Abstract
Microglia are critical in the pathogenesis of neuropathic pain. In this study, we investigated the role of microvesicles (MVs) in neuropathic pain induced by spinal nerve ligation (SNL) in rats. First, we found that MVs shed from microglia were increased in the cerebrospinal fluid and dorsal horn of the spinal cord after SNL. Next, MVs significantly reduced paw withdrawal threshold (PWT) and paw withdrawal latency (PWL). In addition, the P2X7-p38 pathway was related to the bleb of MVs after SNL. Interleukin (IL)-1β was found to be significantly upregulated in the package of MVs, and PWT and PWL increased following inhibition with shRNA-IL-1β. Finally, the amplitude and frequency of spontaneous excitatory postsynaptic currents increased following stimulation with MVs. Our results indicate that the P2X7-p38 pathway is closely correlated with the shedding of MVs from microglia in neuropathic pain, and MVs had a significant effect on neuropathic pain by participating in the interaction between microglia and neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropathic pain is a chronic pain caused by a lesion or disease of the somatosensory nervous system and characterized by hyperalgesia and allodynia [1, 2]. It is an unpleasant sensory consequence of neuronal activities in specific nociceptive pathways that is triggered by noxious stimuli, inflammation, or damage to the nervous system [3]. It is now clear that in addition to neurons playing an important role in the maintenance of common clinical pain, immune and glia cells are also involved in the pathological changes following nerve injury [4]. However, since various pain receptors are involved together with sensitization of sensory neurons, as well as the role of soluble mediators released from non-neuronal cells such as macrophages and mast cells, it is still difficult to understand the complicated spatial and temporal scale of pain development and neuronal disease progression [3, 5]. Thus, neuropathic pain remains an important large clinical and social problem since there is insufficient pain relief from current treatments.
The pro-inflammatory cytokines interleukin (IL)-1β, IL6, IL12, interferon-γ, and tumor necrosis factor (TNF) have been demonstrated to contribute to axonal damage [6]. In addition, they also modulate spontaneous nociceptor activity and stimulus sensitivity [7]. It has been reported that a delayed immune response to nerve injury at the lesion site occurs with an upregulation of IL-1β and TNF-α in macrophages and Schwann cells, indicating a significant effect of inflammatory reactions on peripheral neuropathic pain [8].
In pathological conditions, ATP, one of the most powerful mediators, activates microglia via P2X7 receptors, resulting in increased release of cytokines, including IL-1β, -6, -10, TNF-α, and transforming growth factor-β. Direct modulation of dorsal horn neuron activity driven by these cytokines plays an important role in the development of neuropathic pain [9].
Recently, extracellular vesicles, including exosomes and plasma membrane-derived microvesicles (MVs), also called microparticles, have gained increasing attention as efficient vehicles for the release of signaling molecules in cell communication and information exchange, including the interaction between glia and neurons. This information exchange promotes neuronal survival, the immune response mediated by microglia, and synapse assembly and plasticity [10]. It has been demonstrated that the budding and shedding of microparticles can be triggered by stimulation of the P2X7 ATP receptor that activates the p38 MAPK cascade through src kinase-mediated phosphorylation [11]. In contrast, phosphorylated p38 triggers plasma membrane pore formation and mobilization of acid sphingomyelinases, which further alters membrane fluidity, leading to plasma membrane budding and shedding. IL-1β is released into the extracellular environment through shed microparticles to participate in the communication between microglial and neuronal cells [11].
To date, MVs, an important membrane-enclosed compartment of cells, have not been studied in relation to neuropathic pain. Thus, investigating the effect and molecular mechanism of microvesicles occurring in neuropathic pain may allow the design of appropriate tissue- and cell-targeted approaches to treat neuropathic pain. In this study, we have investigated the role of microvesicles in neuropathic pain induced by spinal nerve ligation (SNL) in rats and its participation in the interaction between microglia and neurons via pro-inflammatory cytokines and neurotransmission.
Materials and methods
Animals
Sprague-Dawley rats (200 g) were purchased from the Laboratory Animal Center of the Second Military Medical University. Rats were randomly assigned to standard cages, with three to four animals per cage. Rats were housed in the Laboratory Animal Center of the Second Military Medical University, 12:12 h light/dark cycle with adequate food and water. The rats were placed in the experimental room 24 h before behavioral test for acclimatization. All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Second Military Medical University and conformed to the American Physiological Society’s Guiding Principles in the Care and Use of Animals. Six rats per group were used for behavioral tests. All efforts were made to minimize animal suffering.
Spinal nerve ligation
Behavioral tests were performed before surgery to establish a baseline. Neuropathic pain was induced by ligating the L5 spinal nerve as in previous research [12]. With sodium pentobarbital anesthesia (50 mg/kg body weight, i.p.), the rat was barbered at the lower back and the skin was sterilized with 0.5 % chlorhexidine and covered with clean gauze. Using sterilized operating instruments, the left paraspinal muscles were separated from the spinous processes at the L5–L6 levels and the L6 transverse process was carefully removed. The L5 spinal nerve was isolated and tightly ligated with 3–0 silk threads. The wound was washed with 5 mL of distilled saline and sutured with 3–0 silk threads. In sham-operated rats, the left L5 spinal nerve was isolated without ligation treatment.
Intrathecal catheter
Implantation of intrathecal cannula was performed following the method described in a previous study [13]. Under anesthesia with sodium pentobarbital, the L4–L5 spinal processes were exposed and the muscles beside the processes were isolated bluntly. Then, a PE-10 polyethylene tubing was inserted into the cavum subarachnoidale through the interval between L4 and L5 vertebrate, approximately 1 cm until the tip of the tubing reached to the lumbar cistern. The outer part of the tubing was fixed onto the muscle of the lumbar and the skin of the head. The fluid within the tubing was about 10 μL. A small volume of lidocaine, approximately 10 μL, was administered in the tubing followed by 10 μL artificial cerebrospinal fluid (CSF) and the rat exhibited motor dysfunction, which indicated the successful implantation of the intrathecal catheter. In the experimental group, a single 10 μL volume of FTY720 (1 mM) or MVs (104 per rat) was injected into the SNL 7-day rat or the naive rat, respectively, followed by a 10 μL dose of artificial CSF. However, in the control group, 20 μL of artificial CSF was administrated directly.
