Abstract
Introduction
Breast cancer is a heterogeneous disease with different prognoses and responses to systemic treatment depending on its molecular characteristics, which makes it imperative to develop new biomarkers for an individualized diagnosis and personalized oncological treatment. Ex vivo high-resolution magic angle spinning proton magnetic resonance spectroscopy (HRMAS 1H MRS) is the most common technique for metabolic quantification in human surgical and biopsy tissue specimens.
Objective
To perform a review of the current available literature on the clinical applications of HRMAS 1H MRS metabolic analysis in tissue samples of breast cancer patients.
Methods
This systematic scoping review included original research papers published in the English language in peer-reviewed journals. Study selection was performed independently by two reviewers and preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines were followed.
Results
The literature search returned 159 studies and 26 papers were included as part of this systematic review. There was considerable variation regarding tissue type, aims, and statistical analysis methods across the different studies. To facilitate the interpretation of the results, the included studies were grouped according to their aims or main outcomes into: feasibility and tumor diagnosis (n = 6); tumor heterogeneity (n = 2); correlation with proteomics/transcriptomics (n = 3); correlation with prognostic factors (n = 11); and response evaluation to NAC (n = 4).
Conclusion
There is a lot of potential in including metabolic information of breast cancer tissue obtained with HRMAS 1H MRS. To date, studies show that metabolic concentrations quantified by this technique can be related to the diagnosis, prognosis, and treatment response in breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Breast cancer is the most common type of cancer in women worldwide (Ferlay et al. 2019). It is a heterogeneous disease, with different prognoses and responses to systemic treatment depending on its molecular characteristics. In clinical practice, breast cancer is currently classified into four main subtypes (luminal A, luminal B, HER2-enriched and triple-negative), based on its expression of two hormone receptors (ER, estrogen receptor; PR, progesterone receptor), expression of human epidermal growth factor receptor 2 (HER2), and proliferation rate (Ki-67 expression and nuclear grade). The classification determines the neoadjuvant and adjuvant therapeutic options for each patient (Waks and Winer 2019).
Because of the genetic heterogeneity of breast cancer and the varied tumor response to treatment, it is imperative to develop new biomarkers for an individualized diagnosis and personalized cancer treatment. Metabolomics is the study of metabolic components in a specific cell or tissue type, reflecting the final product of the preceding gene expression and protein activity as well as the surrounding environment. The quantification of metabolites in a tissue sample can provide additional information to understand cancer biology (Emwas et al. 2013; Kaushik and DeBerardinis 2018).
The two main metabolomics techniques are mass spectrometry (MS) and magnetic resonance spectroscopy (MRS). MRS can be performed in vitro, in vivo or ex vivo. In vitro MRS studies of biopsy breast tissues have been performed using the perchloric acid (PCA) extraction procedure; however, these samples may not exactly reflect the in vivo tissue concentrations of certain metabolites (Thomas et al. 2009). In vivo MRS is non-invasive and has proven to be useful tool for yielding supplemental diagnostic information for various malignancies including breast cancer (Fardanesh et al. 2019); however, because of the lower magnetic field there is spectral overlap between different small metabolite signals. Ex vivo high-resolution magic angle spinning (HRMAS) MRS is the most common technique for metabolic quantification in human surgical and biopsy tissue specimens. While it is less sensitive than MS, it requires minimal sample preparation and enables a high degree of reproducibility; in addition, tissue architectures are preserved, allowing for subsequent histopathology analysis, gene expression profiling, or other investigative methods (Bathen et al. 2010; Fuss and Cheng 2016; Glunde and Bhujwalla 2011).
HRMAS proton (1H) MRS of breast cancer tissue from patients and xenografts has led to the identification of 46 different metabolites (Gogiashvili et al. 2019). Clinical metabolic profiles have been studied for diagnostic and prognostic purposes in breast cancer patients and assessed for correlations with disease subtype, tumor stage/grade, response to neoadjuvant chemotherapy (NAC), and patient survival.(Gogiashvili et al. 2019; Günther 2015; Haukaas et al. 2017; McCartney et al. 2018) We performed a systematic scoping review of the current available literature on the clinical applications of metabolic analysis performed through HRMAS 1H MRS of breast cancer patient tissues.
2 Materials and methods
2.1 Search strategy
A librarian (J.G.) searched Medline (PubMed), Embase.com, the Cochrane Library (Wiley), and the Web of Science Core Collection (Clarivate Analytics) on June 19, 2019. The search strategy incorporated two main concepts. For the first concept, i.e., high resolution magic angle spinning, we combined the keywords HR-MAS, HRMAS and magic angle spinning with the Boolean operator OR. In Embase, we also included the Emtree subject headers “high resolution magic angle spinning nuclear magnetic resonance spectroscopy” and “high resolution magic angle spinning proton nuclear magnetic resonance spectroscopy.” For the second concept, i.e., breast, we included the keyword breast with a truncation operator and subject headers for breast cancer in PubMed and Embase (Higgins and Green 2011). We used the Cochrane Handbook filter for excluding animal-only studies in PubMed and Embase. Results were limited to items in English. A second librarian reviewed the search strategy using the Peer Review of Electronic Search Strategies (PRESS) checklist (McGowan et al. 2016). We saved the search results to the EndNote citation management software and then used the Bramer Method to remove duplicates (Bramer et al. 2016).
