Abstract
Introduction
The native potatoes (Solanum tuberosum ssp. tuberosum L.) cultivated on Chiloé Island in southern Chile have great variability in terms of tuber shape, size, color and flavor. These traits have been preserved throughout generations due to the geographical position of Chiloé, as well as the different uses given by local farmers.
Objectives
The present study aimed to investigate the diversity of metabolites in skin and pulp tissues of eleven native accessions of potatoes from Chile, and evaluate the metabolite associations between tuber tissues.
Methods
For a deeper characterization of these accessions, we performed a comprehensive metabolic study in skin and pulp tissues of tubers, 3 months after harvesting. Specific targeted quantification of metabolites using 96 well microplates, and high-performance liquid chromatography combined with non-targeted metabolite profiling by gas chromatography time-of-flight mass spectrometry were used in this study.
Results
We observed differential levels of antioxidant activity and phenolic compounds between skin and pulp compared to a common commercial cultivar (Desireé). In addition, we uncovered considerable metabolite variability between different tuber tissues and between native potatoes. Network correlation analysis revealed different metabolite associations among tuber tissues that indicate distinct associations between primary metabolite and anthocyanin levels, and antioxidant activity in skin and pulp tissues. Moreover, multivariate analysis lead to the grouping of native and commercial cultivars based on metabolites from both skin and pulp tissues.
Conclusions
As well as providing important information to potato producers and breeding programs on the levels of health relevant phytochemicals and other abundant metabolites such as starch, proteins and amino acids, this study highlights the associations of different metabolites in tuber skins and pulp, indicating the need for distinct strategies for metabolic engineering in these tissues. Furthermore, this study shows that native Chilean potato accessions have great potential as a natural source of phytochemicals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
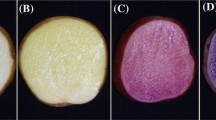
The potato (Solanum tuberosum ssp. tuberosum L.) is currently one of the four most important crops worldwide and is a staple food in the diet of much of the global population (Singh and Saldana 2011). In addition, the Solanaceae family contains many other species that are relevant for human nutrition and health such as tomato, pepper and eggplant. The genus Solanum is composed of wild and cultivated types of potato and tomato, and contains over 1000 species. Wild potatoes are native to America, where they display a wide geographical and ecological distribution (Camandro et al. 2012). The potato is extensively grown in southern Chile, which is considered a sub-center of the origin of the cultivated varieties of this species (Spooner et al. 2005). The native potatoes from Chiloé Island are diverse in size, form, color and flavor, and have been preserved due to the geographical position and the different uses given by farmers (Solano et al. 2013) (Fig. 1). Furthermore, due to its natural conditions and isolation, in Chiloé Island, a great number of native potato accessions have proliferated. Between these accessions, there is substantial variety in quality and suitability for cultivation at different periods in the farming calendar, and different forms of preparation and consumption (Contreras et al. 1981). Hence, this collection represents a rich source of exotic germplasm.
A Diversity in shape and color of native potatoes. (a) Desireé, (b) Meca de gato morada larga, (c) Michuñe azul, (d) Guicoña, (e) Michuñe negro, (f) Cauchau, (g) Guadacho colorado, (h) Murta, (i) Clavela morada, (j) Yagana, (k) Lengua de vaca, (l) Guadacho blanco, (m) Tonta. B Map of Chiloé Island, Chile
Native potatoes in general are rich in phytochemicals such as polyphenolic compounds, flavonoids, anthocyanins, vitamin C and carotenoids (Reyes et al. 2005; Lachman et al. 2009). These metabolites have high free-radical scavenging activity and may help protect proteins, lipids and DNA against reactive oxygen species, which are implicated in age-related neuronal degeneration and many chronic diseases (Ames et al. 1993; Teow et al. 2007). Moreover, extracts derived from tuber skin possess strong antioxidant activity in in vitro models (Singh and Rajini 2004) and are able to protect erythrocytes against oxidative damage (Singh and Rajini 2008). In addition, the presence of phytochemicals such as anthocyanins has been associated with health benefits (Lovat et al. 2016), and many of the most bioactive compounds are located in the skin of the tubers (Scalbert et al. 2005; Albishi et al. 2013; Al-Weshahy et al. 2013). Thus, colored native potatoes are potential sources of natural phytochemical compounds and could thus be used to restore this trait in paler cultivated varieties (Jansen and Flamme 2006; Akyol et al. 2016). Recently, studies aimed at understanding the major metabolic pathways that are active in potato pulp tissues have been performed (Dobson et al. 2008, 2010; Carreno-Quintero et al. 2012). These studies focused on starch content, sugars, glycoalkaloids, and pulp color and quality. Since high natural availability of metabolites was found in pulp and skin of tubers from native potatoes, this set of genotypes represents an important source of genetic variability for breeding programs aimed at improving tuber quality.
Despite its importance for human nutrition and health, it remains unclear how exactly primary and secondary metabolism are regulated and how the final chemical composition of tuber tissues is determined. Here, we used a panel of ten native potatoes from Chiloé Island, and one continental Chile accession with varying tuber sizes, shapes and colors to characterize the metabolite profiles from skin and pulp tissues and investigate their connection with antioxidant activity in tubers. In this study, the importance of these metabolic differences is discussed with respect to the use of native potatoes as a key resource in metabolomics-assisted breeding programs.
2 Materials and methods
2.1 Plant material and growth conditions
We selected ten native accessions of potato (S. tuberosum ssp. tuberosum L.) from different areas of Chiloé Island, and one accession from Los Muermos (Chilean mainland), for characterization at the metabolic level. The chosen native potatoes were selected according to their skin and pulp color, and the size and shape of the tuber (Fig. 1; Table 1). In addition, we analyzed two improved cultivars, Desirée and Yagana, as controls for red- and white-skinned accessions, respectively (Fig. 1; Table 1).
