Abstract
The ease of vegetative propagation by hardwood cuttings is a critical trait for consideration by breeders of woody perennial rootstocks. This is especially so for Pyrus, because most Pyrus rootstock are known to be difficult to propagate. This report presents progress on the identification of loci controlling rooting of hardwood cuttings in European pear (Pyrus communis L.). Quantitative trait loci (QTLs) controlling the development of adventitious roots on hardwood cuttings were identified in both parents of a mapping population developed by crossing “Old Home” and “Louise Bonne de Jersey,” with the goal of investigating the genetic control of several rootstock related traits, which would be useful for rootstock breeding. A QTL for root development was identified on chromosome 7, co-located in both parents and exhibiting male and female additive and dominance effects. These results will assist in developing genetic markers that can be utilized by rootstock breeders for marker-assisted selection for this complex trait.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For commercial pipfruit production, clonal scions are grafted onto rootstocks that beneficially influence scion traits such as vigor and precocity (Itai 2007; Bell and Zimmermann 1990; Lockard and Schneider 1981), as well as fruit yield and quality (Jayawickrama et al. 1991; Webster 2003, 2004; Hancock et al. 2008), and to avoid the necessity of vegetative propagation of scion cultivars. For practical commercial reasons, rootstocks must be easily propagated. Identification of the genetic factors involved in rooting ability will improve the efficiency and horticultural outcomes of rootstock breeding in pear.
Vegetative propagation by rooting of cuttings is widely applied in horticultural crops to multiply established cultivars because it ensures uniformity in growth and cropping (Hartmann et al. 2011), unlike seedling propagation. Vegetative propagation is made possible by the ability of plants to produce adventitious roots (ARs), which are defined as roots growing from plant parts other than root apical meristems. Some plants produce ARs naturally from preformed roots; however in others, ARs must be induced by methods such as wounding (Hartmann et al. 2011). This results in the activation of previously differentiated cells to meristematic cells, followed by the induction phase for root initiation, which leads to cell division, the formation and elongation of root primordia, and finally root elongation and the growth of vascular connections (Hartmann et al. 2011; De Klerk et al. 1999; De Klerk et al. 1995). Endogenous auxin plays a major role in AR growth, activating the differentiated cells and stimulating the primordium growth (Legue et al. 2014). Application of exogenous auxin in the form of indole-3-butyric acid (IBA) is a widely used practice to improve the propagation success in many difficult-to-root cultivars (Fett-Neto et al. 2001; Diaz-Sala et al. 1996; De Klerk et al. 1999; Westwood and Chestnut 1963). Callus (a mass of parenchyma cells) often appears concurrently with ARs. Even though rooting and callus have been thought to be independent processes (Hartmann et al. 2011), it has been shown that ARs can develop out of callus tissue in species that are difficult to propagate vegetatively (Cameron and Thomson 1969; Girouard 1967). Other factors that influence the ability of a shoot cutting to develop roots include the physiological state and treatment of the mother plant (age, health, management), the state of the propagation material (length, thickness, number of nodes, etiolation, position along a shoot), the treatment of the cutting (storage, auxin treatment), and the propagation conditions (humidity, temperature, soil, light) (Webster 1995; Hartmann et al. 2011). Optimizing the conditions for vegetative propagation can enhance the AR formation of some species. However, many fruit tree rootstocks, with otherwise useful traits, exhibit difficulty in development of ARs, even after wounding and when treated under optimal conditions (Webster 1998) and are consequently not used commercially.
While a number of genetic loci related to the control of root development have been identified in Arabidopsis thaliana (Sorin et al. 2005; Sorin et al. 2006; Willemsen et al. 1998; Okushima et al. 2007), little is known about genes involved in AR formation in woody plants and there have been no previous genetic studies in pear. In apple, a gene up-regulated during the induction phase of AR formation, adventitious rooting related oxygenase (ARRO-1), has been identified using gene expression analysis (Butler and Gallagher 1999). Subsequent studies by Smolka et al. (2009) and Li et al. (2012) suggested an involvement of ARRO-1 in auxin regulation during AR induction. However, further investigation is needed to confirm these results and to identify other genes involved in the control of rooting of cuttings from woody plants. An alternative route to the identification of genetic loci controlling complex traits is provided by quantitative trait locus (QTL) analysis. QTLs controlling ease of vegetative propagation have been identified for oak (Scotti-Saintagne et al. 2005), poplar (Han et al. 1994), eucalyptus (Marques et al. 1999; Marques et al. 2005), pine (Shepherd et al. 2006), and citrus (Siviero et al. 2003). In the genus Malus, which is closely related to Pyrus, a large effect QTL controlling the development of ARs was identified on chromosome 17 of Malus prunifolia (Moriya et al. 2015).
