Abstract
Introduction
Induction of tryptophan (TRP) catabolism is an adaptation mechanism to restrict excessive acute immune response in tissues. In the tumour microenvironment, TRP catabolism’s dysregulation plays an important role in local antitumour immune response suppression.
Aim
We investigated changes in the plasma concentrations of TRP and its metabolites in a cohort of colorectal cancer (CRC) patients at different tumour stages and in subjects at risk of developing CRC. TRP metabolites were assessed along kynurenine and serotonin pathways, and the activity of involved enzymes and their tissue expression were monitored.
Method
Plasmatic levels of tryptophan metabolites were quantified in 80 patients’ plasma samples by means of High-Pressure Liquid Chromatography coupled to UltraViolet/Fluorescence Detectors (HPLC-UV/FD), after a simple dilution step. Tissue IDO1 gene expression during to the adenoma-carcinoma sequence and samples were obtained from formalin-fixed and paraffin-embedded (FFPE) normal colon and tumour tissues from a subset of patients (n = 21).
Results
Altered TRP concentrations were detected in plasma samples concomitant to pre-cancerous lesion and persisted during the adenoma-carcinoma transition. Moreover, the anatomical site of cancer lesions (colon or rectum) strongly influences the TRP metabolic profiles. Colon cancer patients exhibited increased TRP catabolism with respect to those affected by rectal cancer, suggesting that TRP’s metabolism alterations play an important role in the onset and progression of colon cancer, but not in those of rectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
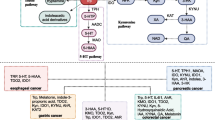
A para-inflammation state is present in tissues exposed to chronic stress and is characterized by a mild to low-grade inflammation. Under normal physiological conditions, the induction of tryptophan (TRP) catabolism restricts excessive acute immune response. In most human tissues, this process occurs through two oxidative pathways named kynurenine and serotonin pathways. The kynurenine pathway is the major route of TRP degradation, with the first step consisting in TRP oxidation by cleavage of the indole-ring. This step can be initiated either by indoleamine-2,3-dioxygenases 1 (IDO1), indoleamine-2,3-dioxygenases 2 (IDO2), or tryptophan-2,3-dioxygenase (TDO). Genes encoding IDO1 and IDO2 proteins are normally expressed in lung, brain, and placenta (Myint et al. 2007), whilst TDO is mainly expressed at hepatic level (Le Floc’h et al. 2011; van Baren and van den Eynde 2015). These enzymes are involved in a variety of physio-pathological processes such as: antimicrobial response and immunoregulation (Schmidt et al. 2009), neuropathology (Vecsei et al. 2013), antitumour defence (van Baren and van den Eynde 2015), and antioxidant activity (Thomas and Stocker 1999). An important TRP metabolite, i.e. serotonin, is a key neurotransmitter in the brain-gut axis and it is synthesized in the serotonin pathway. In the first step of this pathway, TRP is converted to 5-hydroxytryptophan (5-HTP) by the rate-limiting enzyme tryptophan hydroxylase (TPH). Serotonin exhibits growth stimulatory effects via binding to 5HT1 and 5HT2 receptors and promotes tumour growth and survival in several cancers (Sarrouilhe et al. 2015; Siddiqui et al. 2005). However, serotonin can also act in the opposite direction, depending on its levels or on the tumour type (Vicaut et al. 2000). For these reasons, its role in cancer needs to be further elucidated.
Tryptophan is an important link between inflammation and neoplastic progression in many types of cancers and the concomitant alteration of free tryptophan plasma levels has been described (Cascino et al. 1991; Miyagi et al. 2011; Wiggins et al. 2015). In fact, it is now well recognized that TRP catabolism into the tumour microenvironment plays an important role in promoting antitumour immune response, because IDO1 is triggered by the innate response to produce kynurenine (KYN) and to deplete TRP. The ratio KYN/TRP is currently used as a good estimate of IDO1 enzymatic activity (Comai et al. 2011; Favennec et al. 2015; Myint and Kim 2014; Schwarcz et al. 2012). The dysregulated inflammatory response ultimately evades the innate immunity control and induces tumour tolerance through a complex network of pro-inflammatory factors and carcinogens mediators (Mandal et al. 2016; Masaki et al. 2015; Munn and Mellor 2016; Prendergast et al. 2014). The activation and/or deactivation of these molecular mediators is crucial for the inflammation and cancer cross-talk (Chai et al. 2015).