This collection of native potatoes was clonally-propagated in the same field under similar management, climate and soil conditions, at the Experimental Station of the School of Agronomy at the Universidad Católica de Temuco, in the Araucanía Region, Chile. The morphological traits vary widely according to their genotype; elongated, round, oval, fusiform and elliptical tuber shapes are observed (Fig. 1). Each cultivar was grown in four independent blocks that were randomly located in the same field. Each block contained one hundred and twenty plants from which all tubers were harvested. After harvesting tubers free of mechanical and/or physiological damage, samples were maintained in semi dark conditions at 8 °C. After 3 months of storage, samples were prepared for metabolome profiling by discarding the proximal and distal part of the tuber, and dividing the middle part into skin (epidermis and cork cells) and pulp (medulla and perimedulla cells) tissues, which were sliced, immediately frozen in liquid nitrogen, ground to a fine powder and stored at − 80 °C until analysis.
2.2 Determination of total antioxidant activity and phenolic content
The antioxidant activity of skin and pulp was quantified in methanolic extracts using the free radical 2,2-diphenyl-1-picrylhydrazyl scavenging method as described by Chinnici et al. (2004), with some modifications. The absorbance was measured at 515 nm in a spectrophotometer (Thermo Spectronic, GENESYS 10uv, Rochester, New York, USA) using Trolox (Sigma Aldrich, St Louis, MO, USA) as the standard. The total phenolic (TP) content was determined by the Folin–Ciocalteu method (Slinkard and Singleton 1977) using chlorogenic acid as standard. Skin and pulp samples were extracted with methanol (80% v/v), and the extract was centrifuged at 10,000×g for 5 min at 4 °C and the supernatant stored at 4 °C until use. The absorbance was measured at 765 nm and the results expressed in mg of chlorogenic acid equivalents (CAE) per gram fresh weight (FW).
2.3 Determination of total flavonoid and anthocyanin contents
Flavonoids were determined as described by Meyers et al. (2003), with minor modifications. The results are expressed as mg of rutin equivalents per gram FW (mg rutin equivalent g−1 FW) as described in Ribera et al. (2010). The absorbance was measured at 515 nm in a spectrophotometer (ThermoSpectronic, GENESYS 10uv, Rochester, New York, USA) using rutin (Sigma Aldrich, St Louis, MO, USA) as standard.
The extraction of anthocyanins was performed by grinding 0.1 g of fresh material in a cold mortar, and adding 1 mL of acidified methanol. After extraction, the anthocyanin levels were determined by spectrophotometry at 530 nm and at 657 nm with a molar extinction coefficient for cyanidin-3-glucoside of 29,600 as previously described (Nyman and Kumpulainen 2001). The total anthocyanin contents are expressed as mg of cyanidin-3-glucoside equivalents (c3g) per gram FW.
2.4 Determination of metabolite levels
The frozen skin and pulp samples were lyophilized, and metabolites extracted twice with 80% ethanol and once with 50% ethanol. Sucrose, fructose and glucose were determined exactly as described previously by Fernie et al. (2001), and total amino acids as described by Cross et al. (2006). In the insoluble fraction, the starch and total protein contents were determined as indicated by Cross et al. (2006).
For metabolite analysis by gas chromatography-time of flight-mass spectrometry (GC-TOF-MS), the extraction of metabolites from lyophilized samples was performed as described by Lisec et al. (2006). Metabolite derivatization, standard addition, and sample injection for GC-MS were performed as previously described (Osorio et al. 2012). Chromatograms and mass spectra were evaluated using Chroma TOF 1.0 (Leco, http://www.leco.com/) and TAGFINDER 4.0 software (Luedemann et al. 2008). The mass spectra were cross-referenced with those in the Golm Metabolome Database (Kopka et al. 2005). The amount of each metabolite obtained by GC-TOF-MS was determined as relative metabolite abundance, calculated by normalization of signal intensity to that of ribitol, which was added as an internal standard, and then expressed per dry weight (DW) of the material, exactly as previously described (Lisec et al. 2006).
2.5 Statistical analyses
Statistical analyses were performed using the GENES program (Cruz 2013) and R statistical software (http://www.r-project.org). All data were subjected to a one-way analysis of variance (ANOVA), and the means were tested by the Tukey test at a 5% significance level. In order to reduce the dimensionality of the data set, multivariate analysis by Principal Component Analysis (PCA) was performed with Minitab® 17 (Minitab 17 Statistical Software). For PCA, data were normalized to maximize the variance of each component. Furthermore, a graphical representation of the metabolic profiling data set was shown as a heatmap using Multiple Experiment Viewer Software (MeV) version 4.5 (Saeed et al. 2003). Pearson correlation analysis and correlation networks of phytochemical compounds and selected metabolites were obtained using a t-test (5% significance level) to examine the relationships between these variables.
3 Results
3.1 Metabolite levels in skin and pulp tissues of tubers from native potatoes
In order to place the visible differences in tuber skin and pulp from different native potatoes in a metabolic context, we performed a detailed metabolic characterization of these tissues (Figs. 2, 3, Supplementary Table SI). First, we studied the contents of starch, glucose, fructose and sucrose in each genotype. In terms of starch content, we observed more starch in the pulp than in the skin (Fig. 2a, b), particularly in Desireé and Michuñe negro accessions (37.7 and 50.1% greater in pulp, respectively). On the other hand, the starch levels in the Guicoña, Clavela morada, Tonta, Guadacho blanco and Lengua de vaca, were not significantly different between these tissues (Fig. 2a, b).
