Abstract
The objective of this study was to better understand the underlying gene action in eucalyptus, under different plantation densities, for a different set of traits: growth, bark thickness, ecophysiological, and wood chemical property traits. We estimated the magnitude and relative proportion of the various genetic variance components using a eucalyptus genotype by spacing (G × S) interaction experiment. A clonally replicated progeny test including 888 clones belonging to 64 full-sib families of Eucalyptus urophylla × Eucalyptus grandis hybrid was used to estimate genetic parameters using genomic information to assess relationship matrix. Two densities (833 and 2500 trees/ha) were used representing contrasted environments in terms of individual tree available resource. Results showed that for height and circumference, additive-by-spacing (A × S) interaction variance increased from 18 to 55 months old, while dominance-by-spacing (D × S) interaction variance decreased. For bark thickness, specific leaf area, nitrogen, calcium, and magnesium, A × S interaction variance was preponderant. For wood chemical properties, except with Klason lignin, genetic additive effects strongly interacted with spacing compared to non-additive effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forest trees experience huge changes in spatial and temporal environmental conditions, sources of genotype-by-environment (G × E) interactions. The presence of G × E interaction in plant, analyzed in multi-environment trials, is expressed either as inconsistent responses of some genotypes relative to others leading to phenotypic rank change, phenotypic scale change, or both (Li et al. 2017, Issac-Renton et al. 2020). In the perspective of the global changes and the increasing need of agricultural land, the future environmental conditions of the forest plantations will become increasingly severe; plantations will occur in poor soil conditions facing seasonal climatic fluctuations with probably severe droughts. This new context will require forest tree varieties able to deal with both hard and changing conditions.
The exploitation of across-environment information is important and has been demonstrated in plant breeding (Gezan et al. 2017). Genetic variance, heritability, and breeding values may be upwardly biased when they are based on a single trial, because they are potentially confounded with G × E (Willman et al. 2022). The breeders are concerned with testing genotypes across a range of sites to obtain unbiased estimates of genetic effects and to measure the stability of genotypes across different environments (Russell et al. 2015). Improving knowledge and methods for selecting stable genotypes or identifying genotypes suited to specific environments by integrating G × E information remains one of the objectives of research in the tree breeding (Yang et al. 2018).
The most common approaches of analyzing G × E interaction are the variance component estimations, the type B correlations of genetic values in different environments, and the principal component analysis with combination of biplots and regression models (Gaspare et al. 2015; Oakey et al. 2016; Gezan et al. 2017; Ly et al. 2018). The emergence of the genotyping techniques has provided new opportunities to identify G × E patterns and mechanisms across a diversity of phenotypes and environments (Bajgain et al. 2019, 2020). This approach can benefit greatly from using multi-environment models as found by Burgueño et al. (2012). Dense molecular markers can be used to estimate the realized genetic similarity between individuals (Habier et al. 2007; VanRaden 2008), and such information can be incorporated into multi-environment models (de los Campos and Sorensen 2013; Bajgain et al. 2019, 2020; Gouveia et al. 2020).
The plantation spacing in eucalyptus influences the cost of forest production, the growth rate (Bouvet et al. 2003; Brito et al. 2021), and quality of the wood produced (Souza et al. 2020). A longstanding challenge in genetics has been to better understand the mechanisms behind G × E interaction (des Marais et al. 2013), i.e., to relate genetic underlining factors to the magnitude of G × E interactions (Burgueño et al. 2008; Fox et al. 2007; Mathews et al. 2007; Smith et al. 2015; Chen et al. 2017). This question is considered crucial in managing the genetic diversity for adaptation to the global environment change while maintaining genetic gain; thus, several questions arise: Is the magnitude of G × E varying according to different trait categories (growth, ecophysiological, chemical wood properties)? What are the relative parts of additive and non-additive genes effect in G × E interaction?
To address these questions, we used a clonally replicated progeny test using 64 full-sibs families and 888 clones of Eucalyptus urophylla × Eucalyptus grandis established with two plantation spacings, 3 m × 4 m and 2 m × 2 m, leading to the densities 833, the optimal production planting spacing of eucalyptus in the Congo, and 2500 trees ha−1, a planting density contrasting with the first one that can induce G × E interaction. E. urophylla × E. grandis is an artificial hybrid that provides most commercial clones planted in the Congo. This hybrid is derived from a genetic improvement program (Makouanzi et al. 2018), planted on large-scale plantations, and supplies charcoal and firewood to rural populations, but also pulpwood for the paper industry.
In this study, we used near-infrared spectroscopy because of its ability to screen large numbers of individual trees in tree breeding programs to provide breeders with data on a wider range of traits.
The aims of this study were to (i) assess for growth, ecophysiological, and wood quality traits to the magnitude of genotype-by-spacing (G × S) interaction: (ii) assess the part of additive and non-additive gene effects in the G × S interaction and their evolution with age; and (iii) derive recommendations for Eucalyptus hybrid breeding strategy taking into consideration G × E.
Material and methods
Study site and experimental design
The study site was located in the Atlantic coastal zone of the Republic of Congo, in central Africa (11°59′21″E 4°45′51″S). Climate was characterized by high mean annual air humidity and temperature (85% and 25 °C respectively). Annual precipitation averaged 1200 mm year−1.