Cerebrospinal fluid extraction
Rats were anesthetized with sodium pentobarbital (50 mg/kg body weight, i.p.) and fixed on the stereotaxic instrument. The skin was incised at the midline of occipitalia, and the muscles were dissected bluntly. The foramina magnum was exposed, and CSF was extracted with a 100 μL microinjector. Approximately 100 μL volume of CSF was extracted from each rat; the CSF was put in a 1.5 mL epoxy epoxide (EP) tube containing 1 μL of phenylmethanesulfonyl fluoride (PMSF). All EP tubes were placed on ice to reduce CSF temperature and therefore decrease MV rupture.
MV isolation
The MVs were isolated by differential centrifugations according to a previous report [14]. Briefly, CSF or the medium from cultured N9 microglia was subjected to differential centrifugations: firstly, 5 min at 800×g to remove cells and then 5 min at 4500×g to discard large debris. Then, the supernatant was spun down at 20,000×g for 1 h at 4 °C to provide a pellet enriched in MVs.
siRNA targeting rat P2X7R mRNA
P2X7 small interfering RNA (siRNA) was synthesized according to the four sense sequences shown in a previous study [15]: (1) GUACAGUGGCUUCAAGU AU, (2) GGAUGGACCCACAAAGUAA, (3) UUACAGAGGUGGCAGUU CA, (4) GAACGAUGUCUUUCAGUAU. SiGENOME RISC-Free control siRNA was used as non-targeting control.
Cell culture and transfection of IL-1β-shRNA using lentiviral infection
The N9 microglia cell line was obtained from the Department of Neurobiology, Second Military Medical University. N9 cells were cultured in Dulbecco’s modified Eagle media/nutrient mixture (DMEM/F12) with 10 % fetal calf serum (Gibco, Australia), 100 U/mL penicillin, and 100 μg/mL streptomycin. The temperature was 37 °C, and the concentration of CO2 was 5 %. The medium was changed every 2–3 days and cells passaged every 4–5 days. When the number of N9 cells in a T25 bottle was approximately 1 × 106, the medium was removed and the cells were treated with endotoxin lipopolysaccharide (LPS; 10 μg/mL) for 2 h, as described previously [16]. Cells were washed twice with PBS and then treated with 2′(3′)-O-(4-benzoylbenzoyl) ATP (BzATP; 300 μM) in serum-free medium for 30 min. Thereafter, the medium was collected to isolate MVs as described above.
For transfection of IL-1β-short hairpin RNA (shRNA), N9 cell line was grown in a 12-well plate and infected at almost 50 % confluence with lentivirus vectors for IL-1β-shRNA or scrambled-shRNA. After a 12-h treatment, the cultured medium was changed to a conditional medium, and the microglia were further cultured for 48 h and then treated with BzATP (300 μμ) in serum-free medium for 30 min, as described above.
Behavioral tests
The behavioral tests for mechanical allodynia and thermal hyperalgesia were carried out on rats 1 day prior to surgery and 1, 3, 5, 7, 10, and 14 days after surgery until sacrifice. Mechanical allodynia was assessed by measuring the paw withdraw threshold (PWT) in response to probing using von Frey monofilaments (Stoelting, Wood Dale, IL, USA). Briefly, the rat was placed in a plastic cage, which was elevated on a mesh screen. Each rat was allowed to acclimate for 30 min prior to testing. Calibrated filaments, ranging from 1 to 15 g, were applied to the plantar surface of the left hind paw. Each filament was applied over five sessions, two times per session, at least 5 min between two sessions and 5 s between the two applications. The minimum of the filament value causing five positive responses (paw withdrawal or licking) in ten tests was taken as the PWT. Thermal hyperalgesia was assessed by testing the paw withdrawal latency (PWL) using the plantar test (7370, UGO BASILE, Italy). Specifically, the rat was placed beneath the same plastic cage used for the mechanical allodynia test, but this time, upon an elevated glass floor. With the rat standing relatively still, a radiant heat source beneath the glass floor was aimed at the plantar surface of the hind paw. The withdrawal latency was measured to the nearest 0.1 s. Prior to assessment of heat hyperalgesia, the intensity of the radiant heat source was adjusted to yield a mean baseline latency of approximately 11 s from six naive rats with the cutoff automatically set at 22.5 s to avoid tissue damage. The hind paws were tested alternately with at least 5 min between consecutive tests. Three latencies were taken for each hind paw in each test session. The three latencies per side were averaged, and a difference score was computed by subtracting the average latency of the paw on the contralateral side from that of the ipsilateral side. Negative difference scores indicated a hyperalgesic response on the ipsilateral side.
Electrophysiological recording
Spinal cord slices were prepared from Sprague-Dawley rats (120–150 g). In brief, the adult rats were deeply anesthetized with sevoflurane, and then the lumbosacral enlargement of the spinal cord was exposed. The spinal cord was removed immediately and placed in pre-chilled artificial CSF saturated with 95 % O2 and 5 % CO2. The spinal nerve and spinal membrane were modificated. Then, it was mounted on a vibratome, and a 400-μm thick transverse slice was cut from the lumbar segment. The slice was placed in an incubation slot, and the incubation buffer was heated to a temperature of 37 °C. The slice was incubated for 1 h and then electrophysiological recording was performed.