The inflammation-cancer cross-talk appears to be extremely relevant in the case of colorectal cancer (CRC). In fact, chronic bowel inflammations, such as Crohn’s disease and Irritable Bowel Syndrome (IBS), are associated with increased risk of developing tumours in the same anatomical site (Ekbom et al. 1990). Subjects with active Crohn’s disease and IBS patients exhibit lower serum tryptophan levels and higher KYN/TRP ratio as compared with healthy controls (Clarke et al. 2009; Forrest et al. 2003; Gupta et al. 2012), indicating IDO1 contribution to these pathologies. Stress is also a facilitating factor for cancer development (Mantovani et al. 2008). Stress plays an important role in the psychological effects of cancer progression; indeed, reduced tryptophan concentrations and increased KYN/TRP ratio are associated with physical symptoms and sickness (Powell et al. 2013) and decreased quality of life in patients with colorectal liver metastases (Huang et al. 2002).
In colon cancer cell lines, the basal mRNA expression level of IDO1 is very low in the absence of stimuli. Yet, its expression can be strongly induced by IFN-γ (Ferdinande et al. 2012; Ogawa et al. 2012). IDO1 activation in CRC induces tumour progression through the activation of β-catenin pathway and an increase of kynurenine and quinolinic acid production (Thaker et al. 2013). Decreased TRP and increased kynurenine concentrations have been associated with immune system tolerance (Munn et al. 2005) and tumour cells survival (Opitz et al. 2011) in CRC. As a consequence, TRP metabolism in CRC might be proposed as an important prognostic marker, being the increased tumour IDO1 expression associated with a worse clinical outcome (Lob et al. 2009). Moreover, the immunomodulatory effects of TRP and its metabolites may also play a role in the patients’ response to therapy, as recently highlighted in rectal cancer patients who underwent pre-operative chemotherapy (Agostini et al. 2015).
Even though accumulated evidences underscore the importance of TRP metabolism in colorectal pathogenesis, several aspects still need to be clarified. Unlike other tumours, higher kynurenine concentrations in CRC have not been unequivocally associated with IDO1 expression, suggesting that the mere expression of tryptophan catabolic enzymes does not provide sufficient data for optimal immunotherapy (Puccetti et al. 2015). In particular, it is not clear how and when these alterations occur along CRC onset and progression. In particular, there are no data regarding the serotonin pathway and CRC.
In this work, we characterized the TRP metabolism along the kynurenine and the serotonin pathways in a cohort of CRC patients at different tumour stages and in subjects at risk to develop CRC. To do this, we assessed the circulating levels of TRP and its metabolites in relation to the adenoma-adenocarcinoma transition and we correlated the results with tissue IDO1 expression, sex, age, and cancer anatomical site.
2 Materials and methods
2.1 Chemicals
Analytical standard l-tryptophan (TRP, HMDB ID: HMDB 00929) was purchased from EGA-CHEMIE (Steinheim/Albuch W. Germany); 5-hydroxytryptophan (5HTP, HMDB ID: HMDB00504), hydrochloride serotonin (5HT, HMDB ID: HMDB00259), and l-kynurenine sulphate salt (KYN, HMDB ID: HMDB00684) were purchased from Sigma-Aldrich (Saint Louis, MO, USA).
HPLC grade acetonitrile was purchased from Avator Performance Materials BV (Deventer, Netherlands); anhydrous potassium hydrogen phosphate (K2HPO4) and potassium dihydrogen phosphate (KH2PO4), both of analytical grade, were purchased from Fluka Chemie (Buchs, Switzerland).
2.2 Patients’ enrolment
The protocol was approved by ethics committee of institution (Comitato Etico del Centro Oncologico Regionale, Approved Protocol Number: P448) and written informed consent was obtained from every individual. Plasma samples of colorectal cancer (n = 68) and high-risk (> 1 cm2) adenomas (n = 12) patients were obtained from the Tissue Biobank of the First Surgical Clinic of Padua Hospital (Italy). Control samples (n = 31) were obtained from the same institution. Selected samples did not statistically differ by gender and age as verified by the χ2 test of independence and ANOVA. For both tests, the significance threshold was set at α < 5%. The patients’ clinical characteristics are summarized in Table 1.
Blood was withdrawn by DB Vacutainer® Blood Collection Tubes (Becton Dickinson and Company, Franklin Lake, NJ, USA) with K3EDTA as anticoagulant. Within 6 h, plasma was separated by centrifugation of 7 mL of peripheral blood at 1800xg for 10 min at + 4 °C and stored at − 80 °C, following the standardized protocol of the Tissue Biobank.
Formalin-fixed and paraffin-embedded (FFPE) normal colon or/and adenoma and tumour tissue samples were obtained from a subset of enrolled CRC patients (n = 37) for RNA extraction and for inflammatory cell infiltration and histological features evaluation. The lymphocytes, plasma cells, and neutrophils infiltrate were assessed with the following score: 0 = absent, 1 = low, 2 = medium, and 3 = high.
2.3 RNA isolation
Colon tissue samples were manually microdissected from unstained slides to ensure that each sample contained at least 80% of tumour cells. Total RNA was extracted using the RNeasy FFPE kit (Qiagen), according to the manufacturer’s instructions. The concentration and purity of RNAs were determined by NanoDrop 2000 spectrophotometer (Thermo Scientific, USA). The total RNA was considered suitable for the qPCR analysis if the following requirements were met: (1) the optical density (OD) ratio at 260 nm/280 nm was between 1.9 and 2.1; (2) the concentration was higher than 100 ng/µL.