The study was based on a clonally replicated progeny test including 888 clones belonging to 64 full-sibs families of Eucalyptus urophylla × Eucalyptus grandis hybrid (table S1 in supplementary material). Each family has an average of 14 clones. Six copies of each clone were obtained by vegetative propagation and planted according to two contrasted plantation densities (833 and 2500 trees ha−1) generating a G × S interaction experiment. For each density, the field experiment was a complete block design with three replications. Twenty-five trees represented each full-sib family; they were distributed in three blocks within each spacing.
Field measurements and molecular data
Four types of traits were considered: growth, bark thickness, ecophysiological, and wood chemical property traits. Concerning growth traits, total height (HT) and circumference at breast height (C) were measured at different ages, from 4 to 55 months, to realize the trend of genetic parameters with age. The tree bark thickness (BT) was measured in adulthood at 55 months at breast height using the bark thickness gauge. Concerning ecophysiological traits, the structural and functional leaf characters were studied as specific leaf area (SLA) and leaf density (LD). Sampling was carried out at two ages, 8 and 18 months, for practical reasons of ease of leaf collection from eucalyptus trees. At each age, ten mature (fully expanded) leaves per tree were sampled all-round considering the superior and inferior crown section. Therefore, five leaves were taken at each crown section. Each leaf was removed, and its thickness (LT) was measured immediately with a digital micrometer (IP65, Mitutoyo, Japan) at a point mid-way along the length of the leaf and mid-way between the median and the edge of the leaf. After, leaves were taken to the laboratory, and their area was measured after scan using MatLab software (version 8.5 R2015a). The leaves were dried at 65 °C to constant weight. The dry weight was used in conjunction with their measured area to calculate specific leaf area (SLA in m2 kg−1) by the following formula (Sefton et al. 2002):
where Si, Mi, and n are respectively the leaf area, the leaf dry mass, and the number of leaves harvested.
The leaf density (LD in kg m−3) was calculated considering SLA and LT (Sefton et al. 2002):
The leaf macronutrient contents (nitrogen (N), calcium (Ca), magnesium (Mg)) were predicted by near infrared spectroscopy (NIRS) in diffuse reflectance starting from spectra measured on the powder-reduced leaves sampled. The powder size was 500 µm. After the prediction of leaf nitrogen concentration, the following ratio was calculated:
NA (g m−2) is the leaf nitrogen concentration per unit leaf area, which is a good proxy of photosynthetic potential.
Wood chemical property traits including soluble lignin content (LAS), Klason lignin content (LK) α-cellulose content (CEL), holocellulose content (HOLO), syringil/guaiacyl ratio (SG), and extractives content (EXT) were also predicted, in adulthood at 55 months, by NIRS in diffuse reflectance from spectra measured on the sawdust samples collected at 55 months. Spectral acquisition data were processed using a Tango spectrometer and Opus software version 7.0. For the chemical propriety predictions, existing NIRS models of multispecies of eucalypts were used including samples from this study (Denis et al. 2013, Makouanzi et al. 2018, Ryckewaert et al. 2022).
The 888 clones were genotyped according to the GBS technology implemented by diversity arrays technology (DART). Among the 20,000 SNPs identified, 3303 were selected based on the repeatability (see more information about in Bouvet et al. 2016). The estimates of the genetic variance components and their interaction with the environment were obtained using matrices (Af, Am, and D) of genomic relationship between all pairs of individuals.
Data analysis
Eight models were compared using the Akaike information criterion (Akaike 1974) defined as AIC = − 2ln(R) + 2t, where ln(R) is the log-likelihood of the model, and t is the number of variance parameters. The model with the lowest AIC value presented the best data fit (table S2 in supplementary material). This model is based on the equation by Stuber and Cockerham (1966) but without epistasis effects the variance of which had a very high standard error in this considered population (see more information about in Bouvet et al. 2016).
The following model was developed for multisite analysis:
where y is the vector of response variable; µ is a the overall mean; X and Z are the design matrix connecting respectively the fixed and random effects to the data; s was a vector of fixed effects due to the spacing; b(s) was a vector of fixed effects due to the block within each spacing; plot(s) ~ N(0, σ2plot(d)Id) was a vector of random spatial environmental effects due to plot within each spacing, σ2plot(d) being the variance related to spatial effect within each spacing; am ~ N(0, σ2amAm) is a vector of additive random effect due to male σ2am being the male additive variance; am × s ~ N(0, σ2am×sAm) is a vector of random effect due to male additive by spacing interaction, σ2am×s being the male additive variance by spacing interaction; af ~ N(0, σ2afAf) is a vector of additive random effect due to female, σ2af being the female additive variance; af × s ~ N(0, σ2af×sAf) is a vector of random effect due to female additive by spacing interaction, σ2af×s being the female additive variance by spacing interaction; am × af ~ N(0, σ2am×afD) is a vector of random dominance effect, σ2am×af being the dominance variance; and am × af × s ~ N(0, σ2am×af×sD) is a vector of random dominance effect due to dominance by spacing interaction, σ2am×af ×s being the dominance variance by spacing interaction. Af, Am, and D were the matrices of genomic relationship between all pairs of individuals computed using VanRaden (2008) equations (see more information about in Bouvet et al. 2016).