For recording spontaneous excitatory postsynaptic currents (sEPSC), electrodes were made from borosilicate glass capillary tubing (1–2 μm tip diameter) and filled with internal patch solution with a final resistance of 3–6 MΩ. A slice was placed in the recording chamber and perfused at a rate of 5–8 mL/min. Membrane potentials were held at −70 mV in voltage-clamp mode. The membrane patch was ruptured by a brief period of more negative pressure after making a gigaohm seal, further resulting in a whole cell configuration. An MP-285 manipulator was used to move the glass electrode (MP-285, Sutter, Novato, CA). Data acquisition was performed with Clampex 10.2 software. Frequency and amplitude histograms were established using this software.
Immunofluorescence
Immunofluorescence techniques were modified as reported previously [17]. There were five or six rats in each group. Under sodium pentobarbital anesthesia (50 mg/kg body weight, i.p.), rats were perfused through the aorta using 0.01 M phosphate buffered saline (PBS, pH 7.2–7.4, 4 °C) 200 mL, followed by 4 % paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB, pH 7.2–7.4, 4 °C) 200–300 mL. The spinal cords were exposed, and the lumbar segments were dissected out, postfixed in 4 % PFA at 4 °C overnight. Then, the spinal cords were removed to a 20 % sucrose solution in 0.1 M PB at 4 °C overnight, followed by 30 % sucrose solution in 0.1 M PB. The dissected spinal cord was mounted in optimum cutting temperature (OCT) compound, frozen at −20 °C. Transverse spinal cord sections (20 μm) were cut using a cryotome (LEICA CM1900 UV, Germany) and then preserved in 0.01 M PBS containing sodium azide at 4 °C. The sections were washed in 0.01 M PBS three times, 3 min per time, and then incubated with blocking solution containing 10 % normal fetal bovine serum (Gibco, Australia) and 0.2 % Triton X-100 for 1 h at room temperature. Then, the floating tissue sections were incubated in a primary antibody in the same solution overnight for approximately 16 h at 4 °C on a rocker. The primary antibodies used in this experiment were goat polyclonal IBA-1 (1:400, Abcam, USA) and rabbit polyclonal P2X7 (1:100, Abcam, USA). The tissue sections were then rinsed in 0.01 M PBS three times, 3 min each, and incubated for about 1 h at room temperature in a dark humidity chamber with one of the following secondary antibodies: Alexa Fluor®555-conjugated donkey anti-rabbit IgG or Alexa Fluor®488-conjugated donkey anti-goat IgG (1:400, Abcam, USA). The nucleus was stained with Hoechst. The tissue sections were washed three times with 0.01 M PBS for 3 min each and then mounted on glass slides and covered with 50 % glycerol PBS.
Western blot
Animals were deeply anesthetized using sodium pentobarbital (50 mg/kg body weight, i.p.) and decapitated. The lumbar spinal cord segments were dissected out immediately on an ice bag. The ipsilateral L5 spinal segments were homogenized in a lysis buffer containing protease and phosphate inhibitors. Triton X-100 was added in the homogenates to a final concentration of 1 %, and the homogenates were then incubated on ice for 30 min and further separated by centrifugation (20,000×g × 15 min). A BCA Protein Assay (Pierce, USA) was used to quantify the protein concentrations. The supernatant was run through a 12 % SDS-polyacrylamide gel and then wet electro-transferred to a 0.4 μm polyvinylidene fluoride (PVDF) membrane. The reaction was blocked with 10 % evaporated skimmed milk at room temperature for 1 h on a rocker and then incubated overnight for approximately 16 h at 4 °C in either rabbit anti-P2X7 (1:1000, Abcam, USA) or p38 (1:1000, Abcam, USA). Blots were rinsed with 0.01 M PBS three times for 10 min each and incubated in a goat anti-rabbit secondary antibody conjugated with HRP (1:1000, Sigma, USA) for about 1 h. The target proteins were tested by an enhanced chemiluminescence (ECL) detection system (Pierce, USA). The sample was normalized with a monoclonal anti-β-actin antibody produced in mouse (1:1000, Cell Signaling Technology, USA). The specific bands were estimated by molecular size. ImageJ software (NIH, USA) was used to detect the signal intensity.
Flow cytometry
Flow cytometry was performed as described previously [18]. Briefly, MVs obtained from CSF were fixed with 2 % PFA for about 15 min and then permeabilized with 0.1 % PBS-Tween for 20 min. The MVs were isolated by differential centrifugation and then stained with IBA-1 conjugated with FITC (Abcam, USA) and Annexin V conjugated with APC (Sigma, USA) according to the manufacture’s protocol. Thereafter, the MVs were washed with pre-chilled PBS. Then, MVs were isolated through differential centrifugation as described above. Re-suspended with PBS and then analyzed using flow cytometry and Kaluza flow cytometry analysis software (Beckman Coulter).
Enzyme-linked immunosorbent assay
The type and amount of inflammatory factors in the MVs were estimated using an ELISA kit (R&D Systems, USA). The MVs obtained from the microglia cell medium were homogenized using a lysis buffer including protease inhibitor [16]. Then, the protein concentrations were detected using a BCA Protein Assay Kit (Pierce, USA). ELISA was performed, and the standard curve was depicted according to the manufacturer’s protocol.
Electron microscopy
The MVs were fixed with 2.5 % glutaraldehyde for about 30 min, re-suspended, and absorbed to a 400 mesh copper screen until the surface became desiccated. MVs were subjected to negative staining with 1.9 % methylcellulose and 0.1 % uranyl acetate. Then, the samples were observed on a scanning electron microscope.
Statistical analysis
Data are expressed as mean ± standard error (SEM). Repeated measurement was used for detecting differences in mechanical thresholds, thermal latencies, and protein contents over time, between control and ligated animals, or vehicle and drug-treated animals. To further detect significant effects of time points, two-tailed paired Student’s t test was carried out to analyze the differences. ANOVA was used followed by Dunnett’s post hoc test. Pearson correlation coefficients were computed to detect potential correlations between the pain behavior and the amount of MVs following treatment with FTY720. Values of P < 0.05 were considered to be statistically significant.