2.4 Reverse-transcription and quantitative real-time PCR (qPCR)
cDNA was synthesized from 2 µg of total RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s protocol by the Veriti™ 96-well Thermal Cycler instrument. qPCR was performed using the 7500 Fast Real-Time PCR System (Applied Biosystems) with Transferrin receptor (TFRC) gene as endogenous control. The amplification reaction was conducted in a final volume of 20 µL using 4 µl of cDNA, TaqMan® Universal PCR Master Mix 1X (Applied Biosystems) and specific TaqMan® Gene Expression Assay 1X (Applied Biosystems): Hs00984148_m1 (amplicon length 66 bp) for IDO1 and Hs00951083_m1 (amplicon length 66 bp) for TFRC. The thermal condition included one cycle at 50 °C for 2 min for the UNG incubation and at 95 °C for 10 min for the polymerase activation, followed by 40 cycles at 95 °C for 15 s for denaturation and at 60 °C for 1 min for annealing and extension. Each sample was run in duplicate and the threshold cycle (Ct) average was used for the calculations. The results from each sample were compared against one normal tissue cDNA sample as calibrator, using the 2−ΔΔCt calculation method. The fold change was expressed as relative quantification (RQ).
2.5 HPLC-UV/FD analysis for tryptophan and metabolites quantification
We used the Comai et al. (Comai et al. 2011) method to quantify TRP, 5HTP, and 5HT in human plasma samples. Briefly, an HPLC-FD (High-Pressure Liquid Chromatography-Fluorescence Detector) analysis was performed using an LC-10AD equipped with a RF-10AXL detector, both from Shimadzu Corporation (Kyoto, Japan). Excitation and emission wavelengths were 285 and 345 nm, respectively.
The chromatographic separation was carried out at room temperature using an analytical Platinum EPS C18 column (5 μm, 100 Å, 250 mm x 4.6 mm from Alltech Deerfield, IL, USA) and an Alltech guard column with stationary phase RP-8 (25–40 μm Lichroprep, Merck Darmstadt, Germany). The mobile phases were as follows: Phase A, 100% acetonitrile and Phase B, 0.004 M phosphate buffer solution (pH 3.5). The analytes elution was performed with an isocratic gradient (15% Phase A and 85% Phase B, v/v) at 1 mL/min flow rate.
To determine KYN, we employed an LC-10AD coupled to a UV–VIS (Ultraviolet–Visible) detector Model SPD-10A from Shimadzu Corporation (Kyoto, Japan). The absorbance wavelength was set at 360 nm. The chromatographic separation was carried out at room temperature using a GraceSmart RP 18 column (5 μm, 250 mm x 4.6 mm Grace, Deerfield, IL, USA) and with the same isocratic gradient described above. The injection volume was 20 μL for both protocols.
2.6 Statistical analysis
Statistical analysis was performed with GraphPad Prism, version 5.00, 2007 (La Jolla, CA, USA). Groups were compared for statistical differences using univariate statistical analysis. Data normality was evaluated using the D’Agostino-Pearson omnibus test and parametric or non-parametric analyses (Mann–Whitney, Kruskal–Wallis test and Dunn’s post-hoc test, two-way ANOVA) were performed accordingly. For all tests, the significance threshold was set at α < 5%. The Spearman’s rank-order correlation was employed to measure the strength and direction of association between variables.
Univariate receiver operating characteristic (ROC) curves were used to evaluate the sensitivity and specificity. Classification performance was assessed on the basis of the area under the ROC curve (AUC). To generate optimal cut-off points from ROC curves, the Youden’s index was employed.
Data are presented as median and interquartile ranges (Q1, Q3) unless otherwise stated.
3 Results and discussion
Following tryptophan catabolic processes, i.e. the kynurenine and the serotonin pathways, plasma levels of TRP, KYN, 5HTP, and 5HT were quantified in control subjects, high-grade adenomas patients and CRC patients as above described. Our aim was twofold: (1) to verify if the alteration of TRP metabolism was related to the adenoma-adenocarcinoma transition and (2) to correlate it with enzyme expression at tumour tissue level. In addition, we investigated TRP alterations in relation to sex, age, and cancer anatomical site.