Carbon metabolism related compounds in skin (a, c, e, g) and pulp (b, d, f, h) tissues of tubers from 11 Solanum tuberosum ssp. tuberosum L. landraces from Chiloé Island and two commercial cultivars (Desireé and Yagana). a, b starch; c, d glucose; e, f fructose; g, h sucrose. Values are presented as mean ± SE (n = 5). Values marked with a different letters were determined by Tukey test to be significantly different (P < 0.05) from each other. FW fresh weight, DW dry weight
Nitrogen metabolism compounds in skin (a, c) and pulp (b, d) tissues of tubers from 11 native potato (Solanum tuberosum ssp. tuberosum L.) accessions and two commercial cultivars (Desireé and Yagana). a, b total amino acids; c, d total protein levels. Values are shown as mean ± SE (n = 5). Values marked with a different letters were determined by Tukey test to be significantly different (P < 0.05) from each other. FW fresh weight, DW dry weight
High variability was observed between the content of glucose, fructose and sucrose in both tissues (Fig. 2c, h). The highest level of sucrose was observed in pulp of Michuñe azul, Murta and Tonta accessions, with levels that fluctuated between 9 and 11 µmol g−1 DW (Fig. 2h). The lowest level of sucrose was observed in skin and pulp from the Desireé cultivar (Fig. 2g, h), which had the highest concentration of glucose in pulp tissue (Fig. 2d). The level of glucose observed in this cultivar was 1.6 µmol g−1 DW in the skin and 0.8 µmol g−1 DW in the pulp. Similar glucose contents were observed in Lengua de vaca and Guadacho colorado accessions (Fig. 2c, d). The levels of fructose were higher in the skin compared to the pulp tissues in all genotypes analyzed, with the exception of the Lengua de vaca accession (Fig. 2e, f).
With respect to total amino acid levels, substantial variability was observed among genotypes in both tissues (Fig. 3a, b). The Tonta accession had similar levels of amino acids in skin and pulp. The highest levels of total amino acids were found in the skin of Meca de gato morada larga, Michuñe azul and Muchuñe negro (Fig. 3a). Higher protein levels were observed in skin with values varying between 110 and 150 mg g−1 DW (Fig. 3c), and greater than those in the pulp (Fig. 3d). However, similar levels were observed among the 13 potato genotypes in both tissues.
3.2 Anthocyanin content and antioxidant activity in skin and pulp tissues of tubers from native potatoes
Since potatoes are rich sources of phenolic compounds such as anthocyanins and phenolic acids, we extended our characterization of the native potatoes to metabolites related to antioxidant activity. Firstly, we evaluated the total anthocyanin content. For these compounds, significant differences were obtained between skin and pulp (Fig. 4a, b). Some of the native accessions, such as Guicoña and Tonta, displayed remarkably high levels of anthocyanin in both tissues (Fig. 4a, b). In the skin of Clavela morada, the total anthocyanin content was around 10-fold greater in skin compared to pulp, while in other accessions the differences were considerably lower (Fig. 4a). The anthocyanin levels varied in skin from 0.1 to 1.8 mg g−1 FW and in pulp between 0.1 and 0.3 mg g−1 FW (Fig. 4a, b).
Secondary metabolites and antioxidant activity in skin (a, c, e, g) and pulp (b, d, f, h) tissues of tubers from 11 native potato (Solanum tuberosum ssp. tuberosum L.) accessions and two commercial cultivars (Desireé and Yagana). a, b anthocyanin; c, d total phenols; e, f total flavonoids; g, h total antioxidant activity. Values are shown as mean ± SE (n = 5). Values marked with a different letters were determined by Tukey test to be significantly different (P < 0.05) from each other. FW fresh weight, DW dry weight
The total content of phenolics observed in skin fluctuated between 1 and 3 mg g−1 FW, whereas in pulp, levels reached 0.5 mg g−1 FW (Fig. 4c, d). High levels of phenolics were observed in the skin of Guadacho colorado and Lengua de vaca (Fig. 4c). The native potatoes Lengua de vaca, Clavela morada, Tonta and Guadacho colorado displayed higher phenolic contents in the skin in comparison with pulp tissue (Fig. 4c, d).
Concerning the total flavonoid levels, skin tissues possessed more than pulp tissues. These values ranged from 3000 to 7600 mg rutin equivalents g−1 FW in skin and 190–430 mg rutin equivalents g−1 FW in pulp tissues (Fig. 4e, f). High flavonoid levels were observed in the skin of Lengua de vaca, and less in Clavela morada, Guicoña and Michuñe negro, in comparison with Desireé and Yagana (Fig. 4e). In pulp tissues, increased levels were observed only in Meca de gato morada larga and Michuñe azul, compared to both commercial cultivars (Fig. 4f).
Furthermore, we detected differences in antioxidant activity between skin and pulp tissues (Fig. 4g, h). High antioxidant activity was observed in the skin of Lengua de vaca, Michuñe azul, Clavela morada and Tonta in comparison with both commercial cultivars (Fig. 4g). In pulp, higher antioxidant activity was observed in Michuñe azul, Michuñe negro, Murta, Guadacho blanco and Lengua de vaca, and less activity in Cauchau, Guadacho Colorado and Yagana, in comparison with Desireé (Fig. 4h).
3.3 Pearson correlation analysis and integration of primary metabolites and phytochemical traits
To assess the level of association between the main carbon and nitrogen containing compounds and phytochemical traits, we next calculated the Pearson correlation coefficients for all pairs of primary metabolites (protein, total protein, hexoses, sucrose and starch) and phytochemical traits. The full data set of correlation coefficients is presented in a heat map (Fig. 5a, b). In skin tissues, starch content positively correlated with anthocyanin levels, whereas in pulp, starch negatively correlated with anthocyanin (Fig. 5a). Interestingly in pulp tissues, antioxidant activity positively correlated with protein and amino acid levels and negatively correlated with starch levels (Fig. 5b). Furthermore, in skin tissues phenolic content correlated positively with antioxidant activity, and anthocyanin negatively correlated with flavonoids.
