Abstract
Diabetic neuropathic pain (DNP) is a common and destructive complication of diabetes mellitus. The discovery of effective therapeutic methods for DNP is vitally imperative because of the lack of effective treatments. Although 2 Hz electroacupuncture (EA) was a successful approach for relieving DNP, the mechanism underlying the effect of EA on DNP is still poorly understood. Here, we established a rat model of DNP that was induced by streptozotocin (STZ) injection. P2X4R was upregulated in the spinal cord after STZ-injection. The upregulation of P2X4R was mainly expressed on activated microglia. Intrathecal injection of a P2X4R antagonist or microglia inhibitor attenuated STZ-induced nociceptive thermal hyperalgesia and reduced the overexpression of brain-derived neurotrophic factor (BDNF), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in the spinal cord. We also assessed the effects of EA treatment on the pain hypersensitivities of DNP rats, and further investigated the possible mechanism underlying the analgesic effect of EA. EA relieved the hyperalgesia of DNP. In terms of mechanism, EA reduced the upregulation of P2X4R on activated microglia and decreased BDNF, IL-1β and TNF-α in the spinal cord. Mechanistic research of EA's analgesic impact would be beneficial in ensuring its prospective therapeutic effect on DNP as well as in extending EA's applicability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of diabetic patients has been steadily increasing over the last few decades, and it is predicted to reach 629 million by 2045 [1]. One-third of diabetic patients suffer from painful neuropathic symptoms, including paresthesia, hyperalgesia, and allodynia [2], which have a tremendous impact on their daily lives, sleep, and mental health. Although diabetic neuropathic pain (DNP) is a widespread clinical symptom of diabetes, the underlying mechanism is still not properly understood, limiting its effectiveness in adequately treating it. Therefore, there is an imperative need to clarify the pathogenesis of diabetic neuropathy.
The P2X4 receptor (P2X4R), as a subtype of P2X receptors, is one of the key receptors mediating neuropathic pain [3,4,5]. Likewise, numerous reports have discovered that glial cells, particularly microglia, contribute significantly to the emergence and stabilization of neuropathic pain [6,7,8]. Microglia are the resident macrophages in the spinal cord [9]. Once painful peripheral neuropathy occurs, spinal microglia become activated and proliferate in number [6, 10]. When the microglia are activated, they also release microvesicles, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), which contribute to neuropathic pain [11,12,13]. However, the role of spinal microglia P2X4R in DNP still remains unclear. Several studies have found that P2X4R is activated following peripheral nerve injury and may stimulate the microglia to release brain-derived neurotrophic factor (BDNF) by causing a high influx of Ca2+. The outcome of this process may result in an abnormal nociceptive response, which aggravates neuropathic pain [14, 15]. All those studies confirm that spinal microglia P2X4R might represent a key contributor to the pain mechanism of DNP.
Conventional treatment with DNP, such as duloxetine, pregabalin, and opioids, usually lacks effectiveness and has significant side effects [14, 15]. Electroacupuncture (EA), which is rooted on the ideologies of traditional Chinese medicine, is commonly utilised for alleviating neuropathic pain in clinical practice [16, 17]. Our previous study showed that EA can ease pain in a rat model of DNP and we further identified 2 Hz as a better frequency for EA interventions [18]. According to the findings of some clinical studies, EA is an effective treatment for reducing DNP [19]. Nonetheless, the mechanism behind EA's influence on DNP is still poorly understood.
This study's aims to determine whether and how microglia P2X4R contributes to the development of DNP, by demonstrating the anti-allodynic effect of EA on STZ-induced DNP as well as the possible role of microglia P2X4R in EA-mediated analgesia.
Methods and materials
Animals
Male Sprague-Dawley (SPF-grade) rats were acquired from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences. All rats were maintained at a temperature of 25 ± 2 ℃, 55 ± 5% humidity and a 12 h dark/light cycle, with an ad libitum supply of water and food. The Animal Welfare Committee of Zhejiang Chinese Medical University permitted this study (approval number: IACUC-20190805-04).
Experimental design
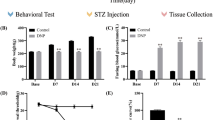
This study was carried out in three steps. In step one, the effects of streptozotocin (STZ) on inducing neuropathic pain were monitored. Rats were randomly assigned into two groups: control and model groups. To test microglia's and P2X4R's effects, control + vehicle, model + vehicle, model + 5-BDBD and model + minocycline groups were established. To test EA’s effect, control, DNP and DNP + EA groups were established.
STZ-induced DNP rat model
To induce diabetes, rats fasted for 16 h before receiving an intraperitoneal injection of STZ (S0130, Sigma-Aldrich, USA) [20, 21]. 7 days after injection, blood was collected from the caudal vein, and fasting blood glucose (FBG) levels were measured. Rats with FBG levels > 13.9 mmol/L were considered type 1 diabetic models [22]. Next, we measured the paw withdrawal latency (PWL) in diabetic rats, and those with a 15% decline in PWL were considered a type 1 DNP model. These were selected for further study.