3.1 Kynurenine pathway in CRC patients
Plasma levels of TRP and KYN were evaluated to assess IDO1 activity. Our results (Table 2) reveal a statistically significant decrease of TRP concentrations in high-grade adenoma and in CRC patients with respect to controls (Kruskal–Wallis, p < 0.0001). The observed decrease is more evident in the presence of cancer lesions with respect to the pre-cancerous ones, namely adenoma. These data indicate an increased IDO1 activity both in adenoma and colorectal cancer patients with respect to control groups (Fig. 1, left panel), calculated as KYN/TRP*1000 ratio. On the contrary, kynurenine levels were comparable between patients affected by colorectal diseases and controls (Kruskal–Wallis, p = 0.1793). Two studies already reported increased kynurenine levels in CRC patients (Cavia-Saiz et al. 2014; Nishiumi et al. 2012). However, the first study was based on plasma KYN quantification performed post-surgery, i.e. in the absence of colorectal tumour mass, whilst the second one was based on serum analysis, not in plasma as we did. Of note, plasma and serum differ in that the latter is obtained after coagulation, which causes protein degradation and peptides/amino acids release (Yu et al. 2011). Moreover, cancer patients exhibit a hypercoagulable state, because tumour cells activate the coagulation system (Caine et al. 2002; Lee et al. 2017; Lima and Monteiro 2013). A hypercoagulable state causes different susceptibility to coagulation between CRC patients and healthy subjects and could introduce a bias during the sampling procedure.
CRC patients were further stratified according to their tumour stage and the anatomical localization (colon or rectum) to reveal correlations with TRP and KYN concentrations and enzymes activity. We report the box-plots related to CRC patients in Fig. 2, divided in three sub-groups depending on the tumour grade staging (TNM stage, see Table 1): tumour in situ (0), early CRC stages (I–II), and late CRC stages (III–IV). Plasma TRP levels were decreased in CRC patients regardless of their tumour stage (Fig. 2, left panel). Colorectal cancer patients presented comparable amount of circulating tryptophan with respect to the adenoma ones, suggesting the early onset of these alterations. Recent studies reported an increase of KYN/TRP in some cancers, with a higher IDO1 activity usually associated with more advanced cancer stages (Suzuki et al. 2010) and lower overall survival (Urakawa et al. 2009). With respect to those observations, the IDO1 activity we calculated was not related to the pathology stage.
Box-plots representing the tryptophan plasma levels during the adenoma-adenocarcinoma transition (left panel) and depending on the anatomical site (right panel). CRC patients were stratified into adenomas, tumour in situ (0), early CRC (stages I–II) and late CRC (stages III–IV) according to their tumour grade. C: control subjects, Kruskal–Wallis with Dunn’s post-test results, *p < 0.05; **p < 0.01; ***p < 0.001. Mann–Whitney, ^^^p < 0.001
Under normal physiological conditions, the plasma KYN/TRP ratio depends on the combination of IDO and TDO activities. However, IDO activity prevails during an active inflammatory state. In our samples, an active inflammatory cell infiltration has been evidenced by hematoxylin and eosin staining on FFPE colorectal cancer tissues (low grade = 43%, high grade = 57%; See Supplementary Figure S1), which could have triggered increased IDO activity. In addition, we investigated the IDO1 gene expression along to the adenoma-carcinoma sequence. To this aim, IDO1 expression was analyzed by qPCR in a set of n = 21 FFPE tissue samples that have passed the quality criteria of purity and concentration as described in the “RNA Isolation” section. In particular, we analyzed: n = 6 normal colon tissues, n = 4 adenoma tissues, and n = 11 colon cancer tissues. The median (and interquartile ranges Q1, Q3) of mRNA expression in normal colon tissues, expressed as Relative Quantitation (RQ), was 0.042 (0.017, 0.066). Although not statistically relevant, probably due to the lower efficiency of RNA extraction from FFPE tissue samples, IDO1 mRNA expression increased about 10-fold, in CRC tissues 0.467 (0.034, 0.739); while for adenoma, it was similar to that of normal tissues RQ = 0.044 (0.009, 0.756).
3.2 Serotonin pathway in CRC patients
5HTP is the intermediate TRP metabolite in the serotonin pathway and it is normally present in plasma at low concentrations. In control samples, a median 5HTP level of 0.06 (0.038, 0.08) µg/mL was measured. For CRC and adenoma patients, 5HTP concentrations decreased with respect to controls (Table 2). This decrease suggests an involvement of the TPH enzyme also in the onset of CRC, although data dispersion impeded statistical significance. Serotonin is the final metabolite of this pathway and it is locally produced in the brain and in the gut or stored in platelets. In our samples, a significant decrease of serotonin was observed in adenoma patients with respect to controls. Similar concentrations were also detected in the plasma of CRC patients, indicating that these alterations persisted during the adenoma-adenocarcinoma transition.
Similar to IDO1, TPH activity may be estimated by the 5HTP/TRP*1000 ratio. The results we report in Fig. 1 (right panel) highlight the increased TPH activity in CRC patients, suggesting the possible contribution of this enzyme in the global TRP reduction observed in cancer patients.