2.2 Selection criteria
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed (Moher et al. 2009; Tricco et al. 2018). Study selection was performed independently by two reviewers (A.G.V.B. and S.B.T.) and disagreements were resolved in consensus. The Covidence software (Veritas Health Innovation, Melbourne, Australia) was used to manage articles while screening. Covidence is a web-based collaboration software platform that streamlines the production of systematic reviews, supporting citation screening, full text review of references, and a number of other processes relevant to the systematic review of scientific literature. First, all titles and abstracts were assessed for relevancy. Then, full texts of potentially relevant articles were reviewed to determine if they met the inclusion criteria for the study. Studies selected for this systematic review comprised: (1) original research papers published in the English language in peer-reviewed journals (conference abstracts and review articles were excluded); and (2) studies with breast cancer patients’ tissue specimen analysis (studies involving only animals/xenograft models were excluded). A search based on the lists of references from included studies was also performed. After selecting the final list of articles to be included in the review, the authors elaborated a classification according to the aims and main outcomes of the studies to facilitate the interpretation of the results. The recorded variables for each article included in the review were: authors and year of publication, country where the study was performed, number of patients or samples, type of tissue specimen, main measured metabolites, use of multivariate statistical analysis models, aims/outcomes, and main results.
3 Results
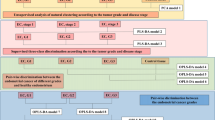
The literature search returned 159 studies, of which 75 were excluded in the screening evaluation and 58 were excluded in the eligibility assessment (Fig. 1). Thus, 26 papers were included as part of this review. Figure 1 shows the PRISMA flow diagram of reviewed and included studies. Included studies were grouped according to their aims or main outcomes into the following: feasibility and tumor diagnosis (n = 6); tumor heterogeneity (n = 2); correlation with proteomics/transcriptomics (n = 3); correlation with prognostic factors (n = 11); and response evaluation to NAC (n = 4). A summary of the included studies is presented in Table 1. An overview of the most prevalent metabolites identified in the included studies is presented in Fig. 2.
Overview of the most prevalent metabolites identified in the included high-resolution magic angle spinning proton magnetic resonance spectroscopy (HRMAS 1H MRS) studies on tissue of breast cancer patients (n = 26). The Y-axis refers to the number of studies that reported each individual metabolite in each column of the bar graph
3.1 Feasibility and tumor diagnosis
The early studies performed by Cheng et al. (1998) and Sitter et al. (2002) showed the feasibility of ex vivo HRMAS 1H MRS to measure tissue metabolism and identify tissue biochemical changes (Cheng et al. 1998; Sitter et al. 2002). Subsequently, these and other authors have compared the metabolic profile between breast cancer and non-cancer tissue (Table 2). In the first pilot study, Cheng et al. (1998) also demonstrated that HRMAS 1H MRS metabolic profiles in malignant tissue differed from benign tissue and that the greatest difference was the absence of the phosphocholine (PC) peak in the normal tissue (Cheng et al. 1998). Sitter et al. (2006) then compared the metabolic spectra from tumor and adjacent non-involved tissue and showed that PC was the dominant choline in the tumor spectra, while glycerophosphocholine (GPC) was the dominant choline in the non-involved breast tissue sample (Sitter et al. 2006). A few years later, Bathen et al. (2013) analyzed 328 tissue samples from 228 breast cancer patients to distinguish between tumor and non-involved adjacent tissue and demonstrated that the choline-containing metabolites were the main biomarkers for tumor content, with PC being especially high in tumor tissues (Bathen et al. 2013).
More recently, Paul et al. (2018) studied the variation in metabolic profile focusing on lipids and showed that a reduction in lipid content was also observed in malignant breast tissue along with a higher fraction of fatty free acids (Paul et al. 2018). They also showed that glycine, taurine, and lactate metabolites exhibited higher signals in tumor tissue.
Different multivariate models on HRMAS 1H MRS spectra have shown good accuracy for discriminating between cancer and non-cancer samples, but in some cases, it was not possible to identify which metabolites were responsible for the discrimination (Bathen et al. 2013; Li and Deng 2017; Paul et al. 2018; Tayyari et al. 2018).
3.2 Tumor heterogeneity
Because HRMAS 1H MRS includes only a small portion of the tumor, to date, the metabolic heterogeneity of the samples has been assessed in only two papers. Park et al. (2016) compared the metabolite concentrations in preoperative core needle biopsy samples with those in central and peripheral surgical tumor specimens. They demonstrated a moderate or higher agreement between the relative concentrations of 94.3% of metabolites between the central and peripheral specimens, 80.0% of metabolites between the preoperative biopsy and central surgical specimens, and 82.9% of metabolites between all three specimen types. Most metabolites showed no significant differences in concentrations between the central and peripheral surgical specimens (97.1%) and between the core needle biopsy and central surgical specimens (Park et al. 2016).