Paw withdrawal latency
PWL was assessed via plantar tests. In all steps of the experiment, PWL was measured 1 day prior to STZ injection (base), then at 7, 14, and 21 days after injection. In addition to these 4 time points, PWL was also measured prior to intraperitoneal injection of 5-BDBD or minocycline, on day 15, as well as 0.5, 1 and 1.5 h post-intraperitoneal injection. Animals were kept in testing chambers and placed on a glass plate for a minimum of 30 min to acclimate. After becoming quiet, a source of infrared radiant heat was pointed at the bottom of each rat's hind paw, and the time required for PWL was measured automatically.
To avoid possible thermal injury, illumination was performed for only 30 s, while exposure to a radiant heat was for 40 s. Three measurements were obtained at 5 min intervals and were averaged for each test. A researcher who was blind to both the experimental groups and the study hypotheses evaluated all of the behavioural tests.
Drug treatment
The P2X4R antagonist 5-BDBD and the microglia inhibitor minocycline were first diluted in 5% dimethyl sulfoxide (DMSO), to prepare a stock solution after which they were diluted to appropriate concentrations before injection. Specifically, 5-BDBD was diluted to 542 µg, 10 µl, while minocycline was 100 µg, 10 µl. Rats in the control + vehicle group and the model + vehicle group were administered with similar 5% DMSO volumes. The drugs were administered through intrathecal injection, once every other day, for four days.
EA treatment
The rats were fixed at first, and then Zusanli (ST36), Kunlun (BL60) acupoints were selected. A 0.25 mm × 13 mm acupuncture needle was put 5 mm deep into acupoints on rats, which were then subjected to 1 mA and 2 Hz electrical stimulation from a HANS acupoint electrical stimulation device (Hans-200A, Jisheng Medical Technology, China) for 30 min. In the DNP + EA group, rats were given EA once a day for 7 days straight. In contrast, rats in the control and DNP groups were immobilized but not subjected to EA stimulation.
Immunofluorescence
Pentobarbital (40 mg/kg) was used to anaesthetize rats. Next, their hearts and ascending aorta were perfused with 4 ℃ pre-cooled normal saline via the left ventricular apex, until the liver turned white. The animals were subjected to a bolus injection of 4% paraformaldehyde. The spinal cords were dissected out, then followed by 6 h of post-fixation in 4% paraformaldehyde. Following a series of hydration steps in 15% and 30% sucrose solutions, tissues were flash-frozen with liquid nitrogen and maintained at -80 ℃. Frozen lumbar spinal cord tissues were sliced into 30-μm-thick sections and washed thrice with TBST (10 min for each wash). 1 h at 37 ℃ with a blocking solution of 10% normal donkey serum. Next, the slices were done for 12 h at 4 ℃ with the following primary antibodies: rabbit anti-P2X4R (1:200) or rabbit anti-BDNF (1:400) respectively and mouse anti-CD11b (1:400) or mouse anti-GFAP (1:400). Rewarming the section at 37 ℃ for 1 h on the second day, Thereafter, sections were washed with TBST, and treated for 1 h at 37 ℃ with the secondary antibodies listed below: Alexa Fluor 594 donkey anti-Mouse IgG (1:200) and Alexa Fluor 488 donkey anti-rabbit IgG (1:400). Imager M2 microscopy was used to take pictures of the sections after they were cleaned with TBST. Mean intensities were calculated in 3 spinal slices from at least three per group using Image J.
Western blotting
Pentobarbital was used to completely anaesthetize the rats (40 mg/kg), then the lumbar spinal cord was isolated and saved at -80 ℃ for later use. Samples were placed in RIPA lysis buffer. The tissue homogenate was centrifuged at 4 ℃ for 20 min. After that, the BCA Protein Assay Kit was used to determine protein concentrations. And then we cooked the protein in loading buffer for 3 min. SDS-PAGE gels were used to separate 20 μg of protein from each sample, which was then moved to polyvinylidene difluoride membranes. Following 1 h of blocking in nonfat milk dilution, the bands were stained for one whole night with the following primary antibodies: rabbit anti-P2X4R (1:1000), rabbit anti-Iba1(1:1000), rabbit anti-TNF-α (1:1000), rabbit anti-IL-1β (1:1000), rabbit anti-BDNF (1:1000) and β-actin (HRP-conjugated) (1:5000). The bands were then washed three times with TBST, treated with HRP-linked antibody anti-rabbit IgG (1:5000) for 2 h, and detected using an enhanced chemiluminescence kit. The Image Quant LAS 4000 system was used to quantify band intensities. Image J was used to determine the thickness of the target bands, and these proteins' expression levels were adjusted to β-actin.
Statistical analysis
SPSS 21.0 was used to process all of the data, which was then shown as the standard error of the mean (\(\overline{x }\) ± SD). We employed a two-way ANOVA followed by Bonferroni tests to see if the groups were different in our studies of body weight (BW), FBG, and PWL across time or with two independent factors. A one-way ANOVA was used to assess the remaining data, and Dunnett’s test was used as a post hoc test. It was deemed statistically significant if P < 0.05.
Results
Establishing a rat model for DNP
A DNP rat model was established which was shown in Fig. 1. We first established a DNP rat model by administering STZ via high-dose intraperitoneal injection [20]. At 1 week after administration, the FBG of model group continued to rise ( P < 0.01), and maintained a high and stable FBG from day 7 to 21 as shown in Fig. 1c. Meanwhile, the BW of rats in model group after administration was lower than that in control group from day 7 ( P < 0.01) ( Fig. 1b). The PWL in model group significantly decreased at day 14 (P < 0.01), and thermal hyperalgesia lasted till the end of the observation time frame ( Fig. 1d). These observations showed that the DNP model was successfully developed at day 14.