3.3 Tryptophan metabolism in relation to anatomical site
When we stratified patients according to the anatomical site of tumour lesion (Fig. 2, right panel), we observed a clear distinction between rectal and colon cancer. Rectal cancer patients exhibited statistically higher levels of TRP with respect to colon cancer patients. The observed TRP levels reflect the higher IDO activity in colon cancer patients with respect to rectal cancer ones: 51.5 (41.05, 72.58) versus 41.05 (35.05, 53.60) respectively (p < 0.05). These differences in TRP levels were still present when we compared colon and rectal cancer patients after a further stratification into early and late stages: colon (I–II) versus rectum (I–II) p < 0.01 and colon (III–IV) versus rectum (III–IV) p < 0.05.
These differences between colon and rectal cancer patients cannot be merely explained by anatomy. In fact, from a biological and clinical points of view, colon and rectum differ for their embryological origin, their function, and their primary tumour histology and molecular characteristics (Tamas et al. 2015). The immunological scenario appears to be different in colon and rectum; as an example, lymphocytic infiltration is associated with better overall survival in colon, but not in rectal cancer patients (Deschoolmeester et al. 2010). In this frame, our data suggest that an immunomodulation process may be present in colon cancer only.
The relationship between TRP levels and colon-rectal cancer patients’ overall survival has been investigated. Colon and rectal cancer patients were divided in two groups, depending on TRP levels. ROC curves were used to generate the optimal cut-off value for TRP levels (see Supplementary Figure S2). For colon cancer patients, the ROC curve yielded an AUC of 0.88, with a cut-off value of 8.5 µg/mL (sensitivity, 80%; specificity, 84%), whilst for rectal cancer patients the AUC was 0.68, with a cut-off value of 9 µg/mL (sensitivity, 67%; specificity, 77%). The poor accuracy observed in the ROC curves for rectal cancer patients was mainly due to TRP levels close to those observed in control subjects. For both colon and rectal cancer patients, no statistical significant correlation between TRP levels and overall survival was present (p = 0.2906 and p = 0.8826, respectively). Similarly, no statistical significant correlation with IDO1 activity and overall survival was present (p = 0.5282 and p = 0.8969, respectively).
3.4 Age and sex-related influences on tryptophan metabolism
Some authors reported that age (Frick et al. 2004; Pertovaara et al. 2006) or sex (Deac et al. 2015) influence tryptophan metabolism. In particular, tryptophan degradation may increase along with immunosenescence (Calder et al. 2017; van der Goot and Nollen 2013). In our cohort, controls and patients were age-matched as described in the “Patients’ enrolment” section. However, we investigated the correlation between age and KYN/TRP ratio in our samples to verify if the wide age dispersion (from 35 to 83 for controls and from 31 to 87 for CRC patients) affected the results and introduced an age bias.
The Spearman’s correlation was calculated to determine the relationship between TRP plasma concentrations and age. In Fig. 3, on the right panel, we show the x, y plot reporting plasma TRP levels with respect to age for CRC patients. A weak, negative correlation was computed for CRC patients (r s = − 0.3623, n = 68, p < 0.001), whilst no correlation was present in control subjects and adenoma patients (r s = − 0.0868, p = 0.648 and r s = 0.232, p = 0.369, respectively). To verify whether age is a confounding factor in the association between TRP and CRC, subjects were divided in three age groups with the same span (31–49, 50–68, and 69–87 years). TRP levels were higher in CRC patients with respect to control subjects for all of these groups, which rules out the possibility of an age confounder (see Supplementary Table S2). Similarly, a weak positive correlation was calculated between IDO1 activity and age in CRC patients (r s = 0.3964, n = 67, p < 0.001). The observed correlation may be explained by immunomodulation processes taking place during cancer development, resulting in more pronounced immunosenescence in CRC patients.
Another variable known to affect immune system activity is sex. In our samples, no differences were found in TRP levels in healthy subjects of both sexes. On the contrary, for both high-grade adenomas and CRC patients, lower plasma levels of TRP were present—with respect to controls—only in females (p < 0.01 and p < 0.001, respectively) as shown in Fig. 3, left panel. In general, females exhibit higher susceptibility to autoimmune diseases such as scleroderma, lupus, and rheumatoid arthritis than males. This occurs because males are more prone to immune suppression (McKean and Nunney 2005), whilst females have increased immune reactivity (Hewagama et al. 2009). These sex-associated differences play a role in cancer incidence, progression, and response to therapy (Dorak and Karpuzoglu 2012). CRC is also characterized by sex-specific differences, because females are more prone than male to right-sided colon cancer (Kim et al. 2015). Our results evidenced a statistically significant decrease of TRP levels in the plasma of women with respect to men both in high-grade adenoma subjects [women: 6.5 µg/mL (4.4, 7.5); men 9.5 µg/mL (7.6, 10.8); Mann Whitney, p < 0.05] and in CRC patients [women: 6.2 µg/mL (3.2, 7.8); men 8.1 µg/mL (6.2, 10.9); Mann Whitney, p < 0.01]. We evaluated such differences as possible confounding factor. We firstly measured the association between TRP levels in both CRC and healthy subjects by comparing the proportion of low-TRP (< 8.5 µg/mL) to the portion of high-TRP people (> 8.5 µg/mL). The calculated relative risk (RR) is 1.9, indicating a high risk of CRC among people having low-TRP levels. The sex-adjusted RR for the TRP/CRC relationship was 2.6, as result of the weighted average of sex-specific estimates (RR = 1.6 for men and RR = 4.2 for women).