Gogiashvili et al. (2018) studied multi-core sampling of tumor tissue and found that intertumoral differences for many metabolites were larger than intratumoral differences, suggesting that the analysis of a single tissue sample may be acceptable for HRMAS 1H MRS studies (Gogiashvili et al. 2018).
3.3 Correlation with proteomics/transcriptomics profiles
A few studies have shown that the metabolic profile from HRMAS 1H MRS can be correlated with the transcriptional profile in breast cancer samples. Borgan et al. (2010) analyzed tissue samples from 46 patients using both HRMAS 1H MRS and gene expression microarrays, showing that it is feasible to correlate metabolic and transcriptional data from the same breast cancer sample. The majority of patients in the study were classified as having Luminal A tumors and multivariate analyses of HRMAS 1H MRS spectra found three Luminal A subgroups. The correlation of metabolic and transcriptional profiles showed that one of the subgroups had metabolic and transcriptional features indicating more proliferation than the other two subgroups (Borgan et al. 2010).
Haukaas et al. (2016) conducted HRMAS 1H MRS as well as protein expression subtyping, mRNA expression profiling, and gene expression subtyping in tumor samples from 228 non-treated breast cancer patients. Following hierarchical cluster analysis, three metabolic clusters were identified. The three clusters were significantly different in the expression breast cancer-related proteins as well as the expression of genes related to the extracellular matrix and metabolic pathways known to be aberrant in cancer (Haukaas et al. 2016).
Cao et al. (2012a, b, c) studied the potential molecular causes of increased choline-containing metabolites in breast cancer and found that breast cancers with malignant choline metabolite profiles (consisting of low GPC and high PC levels) highly co-expressed glycerophosphodiester phosphodiesterase domain 5 (GDPD5), choline kinase alpha (CHKA), and phosphatidylcholine-specific phospholipase D1 (PLD1) (Cao et al. 2012a), suggesting that these genes are probably involved in regulating choline phospholipid metabolism in breast cancer.
3.4 Correlation with prognostic factors
Many of the HRMAS 1H MRS studies in breast cancer samples to date have focused on correlating the metabolic profile from samples with prognostic factors including clinical, histologic, and immunohistochemistry features, as well as recurrence and survival rates (Table 3).
Sitter et al. (2010) classified breast cancer samples into good or poor prognosis groups (based on tumor size, spread to axillary lymph nodes, and expression of ER and PR). At univariate analysis, a trend toward lower concentrations of glycine was found for the good prognosis group. In addition, multivariate models versus single metabolite concentrations showed a stronger correlation with prognosis (Sitter et al. 2010).
Choi et al. (2012) found that several metabolite markers correlated with histopathologic prognostic factors at univariate analysis: ER-negative cancers showed higher choline concentrations than ER-positive cancers; PR-negative cancers showed higher choline, creatine, and taurine concentrations than PR-positive cancers; HER2-positive cancers showed higher taurine, scyllo-inositol, and myo-inositol concentrations than HER2-negative cancers; and triple negative cancers showed higher choline concentrations as well as higher values of choline/creatine and total choline/creatine ratios than non-triple negative cancers. Cancers strongly positive for Ki-67 showed higher total choline and PC concentrations, and higher values of PC/creatine. However, metabolite concentrations did not correlate with histologic grade, tumor size, or lymph node metastasis. When tumors were grouped by prognosis (good vs. poor), the poor prognosis group showed higher glycine and scyllo-inositol concentrations. Multivariate models showed a visible distinction of spectral data by ER, PR, HER2, and Ki-67 status, as well as a reasonable distinction of spectral data by prognosis groups (Choi et al. 2012).
A multivariate model in Shin et al. (2016) predicted indolent tumors, tumors with recurrence, and tumors with lymph node metastases with sensitivities of 100%, 77%, and 82%, and specificities of 68%, 100%, and 96%, respectively (Shin et al. 2016).
Few studies have specifically evaluated the use of metabolite concentrations to discriminate molecular subtypes. Cao et al. (2014) found that triple-negative breast cancers contained a lower level of glutamine and a higher level of glutamate and choline compared with triple-positive breast cancer. High glycine levels were also found in overexpressed HER2 tumors (Cao et al. 2014).
Recently, Tayyari et al. (2018) reported different metabolic profiles for triple-negative and luminal A breast cancers at multivariate analysis. They also found different metabolic profiles between African American and Caucasian women as well as between postmenopausal (> 50 years) and premenopausal (< 50 years) women (Tayyari et al. 2018).