Results
Spinal nerve ligation induces microvesicle shedding from microglia
In order to understand the changes that occur in microglia in neuropathic pain, an experimental animal model for peripheral neuropathic pain by SNL in rats was used in our study. Rapidly appearing (<1 day) and persistent (>3 weeks) neuropathic pain was observed following SNL [19, 20]. MVs separated from CSF after nerve injury were examined by negative staining electron microscopy (EM), which revealed the presence of MVs with different sizes (>100 nm diameter) and shapes (Fig. 1a–c). The size of the MVs was slightly larger on day 7 than on day 3 or day 14.
The existence of microvesicles (MVs) in the cerebrospinal fluid (CSF) and dorsal horn of spinal cord of rats. a–c MVs were isolated from the CSF on different days following spinal nerve ligation (SNL) and analyzed by negative staining electron microscopy. Electron microscopy showed large vesicles with a morphology that was consistent with the characteristics of MVs, including delimitation by a lipid bilayer and a diameter larger than 100 nm. Scale bar = 100 nm. d–i Immunofluorescence labeling of MVs shed from microglia in the dorsal horn of spinal cord of sham group (d, f, h) and SNL 7-day rat (e, g, i). MVs derived from microglia were analyzed by fluorescence microscopy using the microglia marker IBA-1 (d, e) and the nucleus marker Hoechst (f, g). Merged images are shown in the right column (h, i). As indicated by arrows, MVs around microglia were stained by IBA1 without Hoechst (n = 4 rats for each group)
IBA1 is specifically expressed in microglia, and its upregulation is often considered as a marker of “activated microglia” [21–23]. Therefore, it is possible to use IBA1 to detect MVs derived from microglia. The spinal cord slices were stained with IBA1 and Hoechst. Compared to the sham group, the number of MVs around microglia in the dorsal horn was significantly elevated (Fig. 1d–i). In addition, the number of shed MVs in CSF was evaluated by flow cytometry analysis following IBA and Annexin V double labeling. Microvesicles that shed from microglia in CSF were statistically significantly increased, especially on the seventh day after SNL (Fig. 2a, b).
a–e Flow cytometry analysis of microvesicles (MVs) presented in the rat cerebrospinal fluid after spinal nerve ligation, directly stained with IBA1 conjugated with FITC and Annexin V conjugated with APC. f The quantification of MVs stained with IBA-1 conjugated with FITC in flow cytometry experiments. All data are mean ± SEM. *P < 0.05, **P < 0.01, compared with control group. One-way ANOVA, followed by Bonferroni’s post hoc test was applied (n = 3 rats for each group)
Microvesicles are involved in neuropathic pain induced by spinal nerve ligation in rat
FTY720, the first approved oral multiple sclerosis (MS) drug, has been shown to significantly reduce the amount of MVs in the CSF of mice with experimental autoimmune encephalomyelitis, an animal model of MS [24], so we investigated the effect of FTY720 on MVs. Before SNL, no differences in PWT and PWL were observed among the control group (control), sham group (sham), and SNL group (SNL) (Fig. 3a, b). Both the PWT with the strength of the Von Frey monofilament stimulation and the PWL to thermal stimulation significantly decreased following SNL. After injection of MVs into the naive rat, the PWT and PWL were significantly reduced at the beginning of postinjection period (Fig. 3c, d). With increasing time, the PWT reached the same level as the PBS group, becoming not significantly different. However, a significant difference was still present in the PWL after a postinjection period of 6 h. In contrast, we observed a significant elevation of the PWT from 30 min to 2 h and a remarkable increase of the PWL from 30 min to 3 h after injection of FTY720 into rats following SNL for 7 days, both of which slowly declined (Fig. 3e, f). We next examined the number of MVs in the CSF of rats at 3 and 6 h after FTY720 injection. As shown in Fig. 4, FTY720 significantly decreased the number of MVs in CSF. Moreover, the number of MVs was lowest at 3 h after FTY720 injection and began to increase at 6 h (Fig. 4). This result was consistent with the behavioral results (Fig. 3e, f). All the above data indicate that MVs directly participate in the pathology of neuropathic pain induced by SNL in rats.
Time course of changes in paw withdrawal threshold and paw withdrawal latency following spinal nerve ligation (SNL) (a, b) or with injection of 104 microvesicles (MVs) into naive rats (c, d) or 10 μL FTY720 (e, f 1 mM) into rats following SNL for 7 days. All data points are mean ± SEM. *P < 0.05, compared with control group or PBS group; n = 6 rats for each group. Statistical significance was determined by repeated measures ANOVA, followed by Student’s t test
The number of microvesicles (MVs) in cerebrospinal fluid (CSF) decreased after FTY720 intrathecal injection on day 7 after SNL. a–c Flow cytometry analysis of MVs in CSF after PBS (a) or FTY720 injection 3 h (b) or FTY720 injection 6 h (c). d Quantity of IBA1+ MVs in a–c. All data are mean ± SEM. *P < 0.05, compared with PBS group, # P < 0.05; n = 3 rats for each group. Statistical significance was determined by one-way ANOVA, followed by Bonferroni’s post hoc test
The P2X7-p38 pathway is involved in the production of microvesicles in rats following spinal nerve ligation
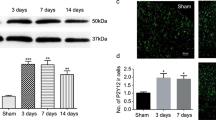
It was thought that the P2X7-p38 pathway was involved in the shedding of MVs [11], and we hypothesized that the pathway was also involved in the shedding of MVs involved in neuropathic pain induced by SNL. To test our hypothesis, tissue lysates following SNL were investigated using western blot analysis. We observed by western blotting that SNL induced a significant increase of P2X7 receptors and p38, both of which reached a peak at day 7 and maintained this high level at day 14 (Fig. 5a, b). Double immunofluorescence indicated that most MVs shed from the microglia were P2X7 receptor positive, and the number of MVs positive for P2X7 receptors and IBA1 increased from days 3 to 7 of neuropathic pain development (Fig. 5c). We then inhibited the expression of P2X7 receptors by intrathecal injection of P2X7 siRNA lentivirus. P2X7 siRNA significantly decreased P2X7 receptors levels in the ipsilateral spinal cord (Fig. 6a) and raised the threshold of mechanical allodynia and thermal hyperalgesia (Fig. 6b, c). Moreover, intrathecal injection of P2X7 siRNA observably decreased the number of MVs in CSF extracted on day 7 after SNL (Fig. 6d–f). Thus, these results suggest that the P2X7-p38 pathway is involved in the shedding of MVs induced by SNL.