To further verify if these differences were related to the sex-specific immune modulation or to the colorectal cancer itself, we analysed a small cohort of ulcerative colitis (UC) patients (n = 12 male and n = 9 female, see Supplementary Table 1 for their clinical characteristics). Chronic inflammation is often associated with malignancies (Fichtner-Feigl et al. 2015). In order to uncover CRC-related differences, we chose UC as a model of chronic inflammation occurring in the same anatomical site. Indeed, any comparison with a systemic inflammatory state is not useful, due to the peculiarities of the gastrointestinal immune system, which is regulated by the microbiota presence and which is constituted by specialized immune cells and structural organization. The results confirmed the presence of a sex-specific immune modulation, with statistically lower TRP levels in women affected by UC as compared with men [women: 8.7 µg/mL (5.7, 9.5); men 11.2 µg/mL (8.9, 12.0); Mann–Whitney p < 0.05]. Likewise, the calculated IDO1 activity was higher in UC women [women: 59.7 (48.9, 74.2); men 41.2 (33.1, 51.8); Mann–Whitney, p < 0.05].
4 Conclusions
In the present work, we investigated the changes in tryptophan and its metabolites in a cohort of CRC patients at different tumour stages. We report an early onset of TRP metabolism alterations, which were detected when pre-cancerous lesions (adenoma) were diagnosed and persisted during CRC progression. Both IDO1 and TPH enzymes showed higher activities in CRC patients than in controls, indicating that not only the kynurenine pathway, but also the serotonin one may be involved in immunomodulation. Moreover, we were able to demonstrate that people affected by colon cancer are more subjected to increased TRP catabolism with respect to those affected by rectal cancer. These findings suggest that early alterations of TRP metabolism can help colon cancer to evade immunosurveillance and to resist immune-mediated suppression.
References
Agostini, M., et al. (2015). An integrative approach for the identification of prognostic and predictive biomarkers in rectal cancer. Oncotarget, 6, 32561–32574. doi:10.18632/oncotarget.4935.
Caine, G. J., Stonelake, P. S., Lip, G. Y., & Kehoe, S. T. (2002). The hypercoagulable state of malignancy: Pathogenesis and current debate. Neoplasia 4, 465–473 doi:10.1038/sj.neo.7900263.
Calder, P. C., et al. (2017). Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Research Reviews, 40, 95–119. doi:10.1016/j.arr.2017.09.001.
Cascino, A., et al. (1991). Increased plasma free tryptophan levels in human cancer: A tumor related effect? Anticancer Research, 11, 1313–1316.
Cavia-Saiz, M., Rodriguez, P. M., Ayala, B., Garcia-Gonzalez, M., Coma-del Corral, M. J., & Giron, C. G. (2014). The role of plasma IDO activity as a diagnostic marker of patients with colorectal cancer. Molecular Biology Reports, 41, 2275–2279. doi:10.1007/s11033-014-3080-2.
Chai, E. Z. P., Siveen, K. S., Shanmugam, M. K., Arfuso, F., & Sethi, G. (2015). Analysis of the intricate relationship between chronic inflammation and cancer. Biochemical Journal, 468, 1–15. doi:10.1042/Bj20141337.
Clarke, G., Fitzgerald, P., Cryan, J. F., Cassidy, E. M., Quigley, E. M., & Dinan, T. G. (2009). Tryptophan degradation in irritable bowel syndrome: evidence of indoleamine 2,3-dioxygenase activation in a male cohort. BMC Gastroenterology, 9, 6–6. doi:10.1186/1471-230x-9-6.
Comai, S., et al. (2011). Effects of PEG-interferon alpha plus ribavirin on tryptophan metabolism in patients with chronic hepatitis C. Pharmacological Research, 63, 85–92. doi:10.1016/j.phrs.2010.10.009.
Deac, O. M., et al. (2015). Tryptophan catabolism and vitamin B-6 status are affected by gender and lifestyle factors in healthy young adults. The Journal of Nutrition, 145, 701–707. doi:10.3945/jn.114.203091.
Deschoolmeester, V., et al. (2010). Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunology, 11, 19. doi:10.1186/1471-2172-11-19.
Dorak, M. T., & Karpuzoglu, E. (2012). Gender differences in cancer susceptibility: an inadequately addressed issue. Frontiers in Genetics, 3, 268. doi:10.3389/fgene.2012.00268.