The following model without spacing effect was derived for estimating genetic parameters within each spacing experiment (see results in supplementary material, table S3 to table S8):
where b is a vector of fixed effects due to the blocks; col ~ N(0, σ2colId) is a vector of random spatial environmental effects due to field design column, σ2col being the variance related to spatial effect, Id the identity matrix; r(b) ~ N(0, σ2r(b)Id) is a vector of random spatial environmental effects due to field design row within block, σ2r(b) being the variance related to spatial effect within block; plot ~ N(0, σ2plotId) is a vector of random spatial environmental effects due the plot, σ2plot being the variance related to spatial effect; am ~ N(0, σ2amAm) is a vector of random additive effects due to E. grandis males, Am being the coancestry coefficient matrix among males {φm} estimated from marker information, σ2am being the additive variance of hybrid population due to alleles from males crossed with females; af ~ N(0, σ2afAf) is a vector of random additive effects due to E. urophylla females, Af being the coancestry coefficient matrix among females {φf} estimated from or marker information, σ2af being the additive variance of hybrid population due to alleles from females crossed with males; am × af ~ N(0, σ2am×af D) is a vector of random dominance effects due to the cross between female and male, D is estimated from marker information, σ2am×af being the dominance variance of hybrid population due to alleles from males crossed with females, ε ~ N(0, σ2ε Id) vector of residual effects.
The Tukey’s HSD test was performed for mean pairwise comparisons.
Broad (H2) and narrow sense (h2) heritabilities, as well as the dominance ratio (d2), were calculated in each spacing design by the following formulas:
The variance component estimation based on the REML method and the BLUP calculations were done using the ASReml version 4.1 package (Gilmour et al. 2006) implemented in R software (R Development Core Team 2011). Approximate standard errors for linear functions of variance components were calculated using the pin.R function. This function, proposed by Ian White in 2013 (http://www.homepages.ed.ac.uk/iwhite/asreml/), applies the delta method for the estimation of approximate standard errors (Oehlert 1992).
Results
Impact of spacing on phenotypes
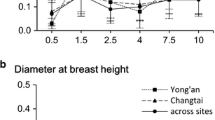
The density effect is significantly different from zero for all traits. Growth and tree bark thickness means were higher in low-spacing plantation compared to high spacing (Table 1). The coefficient of phenotypic variation (CV) of these traits increased in the constrained environment (high spacing). Concerning leaf traits, the decrease in plantation spacing resulted in an increase in the mean and the CV of SLA on the one hand and on the other hand the decrease of these both parameters for LD and NA, while these parameters remained stable for LT. The stability of mean and CV was observed to wood chemical properties. Leaf mineral content was higher in the high-density condition; however, they remained stable (Table 1).
Trends with age in variance components, variance ratios for growth traits, and bark thickness
The results at 55 months for HT and C showed that the variance components were, as expected, higher in large spacing due to the higher mean (Table 2). Variance ratios showed similar trends. For both traits at 55 months, the female additive variance was higher than the male additive variance at 833 trees/ha, when this pattern was inverse at 2500 trees/ha (Table 2). Moreover, the dominance variance was markedly expressed in 833 trees/ha when it was nearly null at 2500 trees/ha (Table 2).
Trends in variance components and variance ratio increased progressively with age at 833 trees/ha when, at 2500 trees/ha, some components, such as \({{\sigma }^{2}}_{af}\) and \({{\sigma }^{2}}_{am\times af}\), decreased to reach a quasi-null value (results shown in supplementary material, tables S3 and S4). A similar trend was observed for the heritabilities and the d2 ratio. They were higher at 833 trees/ha than at 2500 trees/ha. They increased and became stable at 833 trees/ha (tables S3 and S4).
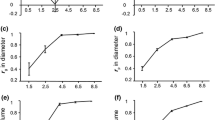
The trend in G × S interaction variances changed according to the genetic effects. The A × S interaction variance increased with age from 18 years old for HT and C (Figs. 1 and 2 showing proportions). Most of the A × S interaction variance was explained by the interaction between the female genetic effects and spacing (Af × S), the interaction variance between male genetic effects and density (Am × S) being null after 4 months. For both traits, the D × S interaction variances decreased with age to reach a null value at 32 months for height and circumference (Figs. 1 and 2).
Concerning bark thickness, all the G × S interaction variance was explained by the A × S interaction (Fig. 3). The proportion of variance explained by the female additive effects (83%) was much higher than the male additive effects (17%).
Trends with age in variance, variance ratio, and G × S variance for leafs traits
For leaf traits, female additive variance presented higher estimates than male additive variance in some cases (tables S5, S6 and S7 in Supplementary material). For the following variables, SLA, N, and LT, the additive variance of the hybrid was exclusively due to the female effect. Trend in genetic variances components changed according to the variables. For example, for SLA, the additive variance decreased with age in both spacings, while the dominance variance increased (table S5).
Heritabilities, as well as the proportion of dominance, varied according to the trait, the age, and the density (tables S5, S6, and S7). Clear trends in heritability and proportion of dominance were not observed. However, for all variables except LT, there was an increase in heritabilities with age at 833 trees/ha. Globally, heritability decreased with age at 2500 trees/ha (tables S5, S6, and S7).
No interaction between spacing plantation and dominance gene effects was detected for the following traits: SLA, N, Ca, and Mg content. Additive gene effects explained all the G × S variance (Table 3). For those traits, the A × S interaction variance decreased from 8 to 18 months. The magnitude of male and female variances by spacing varied according to trait.