STZ-injection increased the expressions of P2X4R, microglia in the spinal cord
P2X4R expression and microglia activation increased in the spinal cord of rats were examined after injection of STZ. Western blotting (WB) analysis at different time points revealed that the level of P2X4R was increased from day 14 (P < 0.01) after STZ-injection compared to the expression in the control group (Fig. 2a). Immunofluorescence (IF) results showed that the P2X4R expression in the spinal cord was increased from day 7 (P < 0.05) after STZ-injection ( Fig. 2c, e). CD11b and Iba1 are microglia activation markers [23]. As shown in Fig. 2b, d and f, significant changes were observed between control and model groups at day 14 in both microglia markers (CD11b and Iba1) by WB (P < 0.05) and IF (P < 0.01). CD11b-positive cells showed a tiny soma with thin-branched or ramified processes during rest, whereas activated microglia displayed a hypertrophy of the cell body with retraction of cytoplasmic processes (Fig. 3a). Moreover, P2X4R expression was presented in CD11b-positive microglia, but not in cells expressing glial fibrillary acidic protein (GFAP), a marker of astrocytes (Fig. 3b). Our findings showed more microglia activation and P2X4R expression in the spinal cords of rats administered with STZ.
STZ-injection increased the expression of P2X4R, Iba1, and CD11b in the spinal cord of DNP rats. Summary data showed the change in P2X4R (a) and Iba1 (b) protein levels in four groups. β-actin was utilized as a control for loading. n = 5. Representative IF images of P2X4R (c) and CD11b (d) in control, 7d, 14d, and 21d groups. Summary of the mean intensity of P2X4R (e) and CD11b (f) immunostaining. n = 3. Data are provided as mean ± SD, * P < 0.05, ** P < 0.01 compared with the control group
STZ-injection increased the expressions of BDNF, TNF-α and IL-1β in the spinal cord
We further examined the role of BDNF, TNF-α and IL-1β in DNP. The IF analyses were used to determine the effect of STZ-injection on BDNF expression in the spinal cord (Fig. 4a, b). We observed an significant increase of BDNF-positive cells in the model group at day 7 (P < 0.05). WB revealed significant elevation of spinal TNF-α and IL-1β at day 14 (P < 0.01) and day 7 (P < 0.01), respectively (Fig. 4c, d). These results revealed that BDNF and proinflammatory cytokines may be implicated in the onset and progression of DNP.
STZ-injection increased the expressions of BDNF, TNF-α and IL-1β in the spinal cord. Typical IF pictures of BDNF (a) in control, 7d, 14d, and 21d groups. Summary of mean intensity of BDNF immunostaining (b). n = 3. Summary data showed the change in TNF-α (c) and IL-1β (d) protein levels in four groups. β-actin was utilized as a control for loading. n = 5. Data are provided as mean ± SD, * P < 0.05, ** P < 0.01 compared with the control group
The P2X4R antagonist 5-BDBD/the microglia inhibitor minocycline alleviated Diabetic-induced pain behaviour
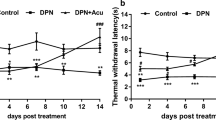
The procedure for this experiment is summarized in Fig. 5a. To determine whether changes in the microglia P2X4R were involved in DNP development, we treated rats with the P2X4R antagonist 5-BDBD or microglia inhibitor minocycline via intrathecal injection. Analysis of PWL, at 0.5, 1, and 1.5 h after either 5-BDBD or the minocycline intrathecal injection, revealed that both antagonists alleviated thermal hyperalgesia relative to the model + vehicle group. The effects on the PWL lasted 0.5 h in the model + 5-BDBD group after 5-BDBD intrathecal injection (P < 0.01), while the effects lasted 1 h in model + minocycline group rats after minocycline intrathecal injection (P < 0.01) (Fig. 5d). Four cycles of 5-BDBD or minocycline treatment significantly improved the PWL of DNP rats (P < 0.01) (Fig. 5e). The above results indicate that microglia P2X4R could be essential in DNP progression. Compared with the model + vehicle group, No significant difference in BW and FBG between neither model + 5-BDBD group nor model + minocycline group at day 21 after administration was detected (P > 0.05), as seen in Fig. 5b, c.
Diabetic-induced pain behaviour was reduced by the P2X4R antagonist 5-BDBD and the microglia inhibitor minocycline. The experimental approach for creating the DNP rat model and injecting 5-BDBD/minocycline (a). Effect of 5-BDBD/minocycline on BW (b), FBG (c) and PWL (d, e) over time. Data are provided as mean ± SD. n = 5. *P < 0.05, **P < 0.01 compared with the control + vehicle group. ## P < 0.01 compared with the model + vehicle group
The P2X4R antagonist 5-BDBD/the microglia inhibitor minocycline reduced the levels of BDNF, TNF-α and IL-1β in the spinal cord
To further verify the establishment of the signaling cascade from microglia P2X4R to BDNF, TNF-α and IL-1β release, we examined the effect of the P2X4R antagonist 5-BDBD or the microglia inhibitor minocycline on BDNF, TNF-α and IL-1β expressions in the spinal cord (Fig. 6). WB revealed that BDNF (P < 0.01), TNF-α (P < 0.01) and IL-1β (P < 0.05) were markedly upregulated in the model + vehicle group, relative to control + vehicle group (Fig. 6b, d, e). Treatment with 5-BDBD effectively reversed levels of BDNF (P < 0.01), TNF-α (P < 0.05) and IL-1β (P < 0.05) in the spinal cord tissue significantly. Similarly, after minocycline treatment, the overexpression of BDNF (P < 0.01), TNF-α (P < 0.01), and IL-1β (P < 0.01) were downregulated. These results suggested that BDNF, TNF-α and IL-1β may be stimulated by P2X4R activation in microglia.