Choi et al. (2017) assessed correlations between metabolic profiles and molecular markers currently used in patients with ER-positive breast cancers. Tumors were grouped by status of HER2, Ki-67, and molecular subtype (luminal A or luminal B). At univariate analysis, the HER2-enriched group showed higher levels of glycine and glutamate, the high Ki-67 group showed higher levels of glutamate, and the Luminal B group showed higher levels of glycine. Multivariate models showed visible discrimination between the groups (Choi et al. 2017).
Giskeødegård et al. (2012) demonstrated that higher levels of glycine and lactate in biopsy samples analyzed using HRMAS 1H MRS were associated with lower survival rates in ER-positive breast cancer (Giskeødegård et al. 2012).
Chae et al. (2016) performed a study using HRMAS 1H MRS to evaluate the differences in metabolic profile between 30 patients with pure ductal carcinoma in situ (DCIS) and 30 patients with DCIS accompanying invasive carcinoma. At univariate analysis, the pure DCIS group showed a higher GPC/PC ratio as well as a higher concentration of myo-inositol and succinate. Multivariate models clearly discriminated between the two groups (Chae et al. 2016).
Yoon et al. (2016) compared metabolic profiles on HRMAS 1H MRS with three prognostic imaging markers, namely signal enhancement ratio (SER) and apparent diffusion coefficient (ADC) on magnetic resonance imaging (MRI) as well as standard uptake value (SUV) on 18F-fluorodeoxiglucose positron emission tomography-computed tomography (18F-FDG PET-CT). The rationale was that high SER, low ADC, and high SUV are related to worse prognosis in breast cancer patients. Multivariate metabolic models showed visible discrimination of high and low values of SER, ADC and SUV. Higher levels of PC, choline, and glycine were found in the high SER group, and a higher level of leucine was found in the low SER group. Higher levels of glycine and PC were found in the low ADC group. Higher levels of PC, choline, and glycine were found in the high SUV group (Yoon et al. 2016).
3.5 Response evaluation to neoadjuvant chemotherapy
Neoadjuvant chemotherapy (NAC) to treat breast cancer is becoming increasingly common, which presents a valuable opportunity for HRMAS 1H MRS (Selli and Sims 2019). Table 4 summarizes the findings of the included studies on this topic.
Choi et al. (2013) used HRMAS 1H MRS to evaluate core needle biopsy specimens from 37 breast cancer patients treated with NAC. The metabolite concentrations were not significantly different between the complete pathologic response group and non-pathologic complete response group at univariate analysis, but multivariate models were able to show visible discrimination between the two groups (Choi et al. 2013).
Cao et al. published two studies in 2012 correlating the metabolic profiles identified using HRMAS MRS with treatment response and 5-year survival. One study evaluated 89 patients receiving either NAC as epirubicin or paclitaxel monotherapy in a randomized open-label multicenter study. They found that tumor metabolism changed significantly in response to NAC treatment, but metabolic responses were similar for the two chemotherapeutic agents. In addition, metabolic responses did not correlate with clinical response (partial response or stable disease) but correlated with patient outcome. Specifically, non-survivors (< 5 years) had increased tumor levels of lactate after treatment, while survivors (≥ 5 years) experienced a decrease in the levels of glycine and choline-containing compounds and an increase in glucose levels (Cao et al. 2012b).
In the other Cao et al. study, in 33 patients with locally advanced breast cancer, there were no statistically significant differences in metabolite concentrations between patients with partial response and patients with stable disease after NAC. Again, different pretreatment MR metabolic profiles characterized by higher levels of total choline and lower levels of lactate were observed in survivors. A significant decrease in GPC post-treatment was associated with long-term survival (≥ 5 years). Multivariate classification models successfully discriminated survivors from non-survivors (Cao et al. 2012c).
Euceda et al. (2017) explored the effect of NAC combined with bevacizumab on metabolic profiles of 122 patients before, during, and after treatment. Lower glucose and higher lactate levels were observed in patients exhibiting a good response (≥ 90% tumor reduction) before treatment, while the opposite was observed after treatment. Multivariate analysis revealed metabolic differences after treatment between pathological complete response and non-pathological complete responders, as well as between pathological minimal residual disease (residual tumor < 1 cm) and pathological non-responders but did not showed differences in samples acquired before or during treatment. It was not possible to discriminate bevacizumab-treated from chemotherapy-only patients at any time point (Euceda et al. 2017).
4 Discussion
Our systematic scoping review shows that HRMAS 1H MRS in the setting of breast cancer has been studied by only a few groups. However, because HRMAS MRS does not damage the tissue for subsequent histologic and immunohistochemistry studies, it could be used more frequently to provide additional metabolic information to help understand breast cancer behavior.
While HRMAS 1H MRS is a standardized technique, as has been discussed extensively in prior reviews (Gogiashvili et al. 2019; Jagannathan and Sharma 2017), there is substantial variation regarding the tissue type and statistical analysis methods used among previous studies.