At 8 months, for LT, the additive by spacing interaction variance was smaller than the dominance one. At 18 months, an opposite situation was observed, with absence of dominance gene effects to G × S interaction. In contrast to growth traits and bark thickness, most of the A × S interaction variance was explained by the male additive effects (Table 3).
The increase in A × S interaction of LD is due to the increase in the male variance by spacing interaction.
The A × S interaction variance increased with age for calcium leaf content, mainly explained by female additive effect. For this trait and NA, the D × S interaction variance was equal to zero. A marked decrease in Af × S variance interaction with age was observed compensated by a strong increase of the Am × S interaction variance (Table 3).
Trends with age in variance, variance ratio, and G × S variance for wood chemical traits
For the wood chemical property traits, the additive variance component was preponderant, whatever the plantation spacing. Except for EXT, the male additive variance was greater than the female additive variance. The genetic variance components decreased with plantation spacing. Similarly, heritability (h2) decreased with spacing varying among traits from 0.35 to 0.45 and from 0.17 to 0.32 at 833 and 2500 trees/ha, respectively (table S8 in Supplementary material).
Except for LK, the genetic additive by spacing interaction variance was higher than the genetic dominance by spacing interaction variance (Table 3). The contribution of male and female effects differed according to the traits. For HOLO, LAS, CEL, and S/G ratio, the contribution of the male additive effects to the G × S interaction variance was higher than the female additive effects. The opposite situation was observed For LK and EXT.
Discussion
Variance components according to parent species and traits
Our results report the predominance of the dominance variance for the growth traits in both spacing designs. This result was consistent with work of Resende et al. (2017) indicating that dominance had substantial contribution to genetic variance for growth in E. grandis × E. urophylla hybrids. Bouvet et al. (2009) with the same hybrid assumed that the higher magnitude of the dominance variance is due to the overdominance effects in hybrid populations, especially when planted in marginal areas as it is the case in the climatic conditions of the Congolese coastal zone. More generally our results are consistent with those obtained from previous studies which clearly corroborate this trend induced by the hybridization (de la Torre et al. 2014; Tan et al. 2018; Tanabe et al. 2019).
The female variability was found higher than the male variability. The more pronounced variability of E. urophylla compared to E. grandis was previously highlighted in the eucalyptus breeding program in the Congo (Bouvet and Vigneron 1996). This result can be explained by the larger genetic basis of the E. urophylla populations introduced in the Congo compared to the E. grandis and in consequence by the larger variability among parent trees (Bouvet and Vigneron 1996; Bouvet et al. 2009).
Concerning the ecophysiological traits, the evolution with age of the genetic variance components changed according to the traits. Additive variance was in most cases higher than dominance variance. For SLA, N, and LT, the hybrid additive variance was exclusively caused by the female origin. The dominance variance remained weak.
This study has shown that in eucalyptus hybrid populations of the Congo, the contribution of dominance effects to the total genetic variance was higher for growth traits than for ecophysiological traits. Such studies focusing on the partition of genetic variance of eucalyptus ecophysiological traits are few. In poplar hybrid population, Li et al. (2002) found that the genetic variance component of leaf traits in male parents was greater than that of female parents. Other studies (Drost et al. 2015; Bijarpasi et al. 2019; Ren et al. 2020) report that leaf morphology traits are under strong genetic control.
The wood chemical trait genetic variances showed the predominance of the additive component. This result is consistent with other experiments with eucalyptus (Rambolarimanana et al. 2018). Our study has shown that the wood chemical properties appeared to be more heritable than growth traits. This is the case in other eucalyptus studies (Hein et al. 2012; Mandrou et al. 2012; Makouanzi et al. 2018; Yang et al. 2018).
Density effect on variances components and variance ratios
Our results showed that the planting spacing affects the genetic parameter estimates. Competition leads to a strong decrease of some additive variance component leading to an almost null estimate for the female component (see Table 2 for σ2af). This would mean that competition affects differently, i.e., according to the parent species, the additive gene expression. This effect was also found by Stonecypher and McCullough (1981), St. Clair and Adams (1991) in Pseudotsuga menziesii, Williams et al. (1983) in Pinus taeda, Kusnandar et al. (1998) in Pinus pinaster, Bouvet et al. (2003) in E. urophylla × E. grandis, Volker et al. (2008) in E. nitens, and Tanabe et al. (2019) in Zelkova serrata. However, working on the progeny tests of Pseudotsuga menziesii var. menziesii, Campbell et al. (1986) found that the structure of genetic variance was not affected by plantation spacing. Patino-Valera and Kageyama (1995) also reported a similar result on E. saligna. These results lead to the assumption that competition due to spacing affects differently the additive gene expression in hybrid depending on the parental origin. This assumption needs to be considered with caution due to the small sample size of the parents and retested in future experiments.
Our results showed that heritabilities for growth traits increased with tree age for each density. Similar results were found for various species: Xie and Ying (1996) in Pinus contorta families, Osorio et al. (2001) in E. grandis clones, Ignacio-Sánchez et al. (2005) in E. urophylla clones. The heritability estimates showed that height was under a stronger genetic control than circumference, result that was observed in eucalyptus tree studies (Bouvet and Vigneron 1996; Botrel et al. 2010; Makouanzi et al. 2018). Our results report clearly that the increase in plantation spacing led to slight increase of wood chemical means, except HOLO and EXT, and to slight decrease of the wood chemical trait heritability. Brito et al. (2021) reported that lignin content of eucalypts was not influenced by the planting spacing. For other traits, evolution of heritability with plantation spacing depends on the tree age. Bouvet et al. (2003) reported for growth traits higher heritability in high density (2500 stems/ha) compared to low density (625 stems/ha) in a conventional progeny test of E. urophylla × E. grandis and E. urophylla × E. pellita. This trend is observed at certain ages in this present study.