P2X7 receptors on microglia increased after spinal nerve ligation (SNL). a Western blot analysis of P2X7 receptors and p38 in spinal cord after SNL. b The quantitative analysis of P2X7 receptors and p38 normalized to β-actin at different times. c Fluorescent images of microglia from spinal cord in rats following SNL, stained for P2X7 receptors (red) and IBA-1 (green). Note the presence of many microvesicles at the cell surface positive for both P2X7 receptors and IBA1. All data points are mean ± SEM. *P < 0.05, a significant difference as compared with the sham group. One-way ANOVA, followed by Bonferroni’s post hoc test was applied. n = 4 rats for each group
Inhibition of P2X7 receptors decreased the number of microvesicles (MVs) derived from microglia and alleviated pain after SNL. a P2X7 receptor expression level in ipsilateral spinal cord on after intrathecal injection of lenti-P2X7 siRNA or lenti-control siRNA. b, c Paw withdrawal threshold to mechanical stimuli (b) and paw withdrawal latency to thermal stimuli (c) of rats tested on day 7 after SNL, following intrathecal injection of lenti-P2X7 siRNA or lenti-control siRNA. d, e Flow cytometry analysis of MVs in cerebrospinal fluid (CSF) acquired after intrathecal injection of lenti-control siRNA (d) or lenti-P2X7 siRNA (e). f The quantification of IBA+ MVs in 100 μL CSF. Lenti-control siRNA or lenti-P2X7 siRNA was injected intrathecally 3 days before SNL, and spinal cord or CSF was acquired on day 7 after SNL. All data points are mean ± SEM. *P < 0.05, compared with basis level (BL) or control siRNA; # P < 0.05. (n = 6 rats for b and c, n = 3 rats for a, d, e, f)
Hyperalgesia induced by microvesicles is mediated by IL-1β
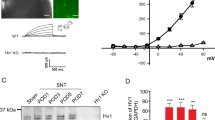
It has been demonstrated that P2X7 receptors are predominantly localized on the cell membrane of immune cells and glia, where they mediate pro-inflammatory cytokine IL-1β release [16, 25]. IL-1β is produced and secreted in pathological conditions, associated with increased pain and hyperalgesia [26]. We hypothesized that IL-1β mediated the painful action of MVs shed from microglia. To confirm our hypothesis, N9 cells were stimulated with BzATP. After exposure to BzATP, the number of shedding MVs on the surface of N9 microglia increased significantly (Fig. 7a, b). Moreover, the presence of proinflammatory cytokines in MVs was detected. As shown in Fig. 7c, IL-1β levels were significantly increased after stimulation with BzATP, whereas TNF-α and IL-6 levels did not alter (Fig. 7c). To test whether IL-1β contributes to the algogenic effect of MVs, we transfected lenti-scrambled shRNA or lenti-IL-1β shRNA into N9 cells and then stimulated them with BzATP. Compared to lenti-scrambled shRNA, treatment with lenti-IL-1β shRNA significantly decreased IL-1β levels in MVs (Fig. 7d). MVs from N9 cells pre-treated with lenti-scrambled shRNA or lenti-IL-1β shRNA were intrathecally injected into naive rats, and PWT and PWL were determined to evaluate pain behavior. Compared to the lenti-scrambled shRNA group, both PWT and PWL were significantly increased in the lenti-IL-1β shRNA group (Fig. 7e, f). This indicated that IL-1β played an important role in the algogenic effect of MVs.
Hyperalgesia induced by microvesicles (MVs) was mediated, at least partly, by IL-1β. a, b Exposure to BzATP induced MV shedding from the N9 cell line (a, control; b, BzATP). c Concentration of IL-1β, TNF-α, and IL-6 in MVs obtained from the microglia cell medium by ELISA. d Concentration of IL-1β in MVs derived from N9 cells transfected with lenti-scrambled shRNA or lenti-IL-1β shRNA followed by stimulation with BzATP. e, f Paw withdrawal threshold to mechanical stimuli (e) and paw withdrawal latency to thermal stimuli (f) of rats administered with MVs (104 per rat) obtained from N9 cells transfected with lenti-scrambled shRNA or lenti-IL-1β shRNA. Shedding MVs are indicated by an arrow in b. All data points are mean ± SEM. *P < 0.05, a significant difference as compared with the control group or the lenti-scrambled shRNA group. One-way ANOVA, followed by Bonferroni’s post hoc test (c) or Student’s t test (d) or repeated measures ANOVA, followed by Student’s t test (e, f) was applied. n = 3 independent cultures for c and d, and n = 6 rats for e and f
Microglia-derived MVs enhance excitatory neurotransmission
Given the fact that inflammatory mediators may affect glutamate transmission [27, 28], we explored the effect of MVs on the modulation of neurotransmission in order to further understand the functional role of MVs in the interaction between microglia and neurons.