Correlation and network analysis of phytochemical compounds and other metabolites in skin and pulp of thirteen native potatoes accessions. Correlation matrix based on Pearson coefficients derived from the data obtained from skin (a) and pulp (b) indicated by color gradient, with positive and negative correlations being distinguished by green and red, respectively. Significant correlation coefficients are indicated by asterisk (*). The correlation network from skin (c) and pulp (d) with trait associated to carbon metabolism shown as blue dots, nitrogen metabolism as green dots and phytochemical compounds yellow colored. Green connecting lines represent positive correlations and red connecting lines negative, respectively. Line width is proportional to the strength of the correlation. AA total amino acids, Antox Act antioxidant activity, Anth anthocyanin, Glu glucose, Fru fructose, Suc sucrose, Phen phenols, Flav flavonoids, Prot total protein levels
For a better understanding of the association between the evaluated traits of all potato genotypes analyzed, the correlation coefficients were arranged in a correlation network (Fig. 5c, d). In this analysis, a large number of associations was observed. In skin tissue, protein and total amino acids showed a negative correlation with those of anthocyanin and phenolics, respectively (Fig. 5c). On the other hand, in pulp tissue there was a positive correlation between nitrogen metabolism and phytochemicals such as flavonoids and antioxidant activity (Fig. 5d).
When we analyzed the skin variables related to carbon metabolism, we observed that starch levels positively correlated with most of the phytochemicals analyzed. Nonetheless, in pulp tissue this correlation was opposite. Although starch seems to play a central role in the relationship between phytochemicals and carbon metabolism in skin tissue, glucose levels also displayed a noticeable correlation with phytochemicals, such as phenolics and flavonoids.
3.4 Primary metabolite profiles in skin and pulp of tubers from native potatoes
To investigate the metabolites of the major primary pathways in both tuber tissues in a broader metabolic context, we performed metabolic profiling analysis. Metabolite profiles were obtained via the relative quantification of 79 and 73 metabolites of known chemical structure in skin and pulp tissues, respectively (Supplementary Tables SII, SIII). These compounds include most plant amino acids, organic acids and sugars. In order to display a general overview of the results, part of the data set from the metabolite profiling study is presented as a heat map (Fig. 6).
Heat map representing the changes in relative levels of primary metabolites in tissues of tubers from 11 Solanum tuberosum ssp. tuberosum L. native potato and two commercial cultivars (Desireé and Yagana). Data are scaled to the mean response measured in the Desireé cultivar for skin and pulp of each metabolite. Values shown are means of four biological replicates. Undetected metabolites are marked in gray. Different letters in each row refer to statistical differences between each genotype according to the Tukey test (P < 0.05)
In total eighteen amino acids were analyzed in the skin and pulp tissues of all 13 accessions (Fig. 6). Low variability between the native potatoes, which were significantly different from the commercial cultivars, was found for alanine, asparagine, glycine, histidine and isoleucine in both skin and pulp tissues. Interestingly, the levels of alanine and glycine were significantly lower in skin of the native potatoes in comparison with Desireé and Yagana cultivars.
The levels of tricarboxylic acid cycle intermediates 2-oxoglutarate, citrate and pyruvate in skin, and fumarate, isocitrate and malate in pulp displayed only minor variations among the genotypes (Fig. 6). The levels of succinate did not vary between the genotypes in both skin and pulp tissues. However, the levels of glycerate, maleate and nicotinate varied between genotypes in skin tissues, and were significantly lower in several of the native accessions compared with the Desireé cultivar.
In addition, we evaluated sugars and related compounds in skin and pulp tissues. Of the sugars identified, isomaltose, melibiose, sorbose and xylose were detected only in pulp tissues. Meanwhile, cellobiose, gentiobiose, maltotriose, palatinose and turanose were quantifiable only in the skin tissue (Fig. 6). Surprisingly the levels of cellobiose, maltose, palatinose and turanose did not vary between the native accessions but were reduced in skins of all accessions in comparison with Desireé. In pulp tissues, reduced levels of maltose, sorbose and xylose were observed in most of the studied accessions. Concerning the levels of hexose phosphates, minor variability in the levels of glucose-6-P and fructose-6-P was found in the skin tissue of the different genotypes. In pulp tissues, fructose-6-P was essentially invariant (Fig. 6). In addition, ribulose-5-P levels in skin tissues were significantly lower in seven native potato accessions.
Moreover, several other metabolites related to different pathways were evaluated. In skin tissues, significant differences in the levels of calystegine, 2-hydroxy-cinnamic acid, putrescine and spermine were observed between the native potatoes and Desireé (Supplementary Table II). In pulp tissue significant differences were observed for some of the native potato accessions in the levels of calystegine, myo-inositol, myo-inositol-1-P and 3-caffeoyl-quinic acid in comparison with Desireé (Supplementary Table III).
3.5 HCA and PCA of the metabolite profiles of skin and pulp tissues of native potatoes
To identify the differences between the metabolite profiles of skin and pulp tissues of native potatoes, the metabolite data set were analyzed using PCA (Fig. 7). In skin tissue, the first principal component (PC1) and the second principal component (PC2) accounted for 25.2% and 16.7% of the total variation, respectively (Fig. 7a). On the other hand, in pulp tissue, PC1 and PC2 explained 30.9% and 16.6% of the total variance, respectively (Fig. 7b). When we compared the PCA scores plot of skin tissues, we observed a clear separation in PC1 of a group of nine native potatoes from the cultivar Desireé and three other accessions (Yagana, Cauchau and Guadacho colorado) (Fig. 7a). In pulp tissues, there was a separation of two groups of accessions and Desireé (Fig. 7b). Interestingly, in both tissues, when the clusters are analyzed in detail, it is clear that the isolation of the cultivar Desireé is more related with sugars than amino acid levels, in comparison with the more distant group of native potatoes (eg Michuñe negro, michuñe azul and Meca de gato). Together these data suggest that the most substantial differences in terms of metabolites in native potatoes are found in amino acid and sugar levels. In addition, this result indicates that, in both skin and pulp, the native potato accessions have very similar metabolite profiles between them, but contrast dramatically with the commercial cultivar Desireé.