Despite the lack of consistency of the results of trend in heritabilities with plant spacing for ecophysiological traits, specific leaf area (SLA), and leaf density (LD), it is found that these traits are under medium to strong genetic control. Our results corroborate those of Bijarpasi et al. (2019) obtained on Fagus orientalis and reporting a high broad-sense heritability of SLA (H2 = 0.88). Ren et al. (2020) reported a high narrow-sense heritability (h2 = 0.77) of the leaf area in Populus simonii × P. nigra hybrid.
Studies addressing the determinism and genetic variability of foliar mineral status and structure are still poorly documented. Our study provides data to feed this issue. Foliar mineral content traits were characterized by a low variability (see CV in Table 1) and a rather high heritability. The integration of these traits in a breeding program could facilitate the selection of the most efficient trees in terms of resource use (Bouvet et al. 2020).
Trends in G × E variance components
Our study showed that the magnitude and trends in G × E interaction change according to tree age, traits, and genetic variance components. Calleja-Rodriguez et al. (2019) and Ling et al. (2021) also detected strong patterns of G × E for growth traits respectively in Pinus sylvestris and Picea koraiensis contrasting environment.
The part of G × E interaction changed with the studied trait. G × E interaction was less pronounced with height than with circumference. This can be explained by the stronger genetic control for height and the weaker influence of the competition effects (Bouvet et al. 2003).
We found that the magnitude of the G × E interaction changed with age without any clear relationship between juvenile and mature stages. Rönneberg-Wästjung et al. (1994) found the gradual decrease of G × E interaction with the age on Salix viminalis and Gwaze et al. (2001) on Pinus taeda. Both results suggested that the G × E interaction observed in the juvenile phase was not conducive to predicting G × E interaction in adulthood.
This study reports that gene expression changes when competition is established according to the traits. For example, growth traits have a greater interaction with planting spacing than chemical wood properties (Isaac-Renton et al. 2020). Yang et al. (2018) also found strong site–site correlation for chemical wood properties and a small one for growth traits. This study also reports that the additive and non-additive effects interact in different ways depending on age and trait. Using the same analysis modeling, Gammal El-Dien et al. (2018) showed the superiority of additive effects in G × E interaction for two traits: height and wood density. Those both traits displayed small dominance variance × environment interaction (4.54 and 7.86% respectively). In contrast to this result, Berlin et al. (2019) have shown that G × E interactions are stronger for non-additive effects for height, and Lai et al. (2017) also found a significant family × site interaction for resin yield, growth traits, and morphologic traits.
Conclusion
This study showed that the additive effects provide the large part or all of G × S for growth, ecophysiological, and tree form traits, as well as wood chemical properties, except Klason lignin. The additive and non-additive genetic effects can be effectively better exploited with the availability of genome-wide markers. Further research is required to examine the non-additive genetic variances such as epistasis and its interaction with environment.
This study showed that the genotype-by-spacing (G × S) interaction plays an essential role in genotypic expression and must be considered in the evaluation and selection of superior genotypes. The importance of G × S with tree age was determined for the growth, ecophysiological, and wood chemical properties traits. The present results offer possibilities to optimize multi-trait selection of eucalyptus varieties in contrasted environment.
Supplementary information.
Data archiving statement
The datasets and haplotypes used during the current study are available from the corresponding author on reasonable request.
References
Akaike H (1974) A new look at the statistical model identification. Trans Autom Control 19:716–723
Bajgain P, Zhang X & Anderson JA (2019) Genome-wide association study of yield component traits in intermediate wheatgrass and implications in genomic selection and breeding. Genes Genomes Genetics: 9https://doi.org/10.1534/g3.119.400073
Bajgain P, Zhang X, Anderson JA (2020) Dominance and G×E interaction effects improve genomic prediction and genetic gain in intermediate wheatgrass (Thinopyrum intermedium). Plant Genome: e20012. https://doi.org/10.1002/tpg2.20012
Berlin M, Jansson G, Högberg K-A, Andreas Helmersson A (2019) Analysis of non –additive genetic effects in Norway spruce. Tree Genet Genomes 15:42. https://doi.org/10.1007/s11295-019-1350-9