To evaluate the effect of microglia-derived MVs on neurotransmission, we examined sEPSC in the SNL 7-day rat and sham rat, before or after exposure to MVs produced from N9 microglia. The MVs were administrated at 5 × 104/mL. Exposure to MVs induced significant increases in sEPSC frequency and amplitude in both groups. Compared to the sham group, the spinal cord slices of SNL 7-day rats had a smaller increase in sEPSC amplitude (Fig. 8b, e). However, MVs increased the frequency of sEPSC by more than twofold in the spinal cord slices from SNL versus sham rats (Fig. 8c, f). This suggested that the spinal cord from SNL rats were more sensitive to MV stimulation than those from sham rats. These results indicate that MVs shed from microglia generated a positive effect on neurotransmission in the development of neuropathic pain.
Microglia-derived microvesicles (MVs) enhance excitatory neurotransmission. Representative recording of spontaneous excitatory postsynaptic current (sEPSC) amplitude and frequency in the dorsal horn of the spinal cord of sham rats (a) or spinal nerve ligation (SNL) 7 day rats (d) before and during MV treatment. Summary of the effect of MVs on the amplitude of sEPSC in sham rats (b) or SNL 7-day rats (e). Summary of the effect of MVs on the frequency of sEPSC in sham rats (c) or SNL 7-day rats (f). Two-tailed paired Student’s t test was applied to analyze the differences, with significance assumed at P < 0.05. Error bars are SEM (n = 3 rats for each group)
Discussion
MVs are small shed vesicles (0.1–1 μm) which bud directly from the plasma membrane and are released into the extracellular environment upon cell activation [29]. However, the majority of studies have focused on exosomes rather than MVs shed from the cell surface of multiple cells originating from the brain. There is some evidence for the shedding of MVs from a neuronal origin [24, 30]. MVs that shed from the cell surface of microglia has attracted interest as microglia, the immune cells of the nervous system, provide the first line of defense during brain injury. Although a study by Gosselin showed that spinal astrocytic activation induced by spared nerve injury (SNI) increased the release of MVs containing EAAT-1 and EAAT-2 [31], the study did not investigate the relationship between MVs and neuropathic pain.
In our studies, we examined the population and morphology of MVs in the CSF and spinal cord of rats. We found that a fraction of large MVs (>100 nm) was pelleted from rat CSF by differential centrifugation (Fig. 1), agreeing with previous findings that microglia are able to produce MVs in vivo [24]. In addition, a statistically significant increased release of MVs was observed at day 7 following SNL. The results suggested that the number of MVs shed from the cell surface of microglia increased in a time-dependent manner following SNL and reached its peak at day 7 (Fig. 2). This phenomenon suggested that MVs might promote the onset of neuropathic pain induced by nerve injury. In previous studies, an early and transient increase in the number of microglia induced by peripheral nerve injury was also observed. Peripheral nerve injury increased the number of dorsal horn microglia by twofold to fourfold [32–34]. The increase in microglia was demonstrated to be associated with proliferation of pre-existing cells, as shown by proliferation markers [35–37]. The proliferation activity of microglia was shown to reach a peak around 2 days after nerve injury and then declined to basal levels [35]. The microglial cell cycle might be critical for the changes in MVs.
Neuropathic pain has been thought to be a chronic pain condition that occurs after nerve damage. Accumulating evidence from studies of animal models of neuropathic pain indicates that neuropathic pain involves aberrant excitability of the nervous system [38–40]. There is evidence that changes occur in neurons as well as spinal microglia, the immune cells of the CNS [3, 4, 41]. After peripheral nerve injured by SNL, microglia change phenotype to the activated form, following the altered expression of diverse molecules, evoking a series of cellular responses involving the secretion of proinflammatory factors. In order to further verify the role of MV shedding from microglia in neuropathic pain, behavioral tests for mechanical allodynia and thermal hyperalgesia were carried out. Following SNL, the sensitivities for mechanical allodynia and thermal hyperalgesia were significantly increased with withdrawal threshold and withdrawal latency times reducing in a time-dependent manner (Fig. 3a,b). In addition, the action of MVs was demonstrated to stimulate neuropathic pain by intrathecal administration of concentrated MVs (Fig. 3c, d). Moreover, intrathecal injection of FTY720 remarkedly alleviated neuropathic pain induced by SNL (Fig. 3e, f), and decreased the number of IBA1+ MVs in CSF (Fig. 4). These results strongly support our hypothesis that MVs from microglia have a key role in the response to neuropathic pain.
A correlation of microglia activation with the development of pain hypersensitivity has also been elucidated in other nerve injury models. Several microglia-specific molecules and signaling pathways have been identified in the development and maintenance of neuropathic pain. There is growing evidence that purines and their receptors are potential regulators of microglial function. It has also been reported that several purinoceptors are expressed in microglia, including P2X4, P2X7, P2Y6, and P2Y12 receptors [38]. Of these receptors, the P2X7 receptor is an ATP-gated ion channel, highly expressed in immune cells and microglia, where it modulates the release of inflammatory cytokines, such as IL-1β and IL-18 [29]. Studies have shown that the P2X7-p38 pathway mediates the release of MVs from microglia [11]. The P2X7-dependent blebbing is preceded by an alteration of the transbilayer lipid distribution and requires p38 MAP kinase activation [42, 43]. Our results indicated that, following SNL, P2X7 receptors and p38 were upregulated in a time-dependent manner (Fig. 5a, b). Furthermore, immunofluorescence studies of microglia after nerve injury confirmed that P2X7 receptors on microglia significantly increased following SNL (Fig. 5c). Intrathecal injection of P2X7 siRNA decreased P2X7 receptors expression in spinal cord and alleviated neuropathic pain after SNL (Fig. 6a–c). Interestingly, P2X7 siRNA significantly decreased the number of IBA1+ MVs, which was consistent with the behavioral results (Fig. 6). This implied that the P2X7-p38 signaling pathway was involved in the mechanism mediating the secretion of MVs from reactive microglial cells.