Tissue types used in the studies ranged from tissues from the tissue bank, surgically obtained tissues, and needle biopsy specimens. Studies comparing tissue samples from different sites of the tumor have demonstrated an acceptable agreement between the intratumoral metabolites concentrations (Gogiashvili et al. 2018; Park et al. 2016). Because percutaneous image-guided needle biopsy is a minimally invasive and widely used procedure for the diagnosis of breast cancer prior to surgery, a promising area of research is to use HRMAS 1H MRS in diagnostic biopsy samples to provide early information of tumor metabolism that may help in patient management.
In terms of statistical analysis, multivariate analysis has become a commonly used strategy for the analysis of large spectral datasets, including that of the metabolic profile, which usually involves multiple metabolites (many of which are correlated). The most commonly used multivariate statistical analysis methods in the studies included in this review were principal component analysis (PCA), partial least square-discriminate analysis (PLS-DA), orthogonal projections to latent structure-discriminant analysis (OPLS-DA), and neural networks. There has been no direct comparison between these methods, but some authors have used more than one type of multivariate modelling method and found that different approaches can have different prediction success (Bathen et al. 2007; Giskeødegård et al. 2010). One problem with multivariate analysis methods is that sometimes it is not possible to identify the exact metabolites that most strongly correlate with a particular clinical parameter, which would be important to understand tumor metabolism and develop targeted treatment.
As an ex vivo technique, HRMAS 1H MRS is more useful than in vivo MRS in the setting of breast cancer. The first studied clinical application of HRMAS 1H MRS metabolite quantification in breast cancer tissue samples involved tumor diagnosis. While in vivo techniques are available for various tissue specimens, it is most difficult to obtain useful metabolic data using conventional in vivo techniques in breast cancer specimens due to the high content of adipose tissue in the breast. In vivo MRS has demonstrated high levels of total choline-containing compounds in malignant breast tissue compared with benign and normal breast tissue (Jagannathan and Sharma 2017). However, in vivo spectroscopy cannot discriminate between individual choline metabolites due to resolution limitations related to the relatively low magnetic field strength used (1.5–3.0 T). In contrast, HRMAS 1H MRS can quantify individual choline metabolites that are involved in biological functions such as cell signaling, lipid metabolism, and cell membrane integrity. Besides choline-containing compounds, HRMAS 1H MRS also allows the identification of other metabolites that have elevated concentrations in breast cancer tissue, such as glycine and taurine (Bathen et al. 2013; Paul et al. 2018; Sitter et al. 2006).
Many studies identified in the present review assessed the correlation between metabolic profiles and prognostic factors in breast cancer patients. Despite the high number of metabolites and different prognostic factors evaluated, some results have been replicated. For example, higher choline concentrations have been demonstrated in hormone-negative and more aggressive tumors in two studies (Cao et al. 2014; Choi et al. 2012). Glycine, which was suggested to be an in vivo biomarker for brain tumor malignancy (Ganji et al. 2016) and was also described in a breast cancer patient in vivo MRS (Bitencourt et al. 2019), has been associated with poor prognostic factors in breast cancer in several ex vivo studies (Cao et al. 2014; Choi et al. 2012, 2017; Yoon et al. 2016). Glutamate levels have been shown be higher in triple-negative and HER2 overexpressed tumors in two studies (Cao et al. 2014; Choi et al. 2017). These findings have also been observed in prior studies in animal models using different techniques for metabolic quantification, and all these metabolites have been shown to be involved in cancer-relevant metabolism reprogramming pathways (Günther 2015; Haukaas et al. 2017; Kaushik and DeBerardinis 2018). In addition to the findings whereby individual metabolites have been correlated with prognostic factors, multivariate modelling has yielded good correlation with prognosis and clinicopathological prognostic factors such as tumor grade, subtype, and lymph node status, across several studies (Choi et al. 2012, 2017; Shin et al. 2016; Sitter et al. 2010; Tayyari et al. 2018).
The analysis of lipid metabolism using MRS has received little attention in the literature until now. In this review, only one article focused on lipids, showing that lipid metabolites enables the discrimination between malignant breast tissue and lymph nodes from adjacent benign breast tissue (Paul et al. 2018). In vivo MRS has also shown that assessment of lipid metabolism enables the differentiation of benign and malignant breast tumors, classification of molecular subtypes, and prediction of long-term survival outcomes in breast cancer patients (Thakur et al. 2019). The correlation between lipid metabolism using HRMAS 1H MRS and in vivo MRS should be explored in future studies.
Using metabolomics techniques to predict and assess treatment response to NAC is a promising area of research. None of the currently published studies using HRMAS 1H MRS have shown a clear correlation between metabolic profiles and pathological complete response after NAC, which is the most relevant clinical outcome so far. However, multivariate and univariate analyses have demonstrated that changes in the metabolic profile after NAC can be associated with long-term survival (Cao et al. 2012b, c). At univariate analysis, a decrease in the levels of glycine and choline-containing compounds after treatment was associated with better prognosis. Another interesting finding was the modification of cell energy metabolism after NAC, characterized by the increase in glucose and decrease in lactate concentrations in responders, which is the opposite of the Warburg effect normally observed in most cancer cells (Euceda et al. 2017).