Principal component analysis (PCA) in skin (a, b) and pulp (c, d) tissues of tubers from 11 native potato (Solanum tuberosum ssp. tuberosum L.) accessions and two commercial cultivars (Desireé and Yagana) based on metabolite profiles. The analysis was performed on the correlation matrix of least square means of averaged accessions. Numbers in parentheses give the percent variation explained by the first and the second principal component. a, b show the scores, and c, d show the loading plots obtained for the resulting distribution of potato genotype and metabolite data, respectively. Text colors indicate the cluster to which metabolites have been assigned using hierarchical clustering (green, amino acid; brown, organic acid; red, sugar; pink, sugar alcohol; blue, miscellaneous)
4 Discussion
4.1 Phytochemical content in native potatoes
Potatoes, specially the purple colored ones, are sources of antioxidant compounds including polyphenols, carotenoids and vitamins, pointing to their relevance not only as a starchy food, but also as a vegetable important for health (Chun et al. 2005; Ezekiel et al. 2013; Chandrasekara and Kumar 2016). Several studies reported high antioxidant activity of potato tubers (Mattila and Hellstron 2007; Lachman et al. 2009), flowers (Sosulski et al. 1982), stems and leaves (Hyon et al. 2008), as well as in tuber skin and pulp (Valcarcel et al. 2015). In this study, we quantified the levels of phenols, flavonols, anthocyanins and the total antioxidant activity (Fig. 4). The levels of these phytochemicals varied among the analyzed potato accessions in both skin and pulp tissues. In general, the highest antioxidant activities were found in purple-skinned and black pulp accessions, like Clavela morada (in skin) and Michuñe azul (in both skin and pulp), and also in the white-skinned and white pulp Lengua de vaca (in both skin and pulp) and Tonta (in skin). In agreement with these results, potato tubers have been described as a rich source of phenolic compounds, with almost 50% of them located in the skin and adjoining tissues (Al-Weshahy et al. 2009; Valcarcel et al. 2015). In our study, the levels of phenolic compounds in skin varied depending on the color and variety of the potato genotypes (Fig. 4). The phenolic compounds are present in the potato skin and flesh; however, the skin is reported to have the highest amounts (Ezekiel et al. 2013). We found particularly high phenolic levels in the skin of Guadacho colorado, Clavela morada, Tonta and Lengua de vaca, mostly associated with high antioxidant activity in these native accessions (Fig. 4). Because of their potential health benefits, in the last decade, there has been increasing attention given to new sources of natural antioxidant phytochemicals (Akyol et al. 2016). That way, these cultivars could be an ideal source to produce potato plants with healthier tubers by means of the transgenic or classical genetic approaches.
The Clavela morada accession possesses a speckled skin coloration, probably attributable to regions exhibiting enrichment of anthocyanins (Fig. 4a). Red and purple skin color of pulp may be partially or entirely due to anthocyanin accumulation (Brown 2005). By contrast, accessions such as Tonta (non-colored skin) and Clavela morada (blue skin) may contain very high levels of phenolic acids and/or other non-anthocyanin flavonoids. Valcarcel et al. (2015) reported that anthocyanins are absent or only present in negligible amounts in the skin of non-colored genotypes. These studies are consistent with the negative correlation observed between flavonoids and anthocyanins in our work (Fig. 5a). Positive correlations were found among total phenolics, total flavonoids and antioxidant activity (Valcarcel et al. 2015). Moreover, our correlation data analyses indicate that phenolic levels in skin tissues are positively correlated with antioxidant activity. Surprisingly, the associations described between these phytochemicals and antioxidant activity were significant in pulp tissues. Together these results indicates that the secondary metabolism taking plance in pulp and skin tissues is quite complex and therefore further research efforts at metabolite and transcript levels should be performed for enhancing our current understanding of the presented associations in tuber tissues of native potatoes.
4.2 Phytochemical compounds and primary metabolites in tuber tissues of native potatoes
In this study, distinct associations were observed between anthocyanin levels and antioxidant capacity with starch, protein and amino acid contents (Fig. 5). In pulp tissues, antioxidant activity was inversely correlated with starch and positively correlated with amino acid and protein levels. Consistently, changes in antioxidant capacities in tubers of potato plants overexpressing and repressing key enzymes of the flavonoid synthesis pathway such as chalcone synthase, chalcone isomerase and dihydroflavonol reductase, increase the synthesis of phenolic compounds accompanied by a decrease in starch and glucose levels in the transgenic plants (Lukaszewicz et al. 2004). Flavonoids and phenolic compounds may also respond to the carbohydrate level by regulatory mechanisms that efficiently divert carbon towards the biosynthesis of aromatic amino acids, precursors of the phenylpropanoid pathway (Obata and Fernie 2012). Thus, these results suggest that in pulp tissues, the antioxidant activity and synthesis of related compounds is associated with the availability of carbohydrates for phenolic compound synthesis. Interestingly, the results observed in skin suggest a weak correlation between starch and antioxidant activity, and a strong positive correlation between starch and anthocyanin levels, indicating that in skin tissues alternative associations between these metabolites take place. Taken together, our results indicate tight and distinct associations between primary and secondary metabolites in skin and pulp tissues. However, since the levels of phenolics and flavonols, as well as antioxidant activity in potato tubers vary according to the season and field of cultivation (Payyavula et al. 2012; Valcarcel et al. 2015), additional experiments and analysis are needed to fully understand these associations.
