., Mohney, R. P., BrosnanSpector, T. D. (2013)Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes, 62(12), 4270-4276https://doi.org/10.2337/db13-0570
Moaddel, R., Fabbri, E., Khadeer, M. A., Carlson, O. D., Gonzalez-Freire, M., Zhang, P., Semba, R. D., & Ferrucci, L. (2016). Plasma biomarkers of poor muscle quality in older men and women from the Baltimore longitudinal study of aging. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 71(10), 1266–1272. https://doi.org/10.1093/gerona/glw046
Morris, C., Grada, C. O., Ryan, M., Roche, H. M., De Vito, G., Gibney, M. J., Gibney, E. R., & Brennan, L. (2013). The relationship between aerobic fitness level and metabolic profiles in healthy adults. Molecular Nutrition & Food Research, 57(7), 1246–1254. https://doi.org/10.1002/mnfr.201200629
Morris, C., O’Grada, C. M., Ryan, M. F., Gibney, M. J., Roche, H. M., Gibney, E. R., & Brennan, L. (2015). Modulation of the lipidomic profile due to a lipid challenge and fitness level: A postprandial study. Lipids in Health and Disease, 14, 65. https://doi.org/10.1186/s12944-015-0062-x
Nie, C., He, T., Zhang, W., Zhang, G., & Ma, X. (2018). Branched chain amino acids: Beyond nutrition metabolism. International Journal of Molecular Sciences, 19(4), 954. https://doi.org/10.3390/ijms19040954
Nikolaidis, M. G., Kyparos, A., & Vrabas, I. S. (2011). F(2)-isoprostane formation, measurement and interpretation: The role of exercise. Progress in Lipid Research, 50(1), 89–103. https://doi.org/10.1016/j.plipres.2010.10.002
Nosarev, A. V., Smagliy, L. V., Anfinogenova, Y., Popov, S. V., & Kapilevich, L. V. (2014). Exercise and NO production: Relevance and implications in the cardiopulmonary system. Frontiers in Cell and Developmental Biology, 2, 73. https://doi.org/10.3389/fcell.2014.00073
Ognjenovic, J., & Simonovic, M. (2018). Human aminoacyl-tRNA synthetases in diseases of the nervous system. RNA Biology, 15(4–5), 623–634. https://doi.org/10.1080/15476286.2017.1330245
Oh, H., Arem, H., Matthews, C. E., Wentzensen, N., Reding, K. W., Brinton, L. A., Anderson, G. L., Coburn, S. B., Cauley, J. A., Chen, C., Goodman, D., Pfeiffer, R. M., Falk, R. T., Xu, X., & Trabert, B. (2017). Sitting, physical activity, and serum oestrogen metabolism in postmenopausal women: The Women’s Health Initiative Observational Study. British Journal of Cancer, 117(7), 1070–1078. https://doi.org/10.1038/bjc.2017.268
Palmnas, M. S. A., Kopciuk, K. A., Shaykhutdinov, R. A., Robson, P. J., Mignault, D., Rabasa-Lhoret, R., Vogel, H. J., & Csizmadi, I. (2018). Serum metabolomics of activity energy expenditure and its relation to metabolic syndrome and obesity. Science and Reports, 8(1), 3308. https://doi.org/10.1038/s41598-018-21585-6
Pang, Y., Kartsonaki, C., Du, H., Millwood, I. Y., Guo, Y., Chen, Y., Bian, Z., Yang, L., Walters, R., Bragg, F., Lv, J., Yu, C., Chen, J., Peto, R., Clarke, R., Collins, R., Bennett, D. A., Li, L., Holmes, M. V., & Chen, Z. (2019). Physical activity, sedentary leisure time, circulating metabolic markers, and risk of major vascular diseases. Circulation: Genomic and Precision Medicine, 12(9), 386–396. https://doi.org/10.1161/CIRCGEN.118.002527
Parikh, S., Goldstein, A., Koenig, M. K., Scaglia, F., Enns, G. M., Saneto, R., Anselm, I., Cohen, B. H., Falk, M. J., Greene, C., Gropman, A. L., Haas, R., Hirano, M., Morgan, P., Sims, K., Tarnopolsky, M., Van Hove, J. L., Wolfe, L., & DiMauro, S. (2015). Diagnosis and management of mitochondrial disease: A consensus statement from the Mitochondrial Medicine Society. Genetics in Medicine, 17(9), 689–701. https://doi.org/10.1038/gim.2014.177
Patel, H., Alkhawam, H., Madanieh, R., Shah, N., Kosmas, C. E., & Vittorio, T. J. (2017). Aerobic vs anaerobic exercise training effects on the cardiovascular system. World Journal of Cardiology, 9(2), 134–138. https://doi.org/10.4330/wjc.v9.i2.134
Pitkanen, H., Mero, A., Oja, S. S., Komi, P. V., Rusko, H., Nummela, A., Saransaari, P., & Takala, T. (2002). Effects of training on the exercise-induced changes in serum amino acids and hormones. Journal of Strength and Conditioning Research, 16(3), 390–398.
PouralijanAmiri, M., Khoshkam, M., Madadi, R., Kamali, K., Faghanzadeh Ganji, G., Salek, R., & Ramazani, A. (2020). NMR-based plasma metabolic profiling in patients with unstable angina. Iranian Journal of Basic Medical Sciences, 23(3), 311–320.