The combination of metabolomics with other—omics fields is another promising area of research. Proteomics, transcriptomics, and genomics have yielded key contributions in the understanding of breast cancer, including the classification of breast cancer into clinically relevant subtypes and the assessment of recurrence risk; these have been incorporated into clinical practice. Combining transcriptional and metabolic data using HRMAS 1H MRS from the same breast carcinoma sample is feasible, can contribute to a more refined subclassification of breast cancers, and reveal relationships at the molecular level, potentially leading to more patient specific treatments (Borgan et al. 2010; Cao et al. 2012a; Haukaas et al. 2016).
Our systematic scoping review has some limitations. We decided to include only studies on breast cancer tissue from patients because our main objective was to evaluate the clinical applications of this technique. However, there are also papers using this technique on breast cancer cell lines and xenograft models, which provide interesting additional information on tumor metabolism but were not included in this review. Lastly, because each study has different outcomes, quantification and statistical analysis methods, it was not possible to perform a quantitative data extraction or meta-analysis of the results.
5 Overall conclusions
In summary, there is a lot of potential in including metabolic information of breast cancer tissue obtained with HRMAS 1H MRS. To date, studies show that metabolic concentrations quantified by this technique can be related to the diagnosis, prognosis, and treatment response in breast cancer patients. However, further studies are needed to standardize data analysis and identify the best scenarios in which this technique can add relevant clinical information.
References
Bathen, T. F., Geurts, B., Sitter, B., Fjøsne, H. E., Lundgren, S., Buydens, L. M., et al. (2013). Feasibility of MR metabolomics for immediate analysis of resection margins during breast cancer surgery. PLoS ONE, 8(4), e61578.
Bathen, T. F., Jensen, L. R., Sitter, B., Fjösne, H. E., Halgunset, J., Axelson, D. E., et al. (2007). MR-determined metabolic phenotype of breast cancer in prediction of lymphatic spread, grade, and hormone status. Breast Cancer Research and Treatment, 104(2), 181–189.
Bathen, T. F., Sitter, B., Sjobakk, T. E., Tessem, M.-B., & Gribbestad, I. S. (2010). Magnetic resonance metabolomics of intact tissue: A biotechnological tool in cancer diagnostics and treatment evaluation. Cancer Research, 70(17), 6692–6696.
Bitencourt, A. G. V., Pinker, K., & Thakur, S. (2019). Elevated glycine detected on in vivo magnetic resonance spectroscopy in a breast cancer patient: Case report and literature review. BJR Case Reports, 5, 20190090.
Borgan, E., Sitter, B., Lingjærde, O. C., Johnsen, H., Lundgren, S., Bathen, T. F., et al. (2010). Merging transcriptomics and metabolomics: Advances in breast cancer profiling. BMC Cancer, 10(1), 628.
Bramer, W. M., Giustini, D., de Jonge, G. B., Holland, L., & Bekhuis, T. (2016). De-duplication of database search results for systematic reviews in EndNote. Journal of the Medical Library Association, 104(3), 240–243.
Cao, M. D., Döpkens, M., Krishnamachary, B., Vesuna, F., Gadiya, M. M., Lønning, P. E., et al. (2012a). Glycerophosphodiester phosphodiesterase domain containing 5 (GDPD5) expression correlates with malignant choline phospholipid metabolite profiles in human breast cancer. NMR in Biomedicine, 25(9), 1033–1042.
Cao, M. D., Giskeødegård, G. F., Bathen, T. F., Sitter, B., Bofin, A., Lønning, P. E., et al. (2012b). Prognostic value of metabolic response in breast cancer patients receiving neoadjuvant chemotherapy. BMC Cancer, 12(1), 39.
Cao, M. D., Lamichhane, S., Lundgren, S., Bofin, A., Fjøsne, H., Giskeødegård, G. F., et al. (2014). Metabolic characterization of triple negative breast cancer. BMC Cancer, 14, 941.
Cao, M. D., Sitter, B., Bathen, T. F., Bofin, A., Lønning, P. E., Lundgren, S., et al. (2012c). Predicting long-term survival and treatment response in breast cancer patients receiving neoadjuvant chemotherapy by MR metabolic profiling. NMR in Biomedicine, 25(2), 369–378.
Chae, E. Y., Shin, H. J., Kim, S., Baek, H.-M., Yoon, D., Kim, S., et al. (2016). The role of high-resolution magic angle spinning 1H nuclear magnetic resonance spectroscopy for predicting the invasive component in patients with ductal carcinoma in situ diagnosed on preoperative biopsy. PLoS ONE, 11(8), e0161038.
Cheng, L. L., Chang, I.-W., Smith, B. L., & Gonzalez, R. G. (1998). Evaluating human breast ductal carcinomas with high-resolution magic-angle spinning proton magnetic resonance spectroscopy. Journal of Magnetic Resonance, 135(1), 194–202.