4.3 Metabolite markers for breeding programs
In potato tubers, different primary metabolites have been proposed to function as predictors for agronomically important traits such as black spot bruising and chip quality (Steinfath et al. 2010) and starch levels (Carreno-Quintero et al. 2012). In our study, we observed variation in the levels of potential metabolite markers among the studied accessions. For example, glucose and fructose, which are involved in the Maillard reaction, which is responsible for the browning of potato chips during frying and also leads to the accumulation of carcinogenic acrylamide (Zhang and Zhang 2007), were drastically reduced in the pulp of some of the native accessions (Fig. 2d). Furthermore, we were able to quantify valine, serine, threonine, glutamine and tyrosine in both skin and pulp tissues (Fig. 6). A large variation in the levels of these amino acids in skin was observed between native potato accessions. These amino acids are associated with susceptibility of potato tubers to black spot bruising (Steinfath et al. 2010). This physiological disorder reduces the commercial value of the tubers and is characterized by the formation of dark-blue to blackish melanin spots, below the skin (Laerke et al. 2002). Another important metabolite marker that displayed different levels compared to the commercial cultivar Desiree was alanine (Fig. 6). This amino acid is needed for the biosynthesis of vitamin B5 (Chakauya et al. 2006) and the formation of other metabolites essential for plant development. Moreover, it has been proposed that β-alanine is associated with the phosphorylation of starch, as well as a number of other metabolites in tubers (Carreno-Quintero et al. 2012). The content of phosphate groups in starch is related with the activity of enzymes involved in starch degradation (Blennow et al. 2002; Meekins et al. 2016) and also with the physico-chemical properties and the end-uses of starches (Blennow et al. 2002). In summary, the characterization of the metabolite composition of native accessions of potatoes from Chiloé revealed that these genotypes might be useful for understanding of metabolic processes associated with phenotypic features of interest.
4.4 Final considerations
The results presented in this study highlight the high nutritional value of the skin from these native potatoes and also indicate that the different accessions described here are promising genotypes for plant metabolic engineering and breeding programs for enhancing beneficial health compounds in potato tubers. Furthermore, we can conclude that there is a very complex relationship between flavonoids, phenolic contents and antioxidant capacity in skin and in pulp tissues, suggesting the participation of other compounds in the antioxidant potential of potato tubers. Moreover, since site and year of cultivation affect the levels of phenolics and flavonoids, as well as antioxidant activity in potato tubers (Payyavula et al. 2012; Valcarcel et al. 2015), further experiments are essential to fully explore the results obtained in the present study. In addition, further profiling of individual phenolic and flavonoid compounds is necessary to elucidate the associations between metabolites and the genetic and mechanistic bases for them. Finally, analyses of a larger potato germplasm collection, containing more of the globe’s genetic diversity, is required to validate the described correlations.
References
Akyol, H., Riciputi, Y., Capanoglu, E., Caboni, M. F., & Verardo, V. (2016). Phenolic compounds in the potato and its byproducts: An overview. International Journal of Molecular Sciences, 17, 835.
Albishi, T., John, J., Al-Khalifa, A., & Shahidi, F. (2013). Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. Journal of Functional Foods, 5, 590–600.
Al-Weshahy, A., El-Nokety, M., Bakhete, M., & Rao, V. (2013). Effect of storage on antioxidant activity of freeze-dried potato peels. Food Research International, 50, 507–512.
Al-Weshahy, A., & Rao, A. V. (2009). Isolation and characterization of functional components from peel samples of six potatoes varieties growing in Ontario. Food Research International, 42, 1062–1066.
Ames, B. N., Shigenaga, M. K., & Hagen, T. M. (1993). Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Sciences of the United States of America, 90, 7915–7922.
Blennow, A., Engelsen, S. B., Nielsen, T. H., Baunsgaard, L., & Mikkelsen, R. (2002). Starch phosphorylation: A new front line in starch research. Trends in Plant Science;7(10), 445–450.
Brown, C. (2005). Antioxidants in potato. American Journal of Potato Research, 82, 163–172.
Camandro, E. L., Erazzú, L. E., Maune, J. F., & Bedogni, M. C. (2012). A genetic approach to the species problem in wild potato. Plant Biology, 14, 543–554.
Carreno-Quintero, N., Acharjee, A., Maliepaard, C., Bachem, C. W., Mumm, R., Bouwmeester, H., et al. (2012). Untargeted metabolic quantitative trait loci analyses reveal a relationship between primary metabolism and potato tuber quality. Plant Physiology, 158, 306–1318.
Chakauya, E., Coxon, K. M., Whitney, H. M., Ashurst, J. L., Abell, C., & Smith, A. G. (2006). Pantothenate biosynthesis in higher plants: Advances and challenges. Physiologia Plantarum, 126, 319–329.
Chandrasekara, A., & Josheph Kumar, T. (2016). Roots and tuber crops as functional foods: A review on phytochemical constituents and their potential health benefits. International Journal of Food Science. https://doi.org/10.1155/2016/3631647.
Chinnici, F., Bendini, A., Gaiani, A., & Riponi, C. (2004). Radical scavenging activities of peels and pulps from cv. Golden delicious apples as related to their phenolic composition. Journal of Agricultural and Food Chemistry, 52, 4684–4689.
Chun, O. K., Kim, D. O., Smith, N., Schroeder, D., Han, J. T., & Lee, C. Y. (2005). Daily consumption of phenolics and total antioxidantcapacity from fruit and vegetables in the American diet. Journal ofthe Science of Food and Agriculture, 85, 1715–1724.
Contreras, A., Banse, J., Fuentealba, J., Aruta, C., & Manquian, N. (1981) Germoplasma chileno de papas (Solanum tuberosum L.). Universidad Austral de Chile, Facultad de Ciencias Agrarias, Instituto de Producción Vegetal. Informe final-1980, 33.
Cross, J. M., von Korff, M., Altmann, T., Bartzetko, L., Sulpice, R., Gibon, Y., et al. (2006). Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiology, 142, 1574–1588.