Ekbom, A., Helmick, C., Zack, M., & Adami, H. O. (1990). Ulcerative colitis and colorectal cancer. A population-based study. The New England Journal of Medicine, 323, 1228–1233. doi:10.1056/NEJM199011013231802.
Favennec, M., et al. (2015). The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity, 23, 2066–2074. doi:10.1002/oby.21199.
Ferdinande, L., et al. (2012). Clinicopathological significance of indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. British Journal of Cancer, 106, 141–147. doi:10.1038/bjc.2011.513.
Fichtner-Feigl, S., Kesselring, R., & Strober, W. (2015). Chronic inflammation and the development of malignancy in the GI tract. Trends in Immunology, 36, 451–459. doi:10.1016/j.it.2015.06.007.
Forrest, C. M., Gould, S. R., Darlington, L. G., & Stone, T. W. (2003). Levels of purine, kynurenine and lipid peroxidation products in patients with inflammatory bowel disease. Advances in Experimental Medicine and Biology, 527, 395–400.
Frick, B., Schroecksnadel, K., Neurauter, G., Leblhuber, F., & Fuchs, D. (2004). Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clinical Biochemistry, 37, 684–687. doi:10.1016/j.clinbiochem.2004.02.007.
Gupta, N. K., et al. (2012). Serum analysis of tryptophan catabolism pathway: Correlation with Crohn’s disease activity. Inflammatory Bowel Diseases, 18, 1214–1220. doi:10.1002/ibd.21849.
Hewagama, A., Patel, D., Yarlagadda, S., Strickland, F. M., & Richardson, B. C. (2009). Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes and Immunity 10, 509–516. doi:10.1038/gene.2009.12.
Huang, A., Fuchs, D., Widner, B., Glover, C., Henderson, D. C., & Allen-Mersh, T. G. (2002). Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. British Journal of Cancer, 86, 1691–1696. doi:10.1038/sj.bjc.6600336.
Kim, S. E., Paik, H. Y., Yoon, H., Lee, J. E., Kim, N., & Sung, M. K. (2015). Sex- and gender-specific disparities in colorectal cancer risk. World Journal of Gastroenterology: WJG, 21, 5167–5175. doi:10.3748/wjg.v21.i17.5167.
Le Floc’h, N., Otten, W., & Merlot, E. (2011). Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 41, 1195–1205. doi:10.1007/s00726-010-0752-7.
Lee, S., et al. (2017). Clinical significance of coagulation factors in operable colorectal cancer. Oncology Letters, 13, 4669–4674. doi:10.3892/ol.2017.6058.
Lima, L. G., & Monteiro, R. Q. (2013). Activation of blood coagulation in cancer: Implications for tumour progression. Bioscience Reports 33, e00064. doi:10.1042/BSR20130057.
Lob, S., et al. (2009). IDO1 and IDO2 are expressed in human tumors: Levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunology, Immunotherapy: CII, 58, 153–157. doi:10.1007/s00262-008-0513-6.
Mandal, R., et al. (2016). The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight, 1, e89829. doi:10.1172/jci.insight.89829.
Mantovani, A., Allavena, P., Sica, A., & Balkwill, F. (2008). Cancer-related inflammation. Nature, 454, 436–444. doi:10.1038/nature07205.
Masaki, A., et al. (2015). Prognostic significance of tryptophan catabolism in adult T-cell leukemia/lymphoma. Rinsho Ketsueki 56, 2295–2304. doi:10.11406/rinketsu.56.2295.
McKean, K. A., & Nunney, L. (2005). Bateman’s principle and immunity: Phenotypically plastic reproductive strategies predict changes in immunological sex differences. Evolution, 59, 1510–1517.
Miyagi, Y., et al. (2011). Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS ONE, 6, e24143. doi:10.1371/journal.pone.0024143.
Munn, D. H., et al. (2005). GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity, 22, 633–642. doi:10.1016/j.immuni.2005.03.013.
Munn, D. H., & Mellor, A. L. (2016). IDO in the tumor microenvironment: Inflammation, counter-regulation, and tolerance. Trends in Immunology, 37, 193–207. doi:10.1016/j.it.2016.01.002.
Myint, A. M., et al. (2007). Tryptophan breakdown pathway in bipolar mania. Journal of Affective Disorders, 102, 65–72. doi:10.1016/j.jad.2006.12.008.
Myint, A. M., & Kim, Y. K. (2014). Network beyond IDO in psychiatric disorders: Revisiting neurodegeneration hypothesis. Progress in Neuro-psychopharmacology & Biological Psychiatry 48, 304–313. doi:10.1016/j.pnpbp.2013.08.008.
Nishiumi, S., et al. (2012). A novel serum metabolomics-based diagnostic approach for colorectal cancer. PLoS ONE, 7, e40459. doi:10.1371/journal.pone.0040459.