Choi, J. S., Baek, H. M., Kim, S., Kim, M. J., Youk, J. H., Moon, H. J., et al. (2012). HR-MAS MR spectroscopy of breast cancer tissue obtained with core needle biopsy: Correlation with prognostic factors. PLoS ONE, 7(12), e51712.
Choi, J. S., Baek, H.-M., Kim, S., Kim, M. J., Youk, J. H., Moon, H. J., et al. (2013). Magnetic resonance metabolic profiling of breast cancer tissue obtained with core needle biopsy for predicting pathologic response to neoadjuvant chemotherapy. PLoS ONE, 8(12), e83866.
Choi, J. S., Yoon, D., Koo, J. S., Kim, S., Park, V. Y., Kim, E.-K., et al. (2017). Magnetic resonance metabolic profiling of estrogen receptor-positive breast cancer: Correlation with currently used molecular markers. Oncotarget, 8(38), 63405–63416.
Emwas, A. H. M., Salek, R. M., Griffin, J. L., & Merzaban, J. (2013). NMR-based metabolomics in human disease diagnosis: Applications, limitations, and recommendations. Metabolomics, 9(5), 1048–1072.
Euceda, L. R., Haukaas, T. H., Giskeødegård, G. F., Vettukattil, R., Engel, J., Silwal-Pandit, L., et al. (2017). Evaluation of metabolomic changes during neoadjuvant chemotherapy combined with bevacizumab in breast cancer using MR spectroscopy. Metabolomics, 13(4), 37.
Fardanesh, R., Marino, M. A., Avendano, D., Leithner, D., Pinker, K., & Thakur, S. B. (2019). Proton MR spectroscopy in the breast: Technical innovations and clinical applications. Journal of Magnetic Resonance Imaging. https://doi.org/10.1002/jmri.26700.
Ferlay, J., Colombet, M., Soerjomataram, I., Mathers, C., Parkin, D. M., Piñeros, M., et al. (2019). Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International Journal of Cancer, 144(8), 1941–1953.
Fuss, T. L., & Cheng, L. L. (2016). Evaluation of cancer metabolomics using ex vivo high resolution magic angle spinning (HRMAS) magnetic resonance spectroscopy (MRS). Metabolites, 6(1), E11.
Ganji, S. K., Maher, E. A., & Choi, C. (2016). In vivo 1 HMRSI of glycine in brain tumors at 3T. Magnetic Resonance in Medicine, 75(1), 52–62.
Giskeødegård, G. F., Grinde, M. T., Sitter, B., Axelson, D. E., Lundgren, S., Fjøsne, H. E., et al. (2010). Multivariate modeling and prediction of breast cancer prognostic factors using MR metabolomics. Journal of Proteome Research, 9(2), 972–979.
Giskeødegård, G. F., Lundgren, S., Sitter, B., Fjøsne, H. E., Postma, G., Buydens, L. M. C., et al. (2012). Lactate and glycine-potential MR biomarkers of prognosis in estrogen receptor-positive breast cancers. NMR in Biomedicine, 25(11), 1271–1279.
Glunde, K., & Bhujwalla, Z. M. (2011). Metabolic tumor imaging using magnetic resonance spectroscopy. Seminars in Oncology, 38(1), 26–41.
Gogiashvili, M., Horsch, S., Marchan, R., Gianmoena, K., Cadenas, C., Tanner, B., et al. (2018). Impact of intratumoral heterogeneity of breast cancer tissue on quantitative metabolomics using high-resolution magic angle spinning 1H NMR spectroscopy. NMR in Biomedicine, 31(2), e3862.
Gogiashvili, M., Nowacki, J., Hergenröder, R., Hengstler, J. G., Lambert, J., & Edlund, K. (2019). HR-MAS NMR based quantitative metabolomics in breast cancer. Metabolites, 9(2), 19.
Günther, U. L. (2015). Metabolomics biomarkers for breast cancer. Pathobiology, 82(3–4), 153–165.
Haukaas, T., Euceda, L., Giskeødegård, G., & Bathen, T. (2017). Metabolic portraits of breast cancer by HR MAS MR spectroscopy of intact tissue samples. Metabolites, 7(2), 18.
Haukaas, T. H., Euceda, L. R., Giskeødegård, G. F., Lamichhane, S., Krohn, M., Jernström, S., et al. (2016). Metabolic clusters of breast cancer in relation to gene- and protein expression subtypes. Cancer & Metabolism, 4(1), 12.
Higgins, J. P. T., & Green, S. (2011). Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London: The Cochrane Collaboration.
Jagannathan, N. R., & Sharma, U. (2017). Breast tissue metabolism by magnetic resonance spectroscopy. Metabolites, 7(2), E25.
Kaushik, A. K., & DeBerardinis, R. J. (2018). Applications of metabolomics to study cancer metabolism. Biochimica et Biophysica Acta: Reviews on Cancer, 1870(1), 2–14.