The P2X4R antagonist 5-BDBD/the microglia inhibitor minocycline reduced the expressions of BDNF, TNF-α and IL-1β in the spinal cord. Representative WB images of TNF-α (a) in four groups. Summary data showed the change in TNF-α (b), BDNF (d) and IL-1β (e) protein level in four groups. β-actin was utilized as a control for loading. n = 5. Representative WB images of BDNF and IL-1β in four groups (c). Data are provided as mean ± SD. *P < 0.05, **P < 0.01 vs. control + vehicle group. # P < 0.05, ## P < 0.01 vs. model + vehicle group
EA reversed thermal hyperalgesia in DNP rats
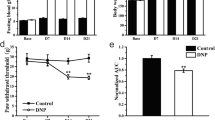
Planned activities for the experiment are depicted in Fig. 7a. PWL considerably elevated in EA-treated DNP rats (P < 0.01) during the 7-day period of EA treatment at “Zusanli” and “Kunlun” acupoints (Fig. 7d), indicating a substantial analgesic effect of EA on DNP rats. However, EA had no significant effect on BW and FBG in DNP rats (P > 0.05) (Fig. 7b, c).
EA reversed DNP-induced thermal hyperalgesia in rats. The protocol of EA administration experiment (a). The impact of STZ injection and EA therapy on BW (b), FBG (c), and PWL (d) over time. n = 8. Data are provided as mean ± SD. **P < 0.01 compared with the control + vehicle group. ##P < 0.01 compared with the model + vehicle group
EA reversed DNP by regulating the expressions of P2X4R, microglia, BDNF, TNF-α and IL-1β in the spinal cord
To determine whether microglia P2X4R mediates the pain-relieving effects of 2 Hz EA, we detected P2X4R expression via IF and WB assays (Fig. 8). The WB results showed that levels of P2X4R and Iba1 in DNP group were increased compared with those in control group (P < 0.01). However, EA could reduce P2X4R and Iba1 expressions of DNP rats (P < 0.01) (Fig. 8a, b). We also found similar results in IF analysis (P < 0.01) (Fig. 8c, d, e, f). Furthermore, the results from the IF assay also indicated morphological alterations in microglia, as microglia branched out with smaller cell bodies in the DNP + EA group, compared with thicker microglia and with bigger cell bodies in the DNP group (Fig. 8e). Next, we analysed BDNF, TNF-α and IL-1β expressions in the spinal cord of rats (Fig. 9). Compared with rats in the control group, DNP rats showed significantly greater BDNF expression in the spinal cord, as determined by IF analysis (P < 0.01), which was significantly inhibited by EA (P < 0.01) (Fig. 9a, b). The analysis of WB revealed significantly increased levels of TNF-α and IL-1β in the spinal cord of the DNP group, compared with the control group (P < 0.05). In contrast to the DNP group, EA could significantly inhibit TNF-α (P < 0.05) and IL-1β (P < 0.01) levels in the spinal cord (Fig. 9c, d). Taken together, we conclude that EA may exert an analgesic effect, and microglial P2X4R, BDNF and proinflammatory cytokines may participate in an analgesic role in EA.
EA reversed DNP through modulating P2X4R, Iba1, and CD11b expressions in the spinal cord. Summary data showed the change in P2X4R (a) and Iba1 (b) protein level in three groups. β-actin was utilized as a control for loading. n = 5. Representative images of P2X4R (c) and CD11b (e) IF in the control group, DNP group, DNP + EA group. Summary of mean intensity of P2X4R (d) and CD11b (f). n = 3. Data are provided as mean ± SD. **P < 0.01 compared with the control group. ##P < 0.01 compared with the DNP group
EA reversed DNP by regulating the expressions of BDNF, TNF-α and IL-1β in the spinal cord. Typical IF pictures of BDNF in the control group, DNP group, DNP + EA group (a). Summary of mean intensity of BDNF (b). n = 3. Summary data showed the change in TNF-α (c) and IL-1β (d) protein levels in three groups. β-actin was used as a loading control. n = 5. Data are provided as mean ± SD. *P < 0.05 compared with the control group. #P < 0.05, ##P < 0.01 compared with the DNP group
Discussion
In the current research, we established an injection of STZ to create a DNP model in rats. Following STZ injection, P2X4R was upregulated in the spinal cord, which occurred mainly in activated microglia. Intrathecal administration of the P2X4R antagonist or microglia inhibitor diminished STZ-induced painful thermal hyperalgesia, and inhibited the overexpression of BDNF, TNF-α and IL-1β in the spinal cord. We also investigated how EA treatment affected the pain hypersensitivities of DNP rats, and further revealed the probable mechanism underlying the analgesic action of EA by proving that EA relieve hyperalgesia of DNP through inhibiting the expression of P2X4R, along with that of BDNF, TNF-α, and IL-1β, in activated microglia in the spinal cord.