Ogawa, K., et al. (2012). (-)-Epigallocatechin gallate inhibits the expression of indoleamine 2,3-dioxygenase in human colorectal cancer cells. Oncology Letters, 4, 546–550. doi:10.3892/ol.2012.761.
Opitz, C. A., et al. (2011). An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature, 478, 197–203. doi:10.1038/nature10491.
Pertovaara, M., et al. (2006). Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mechanisms of Ageing and Development, 127, 497–499. doi:10.1016/j.mad.2006.01.020.
Powell, N. D., Tarr, A. J., & Sheridan, J. F. (2013). Psychosocial stress and inflammation in cancer. Brain Behavior and Immunity, 30, S41–S47. doi:10.1016/j.bbi.2012.06.015.
Prendergast, G. C., et al. (2014). Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunology, Immunotherapy: CII, 63, 721–735. doi:10.1007/s00262-014-1549-4.
Puccetti, P., et al. (2015). Accumulation of an endogenous tryptophan-derived metabolite in colorectal and breast cancers. PLoS ONE, 10, e0122046. doi:10.1371/journal.pone.0122046.
Sarrouilhe, D., Clarhaut, J., Defamie, N., & Mesnil, M. (2015). Serotonin and cancer: What is the link? Current Molecular Medicine, 15, 62–77.
Schmidt, S. K., et al. (2009). Antimicrobial and immunoregulatory properties of human tryptophan 2,3-dioxygenase. European Journal of Immunology, 39, 2755–2764. doi:10.1002/eji.200939535.
Schwarcz, R., Bruno, J. P., Muchowski, P. J., & Wu, H. Q. (2012). Kynurenines in the mammalian brain: When physiology meets pathology. Nature Reviews Neuroscience, 13, 465–477. doi:10.1038/nrn3257.
Siddiqui, E. J., Thompson, C. S., Mikhailidis, D. P., & Mumtaz, F. H. (2005). The role of serotonin in tumour growth (review). Oncology Reports, 14, 1593–1597.
Suzuki, Y., et al. (2010). Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer, 67, 361–365. doi:10.1016/j.lungcan.2009.05.001.
Tamas, K., et al. (2015). Rectal and colon cancer: Not just a different anatomic site. Cancer Treatment Reviews, 41, 671–679. doi:10.1016/j.ctrv.2015.06.007.
Thaker, A. I., et al. (2013). IDO1 metabolites activate beta-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology, 145, 416–425.e1-4. doi:10.1053/j.gastro.2013.05.002.
Thomas, S. R., & Stocker, R. (1999). Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Report, 4, 199–220. doi:10.1179/135100099101534927.
Urakawa, H., Nishida, Y., Nakashima, H., Shimoyama, Y., Nakamura, S., & Ishiguro, N. (2009). Prognostic value of indoleamine 2,3-dioxygenase expression in high grade osteosarcoma. Clinical & Experimental Metastasis, 26, 1005–1012. doi:10.1007/s10585-009-9290-7.
van Baren, N., & van den Eynde, B. J. (2015). Tryptophan-degrading enzymes in tumoral immune resistance. Frontiers in Immunology, 6, 34. doi:10.3389/fimmu.2015.00034.
van der Goot, A. T., & Nollen, E. A. (2013). Tryptophan metabolism: Entering the field of aging and age-related pathologies. Trends in Molecular Medicine, 19, 336–344. doi:10.1016/j.molmed.2013.02.007.
Vecsei, L., Szalardy, L., Fulop, F., & Toldi, J. (2013). Kynurenines in the CNS: Recent advances and new questions. Nature Reviews Drug Discovery, 12, 64–82. http://www.nature.com/nrd/journal/v12/n1/suppinfo/nrd3793_S1.html.
Vicaut, E., Laemmel, E., & Stucker, O. (2000). Impact of serotonin on tumour growth. Annals of Medicine 32, 187–194.
Wiggins, T., Kumar, S., Markar, S. R., Antonowicz, S., & Hanna, G. B. (2015). Tyrosine, phenylalanine, and tryptophan in gastroesophageal malignancy: A systematic review. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 24, 32–38 doi:10.1158/1055-9965.EPI-14-0980.
Yu, Z., et al. (2011). Differences between human plasma and serum metabolite profiles. PLoS ONE, 6, e21230. doi:10.1371/journal.pone.0021230.
Acknowledgements
This work was supported by the Associazione Italiana Ricerca sul Cancro (AIRC), IG 2016 (Grant No. 19104).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This study is conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from every individual and the protocol was approved by ethics committee of institution (Protocol Number:P448).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Crotti, S., D’Angelo, E., Bedin, C. et al. Tryptophan metabolism along the kynurenine and serotonin pathways reveals substantial differences in colon and rectal cancer. Metabolomics 13, 148 (2017). https://doi.org/10.1007/s11306-017-1288-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1288-6