Cruz, C. D. (2013). GENES—a software package for analysis in experimental statistics and quantitative genetics. Acta Scientiarum, Agronomy, 35, 271–276.
Dobson, G., Shepherd, T., Verral, S. R., Conner, S., McNicol, J. W., Ramsay, G., et al. (2008). Phytochemical diversity in tubers of potato cultivars and landraces using a GC-MS metabilomics approach. Journal of Agricultural and Food Chemistry, 56, 10280–10291.
Dobson, G., Shepherd, T., Verral, S. R., Griffiths, W. D., Ramsay, G., McNicol, J. W., et al. (2010). A metabolomics study of cultivated potato (Solanum tuberosum) group Andiggena, Phureja, Stenotomum and Tuberosum using gas chromatography-mass spectrometry. Journal of Agricultural and Food Chemistry, 58, 1214–1213
Ezekiel, B., Singh, N., Sharma, S., & Kaur, A. (2013). Beneficial phytochemicals in potato. A review. Food Research International, 50, 487–496.
Fernie, A. R., Roscher, A., Ratcliffe, G. R., & Kruger, N. J. (2001). Fructose 2,6-bishosphate activates pyrophosphate: Fructose-6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta, 212, 250–263.
Hyon, W. I., Bong-Soon, S., Seung-U, L., Nobuyuki, K., Mayumi, O. K., Carol, E. L., et al. (2008). Analysis of phenolic compounds by high performance liquid chromatography/mass spectrometry in potato flowers, leaves, stem and tubers in home-processed potatoes. Journal of Agricultural and Food Chemistry, 56, 3341–3349.
Jansen, G., & Flamme, W. (2006). Coloured potatoes (Solanum tuberosum L.) anthocyanin content and tuber quality. Genetic Resources and Crop Evolution, 53, 1321–1331.
Kopka, J., Schauer, N., Krueger, S., Birkemeyer, C., Usadel, B., Bergmüller, E., et al. (2005). GMD@CSB.DB: The Golm Metabolome Database. Bioinformatics, 21, 1635–1638.
Lachman, J., Karel, H., Miloslav, Š, Matyáš, O., Vladimír, P., Alena, H., et al. (2009). Cultivar differences of total anthocyanins and anthocyanidins in red and purple-fleshed potatoes and their relation to antioxidant activity. Food Chemistry, 114, 836–843.
Laerke, P. E., Christiansen, J., & Veierskov, B. (2002). Colour of blackspot bruises in potato tubers during growth and storage compared to their discolouration potential. Postharvest Biology and Technology, 26, 99–111.
Lisec, J., Schauer, N., Kopka, J., Willmitzer, L., & Fernie, A. R. (2006). Gas chromatography mass spectrometry-based metabolite profiling in plants. Nature Protocols, 1, 387–396.
Lovat, C., Nassar, A. M., Kubow, S., Li, X. Q., & Donnelly, D. (2016). Metabolic biosynthesis of potato (Solanum tuberosum L.) antioxidants and implications for human health. Critical Reviews in Food Science and Nutrition, 56, 2278–2303.
Luedemann, A., Strassburg, K., Erban, A., & Kopka, J. (2008). TagFinder for the quantitative analysis of gas chromatography-mass spectrometry (GC-MS)-based metabolite profiling experiment. Bioinformatics, 24, 732–737.
Lukaszewicz, M., Matysiak-Kata, I., Skala, J., Fecka, I., Cisowski, W., & Szopa, J. (2004). Antioxidant capacity manipulation in transgenic potato tuber by changes in phenolic compounds content. Journal of Agricultural and Food Chemistry, 52, 1526–1533.
Mattila, P., & Hellström, J. (2007). Phenolic acids in potatoes, vegetables, and some of their products. Journal of Food Composition and Analysis, 20, 152–160.
Meekins, D. A., Vander Kooi, C. W., & Gentry, M. S. (2016). Structural mechanisms of plant glucan phosphatases in starch metabolism. The FEBS Journal., 283, 2427–2447.
Meyers, K. J., Watkins, C. B., Pritts, M. P., & Liu, R. H. (2003). Antioxidant and antiproliferative activities of strawberries. Journal of Food Composition and Analysis, 51, 6887–6892.
Nyman, N. A., & Kumpulainen, J. T. (2001). Determination of anthocyanidins in berries and red wine by high-performance liquid chromatography. Journal of Food Composition and Analysis, 49, 4183–4187.
Obata, T., & Fernie, A. R. (2012). The use of metabolomics to dissect plant responses to abiotic stresses. Cellular and Molecular Life Sciences, 69, 3225–3243.
Osorio, S., Do, P. T., & Fernie, A. R. (2012). Profiling primary metabolites of tomato fruit with gas chromatography/mass spectrometry. Methods in Molecular Biology, 860, 101–109.
Payyavula, R. S., Navarre, D. A., Kuhl, J. C., Pantoja, A., & Pillai, S. S. (2012). Differential effects of environment on potato phenylpropanoid and carotenoid expression. BMC Plant Biology, 12, 39.
Reyes, F. L., Miller, J. C. Jr., & Cisneros-Zevallos, L. (2005). Antioxidant capacity, anthocyanins and total phenolics in purple-and red-fleshed potato (Solanum tuberosum L.) genopypes. American Journal of Potato Research, 82, 271–286.
Ribera, A., Reyes-Díaz, M., Alberdi, M., Zuñiga, G., & Mora, M. L. (2010). Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Vaccinium corymbosum L.) grown in Southern Chile. Journal of Soil Science and Plant Nutrition, 10, 509–536.
Saeed, A. I., Sharov, V., White, J., Li, J., Liang, W., Bhagabati, N., et al. (2003). TM4: A free, open-source system for microarray data management and analysis. BioTechniques, 34, 374.
Scalbert, A., Manach, C., Morand, C., Rémésy, C., & Jiménez, L. (2005). Dietary polyphenols and the prevention of diseases. Critical Reviews in Food Science and Nutrition, 45, 287–306.