A diabetic rat model can be successfully established by STZ injection, either intraperitoneally or intravenously [24,25,26]. A nitrosourea analogue called STZ damages the DNA of the pancreatic beta-cells that secrete insulin [27]. The convenience of STZ-induced diabetic rat model has made it popular for studying the mechanism of DNP and the assessment of prospective treatments. After STZ treatment, rats show behavioural signs of DNP, such as a lower threshold for leaving a painful physical stimulus and shorter time periods before leaving a painful thermal stimulus [28]. In this study, diabetic rats were generated by giving one high-dose injection of STZ into the peritoneum. Our findings demonstrated that from day 7 to 21, decreased BW and higher FBG were induced by a high dose of STZ with intraperitoneal administration. On day 14 following STZ administration, the PWL of the rats decreased significantly, indicating the formation of the DNP rat model.
Microglia, with CD11b and Iba1 as the typical markers [29, 30], are crucial for pain signal transmission at the spinal level [31]. Spinal microglia have been shown to be activated in several types of neuropathic pain, suggesting that they may be involved in the pathophysiology of these disorders [32,33,34,35]. In pathological situations, microglia become more active and more numerous, with alteration of their shape and transcriptional activity [13, 36,37,38]. Our WB results proved that Iba1 expression was significantly increased on 14 days after STZ injection. The IF results showed that the CD11b-positive cells had a tiny soma with thinly branching or ramified processes that was in a resting condition. Yet, after STZ injection, CD11b-positive cells started to exhibit a highly active morphology, characterised by the hypertrophy of cell bodies and the retreat of cytoplasmic processes. P2X4R has been implicated in physiological roles and diseases [39,40,41], and it is selectively upregulated in activated microglia of peripheral nerve injury rats [42]. Antisense-oligonucleotide knockdown or pharmacological blockade of P2X4R stops the peripheral nerve injury-induced increase in sensitivity to mechanical pain[42], and P2X4R-deficient animals did not develop mechanical hypersensitivity caused by peripheral nerve injury [43, 44]. In addition, ATP-stimulated mechanical hypersensitivity induced by cultured microglia, but not unstimulated microglia, in naive rats [42, 45], suggesting that microglia P2X4R activation has an important function in the development of neuropathic pain. Our study demonstrated that after STZ-injection, the expression of P2X4R in the spinal cord of rats increased, and the P2X4R were mainly expressed in the activated microglia. Intrathecal injection of a P2X4R antagonist or microglia inhibitor attenuated STZ-induced nociceptive thermal hyperalgesia.
BDNF is known as a neuromodulator of nociception which plays a vital role as a pain mediator/modulator [46, 47]. A study has demonstrated that P2X4R on the microglia facilitated BDNF mixing and release, which increased central sensitization and persisted in neuropathic pain [48]. Increased BDNF expression acts in a feedforward manner by activating microglia in the spinal cord and inducing nociceptive hypersensitivity. The above evidence indicates the importance of BDNF in DNP rats. The current research indicates an increase in BDNF expression in the DNP rats' spinal cord, which declined rapidly upon administration of the P2X4R antagonist 5-BDBD or the microglia inhibitor minocycline. Previous study has reported that neuroinflammation lead to pain via central sensitization, the activation of microglia upon release of proinflammatory cytokines, such as TNF-α and IL-1β [49]. In our experiment, the WB results indicated an increase in TNF-α and IL-1β expressions in the DNP model rats' spinal cord. Meanwhile, we discovered that 5-BDBD and minocycline could also downregulate TNF-α and IL-1β expressions. Hence, we presume that P2X4R is increased in activated microglia, which facilitates the release of BDNF in the spinal cord, and leads to pain signalling. Activated microglia following DNP may also produce proinflammatory cytokines including TNF-α and IL-1β, which further contribute to DNP.
Numerous studies support the effectiveness of EA in the treatment of several forms of pain, including inflammatory pain and neuropathic pain [50,51,52]. In this study, the EA intervention starting at 14 days after the STZ injection showed substantial pain-relieving effect, in consistent with the previous finding that EA stimulation was capable of alleviating DNP [53]. Another study indicated that EA hindered the initiation and transduction of pain signals [54]. Similarly, EA might relieve pain by reducing the release of interferon-γ and the subsequent production of P2X4R microglia [55]. Nevertheless, the exact mechanism of the action of 2 Hz EA on DNP remains unknown. Our results suggest that 2 Hz EA can exert an analgesic effect in STZ-induced DNP. At the same time, 2 Hz EA not only can inhibit microglia activation, but also can downregulate the overexpression of P2X4R, BDNF, IL-1β, and TNF-α induced by DNP. The results of EA were similar to those of 5-BDBD and minocycline, alleviating DNP and down-regulating the expression of TNF-α and IL-1β. There remains some limitations in this research. We should better pay attention to whether 5-BDBD + EA has a synergistic effect in comparison to EA alone, to observe the effect of EA on DNP when the P2X4R antagonist 5-BDBD was administrated and following P2X4R pathway in the spinal cord of DNP. Time and experimental environment limitations will prevent this from being verified now.