Bijarpasi MM, Shahraji TR, Lahiji HS (2019) Genetic variability and heritability of some morphological and physiological traits in Fagus orientalis Lipsky along an elevation gradient in Hyrcanian forests. Folia Oecologica 46(1):45–53. https://doi.org/10.2478/foecol-2019-0007
Botrel MCG, Trugilho PF, Rosado SCdS, da Silva JRM (2010) Seleção de clones de Eucalyptus para biomassa florestal e qualidade da madeira. Scientia Forestalis, Picacicaba 38(86):237–245
Bouvet J-M, Makouanzi Ekomono CG, Brendel O, Laclau J-P, Bouillet J-P, Epron D (2020) Selecting for water use efficiency, wood chemical traits and biomass with genomic selection in a Eucalyptus breeding program. For Ecol Manage 465:118092. https://doi.org/10.1016/j.foreco.2020.118092
Bouvet J-M, Makouanzi G, Cros D and Vigneron Ph (2016) Modeling additive and non-additive effects in a hybrid population using genome-wide genotyping: prediction accuracy implications. Heredity: 1–12. https://doi.org/10.1038/hdy.2015.78
Bouvet J-M, Saya A, Vigneron Ph (2009) Trends in additive, dominance and environmental effects with age for growth traits in Eucalyptus hybrid populations. Euphytica 165:35–54
Bouvet J-M, Vigneron Ph (1996) Variance structure in Eucalyptus hybrid populations. Silvae Genetica 45(2–3):171–177
Bouvet J-M, Vigneron Ph, Gouma R, Saya A (2003) Trends in variances and heritabilities with age for growth traits in Eucalyptus spacing experiments. Silvae Genetica 52:121–133
Brito AS, Vidaurre GB, Oliveira JTdS, da Silva JGM, Oliveira RF, Júnior AFD, Arantes MDC, Moulin JC, Valin M, De Siqueira L, Zauza EAV (2021). Interaction between planting spacing and wood properties of Eucalyptus clones grown in short rotation. iForest 14 : 12–17. https://doi.org/10.3832/ifor3574-013
Burgueño J, Crossa J, Cornelius PL, Yang R-C (2008) Using factor analytic models for joining environments and genotypes without crossover genotype × environment interaction. Crop Sci. 48https://doi.org/10.2135/cropsci2007.11.0632
Burgueño J, de los Campos G, Weigel K and Crossa J, (2012) Genomic prediction of breeding values when modeling genotype × environment interaction using pedigree and dense molecular markers. Crop Sci 52:707–719
Calleja-Rodriguez A, Gull BA, Wu HX, Mullin TJ, Persson T (2019) Genotype-by-environment interactions and the dynamic relationship between tree vitality and height in northern Pinus sylvestris. Tree Genet Genomes 15:36. https://doi.org/10.1007/s11295-019-1343-8
Campbell RK, Echols RM, Stonecypher RW (1986) Genetic variances and interaction in 9-year-old Douglas fir grown at narrow spacings. Silvae Genetica 35:24–31
Chen Z-Q, Karlsson B, Wu HX (2017) Patterns of additive genotype-by-environment interaction in tree height of Norway spruce in southern and central Sweden. Tree Genet Genomes 13:25. https://doi.org/10.1007/s11295-017-1103-6
de La Torre AR, Wang T, Jaquish B, Aitken SN (2014) Adaptation and exogenous selection in a Picea glauca × Picea engelmannii hybrid zone: implications for forest management under climate change. New Phytol 201(2):687–699
de los Campos G, Sorensen DA, (2013) A commentary on Pitfalls of predicting complex traits from SNPs. Nat Rev Genet 14:894–894
Denis M, Favreau B, Ueno S, Camus-Kulandaivelu L, Chaix G, Gion JM, Nourrisier-Montou S, Polidori J, Bouvet J-M (2013) Genetic variation of wood chemical traits and association with underlying genes in Eucalyptus urophylla. Tree Genet Genomes 9:927–942
des Marais DL, Hernandez KM, Juenger TE (2013) Genotype-by environment interaction and plasticity: exploring genomic responses of plants to the abiotic environment. Annu Rev Ecol Evol Syst 44:5–29. https://doi.org/10.1146/annurev-ecolsys-110512-135806
Drost DR, Puranik S, Novaes E, Novaes CRDB, Dervinis C, Gailing O, Kirst M (2015) Genetical genomics of Populus leaf shape variation. BMC Plant Biol 15:1–10
Fox GP, Bowman J, Kelly A, Inkerman A, Poulsen D, Henry R (2007) Assessing for genetic and environmental effects on ruminant feed quality in barley (Hordeum vulgare). Euphytica 163:249–257. https://doi.org/10.1007/s10681-007-9638-5
Gammal El-Dien O, Ratcliffe B, Klápště J, Porth I, Chen C, El-Kassaby YA (2018) Multienvironment genomic variance decomposition analysis of open-pollinated Interior spruce (Picea glauca × engelmannii). Mol Breeding 38:26. https://doi.org/10.1007/s11032-018-0784-3
Gaspare WJ, Ivkovic´ M, Liepe K, Hamann A, Low CB, (2015) Drivers of genotype by environment interaction in radiata pine as indicated by multivariate regression trees. Forest Ecology Management 353:21–29
Gezan SA, de Carvalho MP, Sherrill J (2017) Statistical methods to explore genotype-by-environment interaction for loblolly pine clonal trials. Tree Genet Genomes 13:1–11. https://doi.org/10.1007/s11295-016-1081-0
Gilmour AR, Gogel BJ, Cullis BR and Thompson R (2006) ASREML user guide, Release 2.0. Hemel Hempstead, UK: VSN International, 342p.