Li, T., & Deng, P. (2017). Nuclear Magnetic Resonance technique in tumor metabolism. Genes and Diseases, 4(1), 28–36.
McCartney, A., Vignoli, A., Biganzoli, L., Love, R., Tenori, L., Luchinat, C., et al. (2018). Metabolomics in breast cancer: A decade in review. Cancer Treatment Reviews, 67(April), 88–96.
McGowan, J., Sampson, M., Salzwedel, D. M., Cogo, E., Foerster, V., & Lefebvre, C. (2016). PRESS peer review of electronic search strategies: 2015 guideline statement. Journal of Clinical Epidemiology, 75, 40–46.
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097.
Park, V. Y., Yoon, D., Koo, J. S., Kim, E.-K., Kim, S. Il, Choi, J. S., et al. (2016). Intratumoral agreement of high-resolution magic angle spinning magnetic resonance spectroscopic profiles in the metabolic characterization of breast cancer. Medicine, 95(15), e3398.
Paul, A., Kumar, S., Raj, A., Sonkar, A. A., Jain, S., Singhai, A., et al. (2018). Alteration in lipid composition differentiates breast cancer tissues: A 1H HRMAS NMR metabolomic study. Metabolomics, 14(9), 119.
Selli, C., & Sims, A. H. (2019). Neoadjuvant therapy for breast cancer as a model for translational research. Breast Cancer: Basic and Clinical Research, 13, 117822341982907.
Shin, H. J., Kim, S., Baek, H. M., Yoon, D., Kim, S., Cha, J. H., et al. (2016). Prediction of indolent breast cancer with favorable prognostic factors by metabolic profiling using in vivo and ex vivo MR metabolomics. Applied Magnetic Resonance, 47(2), 159–174.
Sitter, B., Bathen, T. F., Singstad, T. E., Fjøsne, H. E., Lundgren, S., Halgunset, J., et al. (2010). Quantification of metabolites in breast cancer patients with different clinical prognosis using HR MAS MR spectroscopy. NMR in Biomedicine, 23(4), 424–431.
Sitter, B., Lundgren, S., Bathen, T. F., Halgunset, J., Fjosne, H. E., & Gribbestad, I. S. (2006). Comparison of HR MAS MR spectroscopic profiles of breast cancer tissue with clinical parameters. NMR in Biomedicine, 19(1), 30–40.
Sitter, B., Sonnewald, U., Spraul, M., Fjösne, H. E., & Gribbestad, I. S. (2002). High-resolution magic angle spinning MRS of breast cancer tissue. NMR in Biomedicine, 15(5), 327–337.
Tayyari, F., Gowda, G. A. N., Olopade, O. F., Berg, R., Yang, H. H., Lee, M. P., et al. (2018). Metabolic profiles of triple-negative and luminal A breast cancer subtypes in African-American identify key metabolic differences. Oncotarget, 9(14), 11677–11690.
Thakur, S. B., Horvat, J. V., Hancu, I., Sutton, O. M., Bernard-Davila, B., Weber, M., et al. (2019). Quantitative in vivo proton MR spectroscopic assessment of lipid metabolism: Value for breast cancer diagnosis and prognosis. Journal of Magnetic Resonance Imaging. https://doi.org/10.1002/jmri.26622.
Thomas, M. A., Lipnick, S., Velan, S. S., Liu, X., Banakar, S., Binesh, N., et al. (2009). Investigation of breast cancer using two-dimensional MRS. NMR in Biomedicine, 22(1), 77–91.
Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., et al. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Annals of Internal Medicine, 169(7), 467.
Waks, A. G., & Winer, E. P. (2019). Breast cancer treatment. JAMA, 321(3), 288.
Yoon, H., Yoon, D., Yun, M., Choi, J. S., Park, V. Y., Kim, E. K., et al. (2016). Metabolomics of breast cancer using high-resolution magic angle spinning magnetic resonance spectroscopy: Correlations with 18F-FDG positron emission tomography-computed tomography, dynamic contrast-enhanced and diffusion-weighted imaging MRI. PLoS ONE, 11(7), e0159949.
Funding
This work was partially supported by the NIH/NCI Cancer Center Support Grant (P30 CA008748) and the Breast Cancer Research Foundation
Author information
Authors and Affiliations
Contributions
JG conducted the literature review and constructed the article database. AGVB and SBT performed the study selection and reviewed the selected studies. All authors were involved in writing the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Katja Pinker received payment for activities not related to the present article including lectures including service on speakers bureaus and for travel/accommodations/meeting expenses unrelated to activities listed from the European Society of Breast Imaging (MRI educational course, annual scientific meeting) and the IDKD 2019 (educational course).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bitencourt, A.G.V., Goldberg, J., Pinker, K. et al. Clinical applications of breast cancer metabolomics using high-resolution magic angle spinning proton magnetic resonance spectroscopy (HRMAS 1H MRS): systematic scoping review. Metabolomics 15, 148 (2019). https://doi.org/10.1007/s11306-019-1611-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-019-1611-5