Conclusion
In conclusion, this study proves that microglia P2X4 in spinal cord contributes to the central sensitization of DNP. 2 Hz EA has an efficient antiallodynic effect for DNP. EA can cause the inhibition of microglia and downregulation the expression of P2X4R, BDNF, IL-1β, and TNF-α in the spinal cord of DNP. The above data support EA as a complementary therapy for DNP.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fan W (2017) Epidemiology in diabetes mellitus and cardiovascular disease. Cardiovasc Endocrinol 6(1):8–16. https://doi.org/10.1097/XCE.0000000000000116
Abbott CA, Malik RA, van Ross ERE, Kulkarni J, Boulton AJM (2011) Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 34(10):2220–2224. https://doi.org/10.2337/dc11-1108
Deng Z, Li C, Liu C, Du E, Xu C (2018) Catestatin is involved in neuropathic pain mediated by purinergic receptor P2X4 in the spinal microglia of rats. Brain Res Bull 142:138–146. https://doi.org/10.1016/j.brainresbull.2018.07.003
Teng Y, Zhang Y, Yue S, Du E, Xu C (2019) Intrathecal injection of bone marrow stromal cells attenuates neuropathic pain via inhibition of P2X4R in spinal cord microglia. J Neuroinflammation 16(1):271. https://doi.org/10.1186/s12974-019-1631-0
Yan Y, Liang Y, Ding T, Chu H (2019) PI3K/Akt signaling pathway may be involved in MCP-1-induced P2X4R expression in cultured microglia and cancer-induced bone pain rats. Neurosci Lett 701:100–105. https://doi.org/10.1016/j.neulet.2019.02.024
Tsuda M (2016) Microglia in the spinal cord and neuropathic pain. J Diabetes Investig 7(1):17–26. https://doi.org/10.1111/jdi.12379
Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K (2013) Microglial regulation of neuropathic pain. J Pharmacol Sci 121(2):89–94. https://doi.org/10.1254/jphs.12r14cp
Tsuda M, Inoue K (2016) Neuron-microglia interaction by purinergic signaling in neuropathic pain following neurodegeneration. Neuropharmacology 104:76–81. https://doi.org/10.1016/j.neuropharm.2015.08.042
Ginhoux F, Lim S, Hoeffel G, Low D, Huber T (2013) Origin and differentiation of microglia. Front Cell Neurosci 7:45. https://doi.org/10.3389/fncel.2013.00045
Kim C, Lee JH, Kim W, Li D, Kim Y, Lee K, Kim SK (2016) The Suppressive Effects of Cinnamomi Cortex and Its Phytocompound Coumarin on Oxaliplatin-Induced Neuropathic Cold Allodynia in Rats. Molecules 21(9):1253. https://doi.org/10.3390/molecules21091253
Li J, Li X, Jiang X, Yang M, Yang R, Burnstock G, Xiang ZH, Yuan HB (2017) Microvesicles shed from microglia activated by the P2X7-p38 pathway are involved in neuropathic pain induced by spinal nerve ligation in rats. Purinergic Signal 13(1):13–26. https://doi.org/10.1007/s11302-016-9537-0
Ji RR, Xu ZZ, Gao YJ (2014) Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 13(7):533–548. https://doi.org/10.1038/nrd4334
Inoue K, Tsuda M (2018) Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 19(3):138–152. https://doi.org/10.1038/nrn.2018.2
Peltier A, Goutman SA, Callaghan BC (2014) Painful diabetic neuropathy. BMJ 348:1799. https://doi.org/10.1136/bmj.g1799
Snyder MJ, Gibbs LM, Lindsay TJ (2016) Treating Painful Diabetic Peripheral Neuropathy: An Update. Am Fam Physician 94(3):227–234
Lv ZT, Shen LL, Zhu B, Zhang ZQ, Ma CY, Huang GF, Yin J, Yu LL, Yu SY, Ding MQ, Li J, Yuan XC, He W, Jing XH, Li M (2019) Effects of intensity of electroacupuncture on chronic pain in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Res Ther 21(1):120. https://doi.org/10.1186/s13075-019-1899-6
Seo SY, Lee KB, Shin JS, Lee J, Kim MR, Ha IH, Ko Y, Lee YJ (2017) Effectiveness of Acupuncture and Electroacupuncture for Chronic Neck Pain: A Systematic Review and Meta-Analysis. Am J Chin Med 45(8):1573–1595. https://doi.org/10.1142/S0192415X17500859
He XF, Wei JJ, Shou SY, Fang JQ, Jiang YL (2017) Effects of electroacupuncture at 2 and 100 Hz on rat type 2 diabetic neuropathic pain and hyperalgesia-related protein expression in the dorsal root ganglion. J Zhejiang Univ Sci B 18(3):239–248. https://doi.org/10.1631/jzus.B1600247
Shin KM, Lee S, Lee EY, Kim CH, Kang JW, Lee CK, Seo BN, Kim AR, Jung SY, Kwon O, Choi SM (2018) Electroacupuncture for Painful Diabetic Peripheral Neuropathy: A Multicenter, Randomized, Assessor-Blinded. Controlled Trial Diabetes Care 41(10):141–142. https://doi.org/10.2337/dc18-1254
Wang F, Ma J, Han F, Guo X, Meng L, Sun Y, Jin C, Duan H, Li H, Peng Y (2016) DL-3-n-butylphthalide delays the onset and progression of diabetic cataract by inhibiting oxidative stress in rat diabetic model. Sci Rep 6:19396. https://doi.org/10.1038/srep19396