Gouveia BT, Rios EF, Nunes JAR, Gezan SA, Munoz PR, Kenworthy KE, Unruh JB, Miller GL, Milla-Lewis SR, Schwartz BM, Raymer PL, Chandra A, WherleyBG WuY, Martin D, Moss JQ (2020) Genotype-by-environment interaction for turfgrass quality in bermudagrass across the southeastern United States. Crop Sci 60:3328–3343. https://doi.org/10.1002/csc2.20260
Gwaze DP, Wolliams JA, Kanowski PJ, Bridgwater FE (2001) Interactions of genotype with site for height and stem straightness in Pinus taeda in Zimbabwe. Silvae Genetica 50(3–4):135–140
Habier D, Fernando R, Dekkers J (2007) The impact of genetic relationship information on genome-assisted breeding values. Genetics 177(4):2389–2397
Hein PRG, Bouvet J-M, Mandrou E, Vigneron P, Clair B, Chaix G (2012) Age trends of microfibril angle inheritance and their genetic and environmental correlations with growth, density and chemical properties in Eucalyptus urophylla ST Blake wood. Ann For Sci 69(6):681–691. https://doi.org/10.1007/s13595-012-0186-3
Ignacio-Sánchez E, Vargas-Hernandez JJ, Lopez-Upton JY, Borja-de la Rosa A (2005) Parámetros genéticos del crecimiento y densidad de madera en edades juveniles de Eucalyptus urophylla s.t. Blake Agrociencia 39:469–479
Isaac-Renton M, Stoehr MU, Statland CB, Woods J (2020) Tree breeding and silviculture: Douglas-fir volume gains with minimal wood quality loss under variable planting densities. For Ecol Manage 465:118094. https://doi.org/10.1016/j.foreco.2020.118094
Kusnandar D, Galwer NW, Hertzler GL, Butcher TB (1998) Age trends in variances and heritabilities for diameter and height in maritime pine (Pinus pinaster Ait) in Western Australia. Silvae Genetica 47(2–3):136–141
Lai M, Dong L, Yi M, Sun S, Zhang Y, Fu L, Xu Z, Lei L, Leng C, Zhang L (2017) Genetic variation, heritability and genotype × environment interactions of resin yield, growth traits and morphologic traits for Pinus elliottii at Three Progeny Trials. Forests 8:409. https://doi.org/10.3390/f8110409
Li JH, Zhang QW, Su XH, Gao JS, Lu BM (2002) Multi-level genetic variation in leaf and growth of hybrid system between Populus deltoides and P. cathayana. Forest Res 15:76–82
Li Y, Suontama M, Burdon RD, Dungey HS (2017) Genotype by environment interaction in the forest tree breeding: review methodology and perspectives on research and application. Tree Genet Genomes 13:60. https://doi.org/10.1007/s11295-017-1144-x
Ling J, Xiao Y, Hu J, Wang F, Ouyang F, Wang J, Weng Y, Zhang H (2021) Genotype by environment interaction analysis of growth of Picea koraiensis families at diferent sites using BLUP-GGE. New Forest 52:113–127. https://doi.org/10.1007/s11056-020-09785-3
Ly D, Huet S, Gauffreteau A, Rincent R, Touzy G, Mini A, Jannink J-L, Cormier F, Paux E, Lafarge S, Le Gouis J, Charmet G (2018) Whole-genome prediction of reaction norms to environmental stress in bread wheat (Triticum aestivum L.) by genomic random regression. Field Crop Res 216:32–41
Makouanzi G, Chaix G, Nourissier S, Vigneron Ph (2018) Genetic variability of growth and wood chemical properties in a clonal population of Eucalyptus urophylla × Eucalyptus grandis in the Congo. South Forests: a Journal of Forest Science 80(2):151–158. https://doi.org/10.2989/20702620.2017.1298015
Mandrou E, Hein PRG, Villar E, Vigneron P, Plomion C, Gion J-M (2012) A candidate gene for lignin composition in Eucalyptus: cinnamoyl-CoA reductase (CCR). Tree Genet Genomes 8:353–364. https://doi.org/10.1007/s11295-011-0446-7
Mathews KL, Chapman SC, Trethowan R, PfeifferW GM, Crossa J, Payne T, DeLacy I, Fox PN, Cooper M (2007) Global adaptation patterns of Australian and CIMMYT spring bread wheat. Theor Appl Genet 115:819–835. https://doi.org/10.1007/s00122-007-0611-4
Oakey H, Cullis B, Thompson R, Comadran J, Halpin C & Robbie Waugh R (2016) Genomic selection in multi-environment crop trials. G3: https://doi.org/10.1534/g3.116.027524
Oehlert GW (1992) A note on the delta method. Am Statistician 46(1):27–29
Osorio LF, White TL, Huber DAH (2001) Age trends of heritabilities and genotype by-environment interactions for growth traits and wood density from clonal trials of Eucalyptus grandis HILL ex MAIDEN. Silvae Genetica 50(1):30–37
Patinot-Valera F & Kageyama PY (1995) Parametros geneticos y espaciamieto en progenie de Eucalyptus saligna Smith. Research Paper IPEF, Piracicaba (48/49): 61–76.