MVs have been proposed to mediate IL-1β release from microglia [11]. Upon stimulation of BzATP, N9 microglia cells were induced to release MVs (Fig. 7). The MVs obtained from the microglia cell medium were detected by ELISA, and it was found that the level of IL-1β increased significantly but that of TNF-α and IL-6 exhibited no significant alteration (Fig. 7c). The change in IL-1β was consistent with previous studies, in which IL-1β was packaged in the bleb of MVs and secreted through the activation of P2X7 receptors [11, 44]. However, the other two cytokines, TNF-α and IL-6, displayed no distinct changes. It was reported that various P2X7 receptor antagonists blocked the P2X7-dependent release of IL-6 but surprisingly had no effect on BzATP-induced release of TNF-α in cultured microglia [45]. In addition, the IL-1β levels in concentrated MVs, induced by BzATP, reduced promptly when N9 microglial cells were transfected with IL-1β shRNA lentivirus (Fig. 7d). Subsequently, mechanical allodynia and thermal hyperalgesia were investigated by intrathecal administration of MVs concentrated from N9 microglial cells transfected with lenti-shRNA-IL-1β. As shown in Fig. 7, intrathecal injection of MVs from lenti-shRNA-IL-1β transfected N9 microglia, which increased both the withdrawal threshold and the withdrawal latency (Fig. 7e, f). This indicated that the algogenic effect of microglia-derived MVs was mediated, at least partly, by IL-1β. This is in agreement with previous reports that IL-1β acts as a mediator of supraspinal circuits of pain in neuropathic conditions [46–48].
Excitatory postsynaptic currents (EPSCs) are closely related to pain [49]. Activation of low-threshold afferent fibers only generates typical AMPA receptor-mediated EPSCs; however, high-intensity single-shock stimulation of primary afferent sensory fibers produces fast, kainate receptor-mediated EPSCs in the superficial dorsal horn of the spinal cord, indicating that kainate receptors may be restricted to high-threshold nociceptive and thermoreceptive primary afferent fibers [49]. In our study, we also observed that the MVs derived from microglia enhanced the frequency and amplitude of sEPSC within 1 min after treatment with MVs (Fig. 8), as seen in a previous study [50]. However, mechanical and thermal hypersensitivities were induced by MVs from 1 h but not at 30 min after their intrathecal administration (Fig. 3c, d). This may be because painful behavior mediated by MVs was the result of the accumulation of EPSCs. Based on our results, MVs shed from the surface of microglia contributed to the release of the inflammatory cytokine IL-1β. The effect of IL-1β may be involved in the interaction of macrophages and neurons. However, the IL-1β may also elicit an increase in the metabolism of sphingolipids in neuronal cells, resulting in excitatory neurotransmitter release [29]. Our investigation has provided preliminary evidence that MVs involved in neuropathic pain also regulate the frequency and amplitude of sEPSC. This suggests another possible pathway by which microglia have an effect on neuronal activity.
In conclusion, we have demonstrated that MVs can be shed from the surface of microglia involved in neuropathic pain induced by SNL. The released MVs enhance the sensitivity of mechanical allodynia and thermal hyperalgesia induced by SNL, with a significant decrease in the withdrawal threshold and withdrawal latency time. In addition, the pivotal role of the P2X7-p38 pathway has been demonstrated in the bleb of MVs in the neuropathic model. Furthermore, released IL-1β packaged in the MVs has been illustrated to have a positive effect on neuropathic pain. Thus targeting the P2X7-p38 pathway, involved in the shedding of MVs equipped with IL-1β, may provide a novel therapeutic approach for the treatment of neuropathic pain.
Abbreviations
- ANOVA:
-
Analysis of variance
- BCA:
-
Bicinchoninic acid
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- ELISA:
-
Enzyme-linked immunosorbent assay
- EM:
-
Electron microscopy
- MVs:
-
Microvesicles
- PBS:
-
Phosphate buffered saline
- PFA:
-
Paraformaldehyde
- PWT:
-
Paw withdrawal threshold
- PWL:
-
Paw withdrawal latency
- SEM:
-
Standard error
- SNL:
-
Spinal nerve ligation
- sEPSC:
-
Spontaneous excitatory postsynaptic currents.