Singh, N., & Rajini, P. S. (2004). Free radical scavenging activity of an aqueous extract of potato peel. Food Chemistry, 85, 611–616.
Singh, N., & Rajini, P. S. (2008). Antioxidant-mediated protective effect of potato peel extract in erythrocytes against oxidative damage. Chemico-Biological Interactions, 173, 97–104.
Singh, P. P., & Saldana, M. D. A. (2011). Subcritical water extraction of phenolic compounds from potato peel. Food Research International, 44, 35–38.
Slinkard, K., & Singleton, V. L. (1977). Total phenol analysis: Automation and comparison with manual methods. American Journal of Enology and Viticulture, 28, 29–55.
Solano, J., Mathias, M., Esnault, F., & Brebant, P. (2013). Genetic diversity among native varieties and commercial cultivars of Solanum tuberosum ssp tuberosum L. present in Chile. Electronic Journal of Biotechnology, 16, 6.
Sosulski, F., Krygier, K., & Hogge, L. (1982). Free, esterified, and insoluble-bound phenolic acids. 3. Composition of phenolic acids in cereal and potato flours. Journal of Agricultural and Food Chemistry, 30, 337–340.
Spooner, D. M., McLean, K., Ramsay, G., Waugh, R., & Bryan, G. J. (2005). A single domestication for potato based on multilocus amplified fragment length polymorphism genotyping. Proceedings of the National Academy of Sciences of the United States of America, 102, 14694–14699.
Steinfath, M., Strehmel, N., Peters, R., Schauer, N., Groth, D., Hummel, J., Steup, M., Selbig, J., Kopka, J., Geigenberger, P., & Van Dongen, J. T. (2010). Discovering plant metabolic biomarkers for phenotype prediction using an untargeted approach. Plant Biotechnol Journal, 8, 900–911.
Teow, C. C., Truong, V. D., McFeeters, R. F., Thompson, R. L., Pecota, K. V., & Yencho, G. C. (2007). Antioxidant activities, phenolic and b-carotene contents of sweet potato genotypes with varying flesh colours. Food Chemistry, 103, 829–838.
Valcarcel, J., Reilly, K., Gaffney, M., & O’Brien, N. M. (2015). Antioxidant activity, total phenolic and total flavonoid content in sixty varieties of potato (Solanum tuberosum L.) grown in Ireland. Potato research, 58, 221–244.
Zhang, Y., & Zhang, Y. (2007). Formation and reduction of acrylamide in maillard reaction: A review based on the current state of knowledge. Critical Reviews in Food Science and Nutrition, 47, 521–542.
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) [grant number CBB - AUC-00018-16]. Research fellowships were granted by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to ANN and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) [Grant Number CBB-BPD- 00019-16] to FMOS. ANN, MRD and CIB also acknowledge the support from the Chilean Ministerio de Educación (MEC-CONICYT; Grant PAI80160036). The authors wish to thank the NUBIOMOL-UFV for providing the facilities for the analysis of this work, and Michael Handford (Universidad de Chile) for language support.
Author information
Authors and Affiliations
Contributions
CIB, MRD, and ANN conceived and designed the research. FD and CIB performed the experiments. TO, MM and FMdeOS contributed powerful analytical tools. CIB, FMdeOS and MM analyzed data. CIB and ANN wrote the manuscript. MRD, TO, JS and ARF revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11306_2018_1428_MOESM1_ESM.xlsx
SI Table 1. Antioxidant activity, starch content, total amino acid, total anthocyanin, glucose content, fructose content, sucrose content, total phenol, total flavonoids and total protein in skin and pulp tissues of tubers from 11 native potato (Solanum tuberosum ssp. tuberosum L.) varieties and two commercial cultivars (Desireé and Yagana). Values are mean ± SE (n=5). Values in the same row followed by different letters are statistically different (P < 0.05). FW= fresh weight; DW= dry weight Supplementary material 1 (XLSX 24 KB)
11306_2018_1428_MOESM2_ESM.xlsx
SI Table 2. Relative abundance of primary skin metabolites of 11 native potato (Solanum tuberosum ssp. tuberosum L.) varieties and two commercial cultivars (Desireé and Yagana). Values are mean ± SE (n=5). Values in the same row followed by different letters are statistically different (P < 0.05). Abbreviations: D, Desireé; MGML, Meca de gato morada larga; MA, Michuñe azul; C, Cauchau; G, Guicoña; MN, Michuñe negro; GC, Guadacho colorado; M, Murta; CM, Clavela morada; Y, Yagana; T, Tonta; GB, Guadacho blanco; LV, Lengua de vaca. Supplementary material 2 (XLSX 61 KB)
11306_2018_1428_MOESM3_ESM.xlsx
SI Table 3. Relative abundance of primary pulp metabolites of 11 native potato (Solanum tuberosum ssp. tuberosum L.) varieties and two commercial cultivars (Desireé and Yagana). Values are mean ± SE (n=5). Values in the same row followed by different letters are statistically different (P < 0.05). Abbreviations: D, Desireé; MGML, Meca de gato morada larga; MA, Michuñe azul; C, Cauchau; G, Guicoña; MN, Michuñe negro; GC, Guadacho colorado; M, Murta; CM, Clavela morada; Y, Yagana; T, Tonta; GB, Guadacho blanco; LV, Lengua de vaca. Supplementary material 3 (XLSX 52 KB)
Rights and permissions
About this article
Cite this article
Inostroza-Blancheteau, C., de Oliveira Silva, F.M., Durán, F. et al. Metabolic diversity in tuber tissues of native Chiloé potatoes and commercial cultivars of Solanum tuberosum ssp. tuberosum L.. Metabolomics 14, 138 (2018). https://doi.org/10.1007/s11306-018-1428-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-018-1428-7