R Development Core Team (2011) R: a language and environment for statistical computing. Austria, R Foundation for Statistical Computing, Vienna
Rambolarimanana T, Ramamonjisoa L, Verhaegen D, Leong Pock Tsy J-M, Jacquin L, Cao-Hamadou T-V, Makouanzi G, Bouvet J-M (2018) Performance of multi-trait genomic selection for Eucalyptus robusta breeding program. Tree Genetics & Genomes 14:71. https://doi.org/10.1007/s11295-018-1286-5
Ren J, Ji X, Wang C, Hu J, Nervo G and Li J (2020) Variation and genetic parameters of leaf morphological traits of eight families from Populus simonii × P. nigra. Forests 11 (1319): 1–17. https://doi.org/10.3390/f11121319
Resende RT, Resende MDV, Silva FF, Azevedo CF, Takahashi EK, Silva-Junior OB, Grattapaglia D, D, (2017) Assessing the expected response to genomic selection of individuals and families in Eucalyptus breeding with an additive-dominant model. Heredity 1:11
Rönneberg-Wästjung AC, Gullberg U, Nilsson C (1994) Genetic parameters of growth characters in Salix viminalis grown in Sweden. Can J for Res 24:1060–1969
Russell DJF, Wanless S, Collingham YC, Huntley B, Hamer KC (2015) Predicting future European breeding distributions of British seabird species under climate change and unlimited/no dispersal scenarios. Diversity 7:342–359
Ryckewaert P, Chaix G, Héran D, Zgouz A, Bendoula R (2002) Evaluation of a combination of NIR micro-spectrometers to predict chemical properties of surgarcane forage using a multi-block approach. Biosys Eng 217:18–25. https://doi.org/10.1016/j.biosystemseng.2022.02.019
Sefton CA, Montagu K, Atwell BJ, Conroy JP (2002) Anatomical variation in juvenile eucalypt leaves accounts for differences in specific leaf area and CO2 assimilation rates. Austrian Journal of Botany 50:301–310
Smith AB, Ganesalingam A, Kuchel H, Cullis BR (2015) Factor analytic mixed models for the provision of grower information from national crop variety testing programs. Theor Appl Genet 128:55–72. https://doi.org/10.1007/s00122-014-2412-x
St Clair JB, Adams WT (1991) Relative family performance and variance structure of open pollinated Douglas-fir seedlings grown in three competitive environments. Theor Appl Genet 81:541–550
Stonecypher R, McCullough R (1981) Evaluation of full-sib families of Douglas fir in a nelder design. Proc South For Tree Improv Conf 16:56–76
Stuber CW, Cockerham CC (1966) Gene effects and variances in hybrid populations. Genetics 54:1279–1286
Souza CO, Silva JGM, Arantes MDC, Vidaurre GB, Dias Júnior AF, Oliveira MP (2020) Pyrolysis of Anadenanthera peregrina wood grown in different spacings from a forest plantation in Brazil aiming at the energy production. Environ Dev Sustain 22:5153–5168. https://doi.org/10.1007/s10668-019-00418-0
Tan B, Grattapaglia D, Wue HX, Ingvarsson PK (2018) Genomic relationships reveal significant dominance effects for growth in hybrid Eucalyptus. Plant Sci 267:84–93
Tanabe J, Endo R, Kuroda S, Ishiguri F, Narisawa T, Takashima Y (2019) Variance components and parent-offspring correlations of growth traits vary among the initial planting spacings in Zelkova serrata. Silvae Genetica 68:45–50
VanRaden PM (2008) Efficient methods to compute genomic predictions. J Dairy Sci 91(11):4414–4423
Volker PW, Potts BM, Borralho NMG (2008) Genetic parameters of intra- and inter-specific hybrids of Eucalyptus globulus and E. nitens. Tree Genet Genomes 4:445–460
Williams CG, Bridgwater FE, Lambeth CC (1983) Performance of single versus mixed family plantation blocks of loblolly pine. Proc South Forest Tree Improv Conf 17:56–76
Willman MR, Bushakra JM, Bassil N, Finn CE, Dossett M, Perkins-Veazie P, Bradish CM, Fernandez GE, Weber CA, Sheerens JC, Dunlap L, Fresnedo-Ramirez J (2022) Analysis of a multi- environment trial for black raspberry (Rubus occidentalis L.) quality traits. Genes (Basel) 13(3): 418. https://doi.org/10.3390/genes13030418
Xie CY, Ying CC (1996) Heritabilities, age-age correlations and early selection in lodgepole pine (Pinus contorta ssp. Latifolia). Sivae Genetica 45:101–107
Yang H, Weng Q, Li F, Zhou C, Li M, Chen S, Ji H & Gan S (2018) Genotypic variation and genotype-by-environment interactions in growth and wood properties in a cloned Eucalyptus urophylla × E. tereticornis family in Southern China. For Sci. 64 3 225 232
Acknowledgements
We are grateful to Philippe Vigneron for his contribution to the designed trial. We thank Crisley Ulrich Mayinguidi and all the technical team of CRDPI for maintaining the trial and for data collection. We would like also to thank Anne Clément-Vidal and Armelle Soutiras for their assistance with NIRS measurement, and Gilles Chaix for his assistance with the NIRS analysis. The authors thank the reviewers for their comments, which helped us improve the manuscript.
Author information
Authors and Affiliations
Contributions
CGME designed the trial. CGME and JMB supervised the collection of the field data. Near-infrared spectroscopy analyses were performed by CGME. CGME and JMB performed the statistical analyses and wrote the manuscript with the help of TR.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by V. Decroocq.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Makouanzi Ekomono, C.G., Rambolarimanana, T. & Bouvet, JM. Preponderance of additive and non-additive variances for growth, ecophysiological and wood traits in Eucalyptus hybrid genotype-by-spacing interaction. Tree Genetics & Genomes 18, 32 (2022). https://doi.org/10.1007/s11295-022-01563-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-022-01563-w