Zhou R, Xu T, Liu X, Chen Y, Kong D, Tian H, Yue M, Huang D, Zeng J (2018) Activation of spinal dorsal horn P2Y13 receptors can promote the expression of IL-1β and IL-6 in rats with diabetic neuropathic pain. J Pain Res 11:615–628. https://doi.org/10.2147/JPR.S154437
Erbaş O, Oltulu F, Yılmaz M, Yavaşoğlu A, Taşkıran D (2016) Neuroprotective effects of chronic administration of levetiracetam in a rat model of diabetic neuropathy. Diabetes Res Clin Pract 114:106–116. https://doi.org/10.1016/j.diabres.2015.12.016
Manitz MP, Plümper J, Demir S, Ahrens M, Eßlinger M, Wachholz S, Eisenacher M, Juckel G, Friebe A (2016) Flow cytometric characterization of microglia in the offspring of PolyI: C treated mice. Brain Res 1636:172–182. https://doi.org/10.1016/j.brainres.2016.02.004
Hao L, Mi J, Song L, Guo Y, Li Y, Yin Y, Zhang C (2021) SLC40A1 Mediates Ferroptosis and Cognitive Dysfunction in Type 1 Diabetes. Neuroscience 463:216–226. https://doi.org/10.1016/j.neuroscience.2021.03.009
El-Hossary N, Hassanein H, El-Ghareeb AW, Issa H (2016) Intravenous vs intraperitoneal transplantation of umbilical cord mesenchymal stem cells from Wharton’s jelly in the treatment of streptozotocin-induced diabetic rats. Diabetes Res Clin Pract 121:102–111. https://doi.org/10.1016/j.diabres.2016.09.008
Qin Z, Wang L, Li G, Qian X, Zhang J, Guo Y, Liu G (2020) Analysis of the analgesic effects of tricyclic antidepressants on neuropathic pain, diabetic neuropathic pain, and fibromyalgia in rat models. Saudi J Biol Sci 27(9):2485–2490. https://doi.org/10.1016/j.sjbs.2020.05.043
Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50(6):537–546
Morrow TJ (2004) Animal models of painful diabetic neuropathy: the STZ rat model. Curr Protoc Neurosci Chapter 9:Unit 9.18 https://doi.org/10.1002/0471142301.ns0918s29
Cheng KI, Wang HC, Chuang YT, Chou CW, Tu HP, Yu YC, Chang LL, Lai CS (2014) Persistent mechanical allodynia positively correlates with an increase in activated microglia and increased P-p38 mitogen-activated protein kinase activation in streptozotocin-induced diabetic rats. Eur J Pain 18(2):162–173. https://doi.org/10.1002/j.1532-2149.2013.00356.x
Sun JS, Yang YJ, Zhang YZ, Huang W, Li ZS, Zhang Y (2015) Minocycline attenuates pain by inhibiting spinal microglia activation in diabetic rats. Mol Med Rep 12(2):2677–2682. https://doi.org/10.3892/mmr.2015.3735
Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF (1997) Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol 79(2):163–175. https://doi.org/10.1016/s0165-5728(97)00119-7
Schwab JM, Guo L, Schluesener HJ (2005) Spinal cord injury induces early and persistent lesional P2X4 receptor expression. J Neuroimmunol 163(1–2):185–189. https://doi.org/10.1016/j.jneuroim.2005.02.016
Vázquez-Villoldo N, Domercq M, Martín A, Llop J, Gómez-Vallejo V, Matute C (2014) P2X4 receptors control the fate and survival of activated microglia. Glia 62(2):171–184. https://doi.org/10.1002/glia.22596
Zabala A, Vazquez-Villoldo N, Rissiek B, Gejo J, Martin A, Palomino A, Perez-Samartín A, Pulagam KR, Lukowiak M, Capetillo-Zarate E, Llop J, Magnus T, Koch-Nolte F, Rassendren F, Matute C, Domercq M (2018) P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol Med 10(8):8743. https://doi.org/10.15252/emmm.201708743
Zhang Z, Zhang ZY, Fauser U, Schluesener HJ (2008) Mechanical allodynia and spinal up-regulation of P2X4 receptor in experimental autoimmune neuritis rats. Neuroscience 152(2):495–501. https://doi.org/10.1016/j.neuroscience.2007.12.042
Butovsky O, Weiner HL (2018) Microglial signatures and their role in health and disease. Nat Rev Neurosci 19(10):622–635. https://doi.org/10.1038/s41583-018-0057-5
Salter MW, Stevens B (2017) Microglia emerge as central players in brain disease. Nat Med 23(9):1018–1027. https://doi.org/10.1038/nm.4397
Zhang J, Yi S, Xiao C, Li Y, Liu C, Jiang W, Yang C, Zhou T (2020) Asperosaponin VI inhibits LPS-induced inflammatory response by activating PPAR-γ pathway in primary microglia. Saudi J Biol Sci 27(11):3138–3144. https://doi.org/10.1016/j.sjbs.2020.07.013
Burnstock G (2008) Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov 7(7):575–590. https://doi.org/10.1038/nrd2605
Khakh BS, North RA (2012) Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron 76(1):51–69. https://doi.org/10.1016/j.neuron.2012.09.024
Inoue K (2021) Nociceptive signaling of P2X receptors in chronic pain states. Purinergic Signalling 17(1):41–47. https://doi.org/10.1007/s11302-020-09743-w
Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424(6950):778–783. https://doi.org/10.1038/nature01786
Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K (2009) Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain 5:28. https://doi.org/10.1186/1744-8069-5-28
Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F (2008) Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28(44):11263–11268. https://doi.org/10.1523/JNEUROSCI.2308-08.2008
Tozaki-Saitoh H, Masuda J, Kawada R, Kojima C, Yoneda S, Masuda T, Inoue K, Tsuda M (2019) Transcription factor MafB contributes to the activation of spinal microglia underlying neuropathic pain development. Glia 67(4):729–740. https://doi.org/10.1002/glia.23570
Miao J, Ding M, Zhang A, Xiao Z, Qi W, Luo N, Di W, Tao Y, Fang Y (2012) Pleiotrophin promotes microglia proliferation and secretion of neurotrophic factors by activating extracellular signal-regulated kinase 1/2 pathway. Neurosci Res 74(3–4):269–276. https://doi.org/10.1016/j.neures.2012.09.001
Smith PA (2014) BDNF: no gain without pain? Neuroscience 283:107–123. https://doi.org/10.1016/j.neuroscience.2014.05.044
Trang T, Beggs S, Wan X, Salter MW (2009) P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci 29(11):3518–3528. https://doi.org/10.1523/JNEUROSCI.5714-08.2009
Ji RR, Nackley A, Huh Y, Terrando N, Maixner W (2018) Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 129(2):343–366. https://doi.org/10.1097/ALN.0000000000002130
Gu Y, Chen S, Mo Y, Tu Y, Chen N, Zhao X, Li S, Yu Q, Dai Q, Wang J (2020) Electroacupuncture Attenuates CFA-Induced Inflammatory Pain by Regulating CaMKII. Neural Plast 2020:8861994. https://doi.org/10.1155/2020/8861994
Zhang RY, Zhu BF, Wang LK, Song Y, Zhao JG, Guo Y, Zhao L, Chen S (2020) Electroacupuncture alleviates inflammatory pain via adenosine suppression and its mediated substance P expression. Arq Neuropsiquiatr 78(10):617–623. https://doi.org/10.1590/0004-282X20200078
Zhao X, Liu L, Wang Y, Wang G, Zhao Y, Zhang Y (2019) Electroacupuncture enhances antioxidative signal pathway and attenuates neuropathic pain induced by chemotherapeutic paclitaxel. Physiol Res 68(3):501–510. https://doi.org/10.33549/physiolres.934084
Hu QQ, He XF, Ma YQ, Ma LQ, Qu SY, Wang HZ, Kang YR, Chen LH, Li X, Liu BY, Shao XM, Fang JF, Liang Y, Fang JQ, Jiang YL (2023) Dorsal root ganglia P2X4 and P2X7 receptors contribute to diabetes-induced hyperalgesia and the downregulation of electroacupuncture on P2X4 and P2X7. Purinergic Signal 19(1):29–41. https://doi.org/10.1007/s11302-022-09844-8
Lee JY, Choi HY, Park CS, Pyo MK, Yune TY, Kim GW, Chung SH (2019) GS-KG9 ameliorates diabetic neuropathic pain induced by streptozotocin in rats. J Ginseng Res 43(1):58–67. https://doi.org/10.1016/j.jgr.2017.08.004
Chen XM, Xu J, Song JG, Zheng BJ, Wang XR (2015) Electroacupuncture inhibits excessive interferon-γ evoked up-regulation of P2X4 receptor in spinal microglia in a CCI rat model for neuropathic pain. Br J Anaesth 114(1):150–157. https://doi.org/10.1093/bja/aeu199
Funding
This research was supported by the National Natural Science Foundation of China (81804181 to X.F.H. and 81774389 to Y.L.J.), the Natural Science Foundation of Zhejiang Province of China (LY22H270006 to Y.L.J.), the Research Fund for Zhejiang Traditional Chinese Medicine University Affiliated Hospital (2022FSYYZZ09 to Y.L.J.), and the Key Laboratory of Acupuncture and Neurology of Zhejiang Province (2019E10011).
Author information
Authors and Affiliations
Contributions
X.H., J.F. and Y.J. conceived and designed the experiments. S.Q., H.W., Q.H., Y.K., X.L., L.C. and L.M. performed animal experiments. Q.H., Y.M., and L.M. performed western blotting experiments. S.Q. and H.W. performed immunofluorescence labeling experiments. B.L. and H.Z. performed data analysis. X.H., S.Q. and H.W. wrote the manuscript. X.S., Y.L., J.D. and B.L. performed revising. All authors reviewed and revised this manuscript and reviewed the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This study was permitted by The Animal Welfare Committee of Zhejiang Chinese Medical University (Approval number: IACUC-20190805-04). The institution agreed to participate and agreed to publish.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The article has been submitted in preprint (https://doi.org/10.21203/rs.3.rs-1613857/v1).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qu, Sy., Wang, Hz., Hu, Qq. et al. Electroacupuncture may alleviate diabetic neuropathic pain by inhibiting the microglia P2X4R and neuroinflammation. Purinergic Signalling (2023). https://doi.org/10.1007/s11302-023-09972-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11302-023-09972-9