References
Gu N, Eyo UB, Murugan M et al (2016) Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav Immun 55:82–92
Baron R (2006) Mechanisms of disease: neuropathic pain—a clinical perspective. Nat Clin Pract Neurol 2(2):95–106
Scholz J, Woolf CJ (2007) The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10:1361–1368
Calvo M, Dawes JM, Bennett DL (2012) The role of the immune system in the generation of neuropathic pain. Lancet Neurol 11:629–642
Bele T, Fabbretti E (2015) P2X receptors, sensory neurons and pain. Curr Med Chem 22:845–850
Melli G, Keswani SC, Fischer A, Chen W, Höke A (2006) Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain 129:1330–1338
Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A (2003) Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol 54:287–296
Sommer C, Schäfers M (1998) Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res 784:154–162
Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA (2001) Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol 439:127–139
Budnik V, Ruiz-Cañada C, Wendler F (2016) Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci 17:160–172
Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C (2009) Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J 28:1043–1054
Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K (2002) VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain 99:111–120
Peng HY, Chen GD, Tung KC, Chien YW, Lai CY, Hsieh MC, Chiu CH, Lai CH, Lee SD, Lin TB (2009) Estrogen-dependent facilitation on spinal reflex potentiation involves the Cdk5/ERK1/2/NR2B cascade in anesthetized rats. Am J Physiol Endocrinol Metab 297:E416–E426
Marzesco AM, Janich P, Wilsch-Bräuninger M, Dubreuil V, Langenfeld K, Corbeil D, Huttner WB (2005) Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci 118:2849–2858
Zhou D, Chen ML, Zhang YQ, Zhao ZQ (2010) Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats. J Neurosci 30:8042–8047
MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A (2001) Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 15:825–835
Liu X, Liu H, Xu S, Tang Z, Xia W, Cheng Z, Li W, Jin Y (2016) Spinal translocator protein alleviates chronic neuropathic pain behavior and modulates spinal astrocyte-neuronal function in rats with L5 spinal nerve ligation model. Pain 157:103–116
Shefler I, Pasmanik-Chor M, Kidron D, Mekori YA, Hershko AY (2014) T cell-derived microvesicles induce mast cell production of IL-24: relevance to inflammatory skin diseases. J Allergy Clin Immunol 133:217–224.e1-3
Kim SH, Chung JM (1992) An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50:355–363
Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR (2006) A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci 26:3551–3560
Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW (2007) Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem 55:687–700
Ji RR, Suter MR (2007) p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain 3:33
Beggs S, Salter MW (2013) The known knowns of microglia-neuronal signalling in neuropathic pain. Neurosci Lett 557(Pt A):37–42
Verderio C, Muzio L, Turola E, Bergami A, Novellino L, Ruffini F, Riganti L, Corradini I, Francolini M, Garzetti L, Maiorino C, Servida F, Vercelli A, Rocca M, Dalla LD, Martinelli V, Comi G, Martino G, Matteoli M, Furlan R (2012) Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol 72:610–624
Bianco F, Pravettoni E, Colombo A, Schenk U, Möller T, Matteoli M, Verderio C (2005) Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol 174:7268–7277
Sommer C, Kress M (2004) Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 361:184–187
Vezzani A, Ravizza T, Balosso S, Aronica E (2008) Glia as a source of cytokines: implications for neuronal excitability and survival. Epilepsia 49(Suppl 2):24–32
Viviani B, Gardoni F, Marinovich M (2007) Cytokines and neuronal ion channels in health and disease. Int Rev Neurobiol 82:247–263
Turola E, Furlan R, Bianco F, Matteoli M, Verderio C (2012) Microglial microvesicle secretion and intercellular signaling. Front Physiol 3:149
Schiera G, Proia P, Alberti C, Mineo M, Savettieri G, Di LI (2007) Neurons produce FGF2 and VEGF and secrete them at least in part by shedding extracellular vesicles. J Cell Mol Med 11:1384–1394
Gosselin RD, Meylan P, Decosterd I (2013) Extracellular microvesicles from astrocytes contain functional glutamate transporters: regulation by protein kinase C and cell activation. Front Cell Neurosci 7:251
Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424:778–783
Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB (2009) CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain 13:263–272
Beggs S, Salter MW (2007) Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav Immun 21:624–633
Echeverry S, Shi XQ, Zhang J (2008) Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain 135:37–47
Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S (2007) Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci 27:12396–12406
Narita M, Yoshida T, Nakajima M, Narita M, Miyatake M, Takagi T, Yajima Y, Suzuki T (2006) Direct evidence for spinal cord microglia in the development of a neuropathic pain-like state in mice. J Neurochem 97:1337–1348
Inoue K, Tsuda M (2009) Microglia and neuropathic pain. Glia 57:1469–1479
Woolf CJ, Salter MW (2000) Neuronal plasticity: increasing the gain in pain. Science 288:1765–1769
Scholz J, Woolf CJ (2002) Can we conquer pain. Nat Neurosci 5(Suppl):1062–1067
Tsuda M, Inoue K, Salter MW (2005) Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci 28:101–107
Piccin A, Murphy WG, Smith OP (2007) Circulating microparticles: pathophysiology and clinical implications. Blood Rev 21:157–171
Pap E, Pállinger E, Pásztói M, Falus A (2009) Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res 58:1–8
Stock C, Schilling T, Schwab A, Eder C (2006) Lysophosphatidylcholine stimulates IL-1beta release from microglia via a P2X7 receptor-independent mechanism. J Immunol 177:8560–8568
Shieh CH, Heinrich A, Serchov T, van Calker D, Biber K (2014) P2X7-dependent, but differentially regulated release of IL-6, CCL2, and TNF-α in cultured mouse microglia. Glia 62:592–607
Apkarian AV, Lavarello S, Randolf A, Berra HH, Chialvo DR, Besedovsky HO, del RA (2006) Expression of IL-1beta in supraspinal brain regions in rats with neuropathic pain. Neurosci Lett 407:176–181
del RA, Apkarian AV, Martina M, Besedovsky HO (2012) Chronic neuropathic pain-like behavior and brain-borne IL-1β. Ann N Y Acad Sci 1262:101–107
Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, Iwakura Y, de Rivero Vaccari JP, Keane RW, Lacroix S (2011) Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: implications for neuropathic pain. J Neurosci 31:12533–12542
Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M (1999) Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature 397:161–164
Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, Novellino L, Clementi E, Giussani P, Viani P, Matteoli M, Verderio C (2012) Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J 31:1231–1240
Acknowledgments
This work was funded by the National Natural Science Foundation of China (81371253), the Shanghai Municipal Education Commission (14ZZ083), and the Shanghai Changzheng Hospital (2015CZQN01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Second Military Medical University and conformed to the American Physiological Society’s Guiding Principles in the Care and Use of Animals.
Conflict of interest
The authors declared no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Jian Li, Xiangnan Li, and Xin Jiang contributed equally to this work and should be considered as co-first authors.
Rights and permissions
About this article
Cite this article
Li, J., Li, X., Jiang, X. et al. Microvesicles shed from microglia activated by the P2X7-p38 pathway are involved in neuropathic pain induced by spinal nerve ligation in rats. Purinergic Signalling 13, 13–26 (2017). https://doi.org/10.1007/s11302-016-9537-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-016-9537-0