Riddell, M., & Perkins, B. A. (2009). Exercise and glucose metabolism in persons with diabetes mellitus: Perspectives on the role for continuous glucose monitoring. Journal of Diabetes Science and Technology, 3(4), 914–923. https://doi.org/10.1177/193229680900300439
Riddle, E. S., Stipanuk, M. H., & Thalacker-Mercer, A. E. (2016). Amino acids in healthy aging skeletal muscle. Frontiers in Bioscience, Elite, 8, 326–350.
Robinson, M. M., Dasari, S., Konopka, A. R., Johnson, M. L., Manjunatha, S., Esponda, R. R., Carter, R. E., Lanza, I. R., & Nair, K. S. (2017). Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metabolism, 25(3), 581–592. https://doi.org/10.1016/j.cmet.2017.02.009
Sakaguchi, C. A., Nieman, D. C., Signini, E. F., Abreu, R. M., & Catai, A. M. (2019). Metabolomics-based studies assessing exercise-induced alterations of the human metabolome: A systematic review. Metabolites, 9(8), 164. https://doi.org/10.3390/metabo9080164
Schranner, D., Kastenmuller, G., Schonfelder, M., Romisch-Margl, W., & Wackerhage, H. (2020). Metabolite concentration changes in humans after a bout of exercise: A systematic review of exercise metabolomics studies. Sports Medicine—Open, 6(1), 11. https://doi.org/10.1186/s40798-020-0238-4
Shimomura, Y., Murakami, T., Nakai, N., Nagasaki, M., & Harris, R. A. (2004). Exercise promotes BCAA catabolism: Effects of BCAA supplementation on skeletal muscle during exercise. Journal of Nutrition, 134(6 Suppl), 1583S-1587S. https://doi.org/10.1093/jn/134.6.1583S
Smith, A. J., Phipps, W. R., Thomas, W., Schmitz, K. H., & Kurzer, M. S. (2013). The effects of aerobic exercise on estrogen metabolism in healthy premenopausal women. Cancer Epidemiology, Biomarkers & Prevention, 22(5), 756–764. https://doi.org/10.1158/1055-9965.EPI-12-1325
Thalacker-Mercer, A., Riddle, E., & Barre, L. (2020). Protein and amino acids for skeletal muscle health in aging. Advances in Food and Nutrition Research, 91, 29–64. https://doi.org/10.1016/bs.afnr.2019.08.002
Tsukiyama, Y., Ito, T., Nagaoka, K., Eguchi, E., & Ogino, K. (2017). Effects of exercise training on nitric oxide, blood pressure and antioxidant enzymes. Journal of Clinical Biochemistry and Nutrition, 60(3), 180–186. https://doi.org/10.3164/jcbn.16-108
Tworoger, S. S., Missmer, S. A., Eliassen, A. H., Barbieri, R. L., Dowsett, M., & Hankinson, S. E. (2007). Physical activity and inactivity in relation to sex hormone, prolactin, and insulin-like growth factor concentrations in premenopausal women—exercise and premenopausal hormones. Cancer Causes and Control, 18(7), 743–752. https://doi.org/10.1007/s10552-007-9017-5
Valerio, A., D’Antona, G., & Nisoli, E. (2011). Branched-chain amino acids, mitochondrial biogenesis, and healthspan: An evolutionary perspective. Aging (albany NY), 3(5), 464–478. https://doi.org/10.18632/aging.100322
Wang, Y., & Xu, D. (2017). Effects of aerobic exercise on lipids and lipoproteins. Lipids in Health and Disease, 16(1), 132. https://doi.org/10.1186/s12944-017-0515-5
Wientzek, A., Floegel, A., Knuppel, S., Vigl, M., Drogan, D., Adamski, J., Pischon, T., & Boeing, H. (2014). Serum metabolites related to cardiorespiratory fitness, physical activity energy expenditure, sedentary time and vigorous activity. International Journal of Sport Nutrition and Exercise Metabolism, 24(2), 215–226. https://doi.org/10.1123/ijsnem.2013-0048
Wishart, D. S. (2019). Metabolomics for investigating physiological and pathophysiological processes. Physiological Reviews, 99(4), 1819–1875. https://doi.org/10.1152/physrev.00035.2018
Wurtz, P., Makinen, V. P., Soininen, P., Kangas, A. J., Tukiainen, T., Kettunen, J., Savolainen, M. J., Tammelin, T., Viikari, J. S., Ronnemaa, T., Kahonen, M., Lehtimaki, T., Ripatti, S., Raitakari, O. T., Jarvelin, M. R., & Ala-Korpela, M. (2012). Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes, 61(6), 1372–1380. https://doi.org/10.2337/db11-1355
Xiao, Q., Moore, S. C., Keadle, S. K., Xiang, Y. B., Zheng, W., Peters, T. M., Leitzmann, M. F., Ji, B. T., Sampson, J. N., Shu, X. O., & Matthews, C. E. (2016). Objectively measured physical activity and plasma metabolomics in the Shanghai Physical Activity Study. International Journal of Epidemiology, 45(5), 1433–1444. https://doi.org/10.1093/ije/dyw033
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute on Aging.
Author information
Authors and Affiliations
Contributions
QT: Concept, Design, Methods, Data collection, Data interpretation, Writing–original draft, reviewing, and editing. AC: Methods, Data collection, Visualization, Data interpretation, Writing-methods, reviewing, and editing. RM: Methods, Data interpretation, Visualization, Writing–discussion, reviewing, and editing. LF: Supervision, Concept, Design, Data interpretation, Writing-Reviewing, and Editing.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tian, Q., Corkum, A.E., Moaddel, R. et al. Metabolomic profiles of being physically active and less sedentary: a critical review. Metabolomics 17, 68 (2021). https://doi.org/10.1007/s11306-021-01818-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-021-01818-y