In the present study on Pyrus communis, we have analyzed the genetic control of root development in dormant hardwood cuttings, which is a frequently used, low-cost, and fast method of vegetative propagation of woody perennials (Webster 1995, 1998), including apple rootstocks. Our study utilized an F1 segregating population derived from a cross between “Old Home” and “Louise Bonne de Jersey” (OH × LBJ) created for studying a range of traits relevant to rootstock breeding (Knäbel et al. 2015), as well as for the development of new rootstocks. Old Home is an important parent for Pyrus rootstock breeding as it is fire blight-, wooly pear aphid-, and phytophthora-resistant as well as being tolerant to pear decline, high pH soils, and drought (Jacob 2002; Wertheim 2002). In addition, Knäbel et al. (2015) found a QTL on LG5 of Old Home controlling scion vigor and precocity. The detection of QTLs influencing the ease of vegetative propagation derived from Old Home would, therefore, be of great interest for commercial rootstock breeding.
Materials and methods
Plant material and rooting assessment
A mapping population comprised of a rootstock population of 421 F1 individuals from an OH × LBJ cross, established as a breeding population for pear rootstocks and for genetic analysis of vigor and precocity control of such rootstocks (Knäbel et al. 2015). Progeny were grown in a New Zealand orchard as described by Knäbel et al. (2015) and grafted onto Pyrus calleryana Decne. seedling rootstocks in 2009. Such practice enabled mother trees to become established for the provision of both cutting material and leaves for DNA extraction, with cuttings being collected when trees were dormant during June/July. The timing of the first cutting collection coincided with the completion of the third year of growth after grafting and was repeated over four successive years. The mother plants were grown to a height of 3 m so as to avoid collection of juvenile wood (assessed by the presence of thorns). The top 500 mm of each tree was pollared to encourage the production of numerous medium vigor, non-juvenile, proleptic, vegetative shoots. Lateral shoots were removed from the primary axis of the tree preserving as many of the proximal most non-extended internodes as possible. Ten 1-year-old shoots, with a base diameter of 7–12 mm, from each individual tree were stored in plastic bags at 4 °C for 0–4 days before preparation of cuttings.
Cuttings, approximately 200 mm in length, were taken from the basal-most portion of each shoot, cutting above a node at the top and just below a node at the bottom. A scalpel was used to wound the base of each cutting by making two 10-mm long shallow slices on opposite sides of the cutting (taking care to keep the bud intact) (Van Huyssteen 1992), to expose a greater area of cambium, thus maximizing the surface area for absorption of rooting hormone. The basal 20 mm of each cutting was dipped into a 1:1 solution of water:10 g/L indolebutyric acid (LIBA™ 10000, Zelam Ltd., New Plymouth, New Zealand) for 30 s to stimulate AR formation. Following this, Greenseal™ ULTRA, a pruning paint, was applied to the cut apical surface of each cutting to prevent desiccation. The prepared cuttings were placed approximately 60 mm deep in a bed containing 150 mm of sand in a polythene tunnel house, arranged by individual with all ten cuttings placed together. The bed was constantly heated to provide cuttings with basal heat of 19 °C with an electric heating cable system and regularly irrigated by hand. Air temperature inside the tunnel house was cooled with a fogging system.
After 6 weeks, the cuttings were evaluated for rooting ability. The absence or presence of callus and roots was recorded for all ten cuttings from each genotype (Fig. 1). The percentage of cuttings that developed roots, callus, or both was calculated for each F1 individual and used for data analysis.
Assessment of ability to form adventitious roots in a pear segregating population. Examples of successful rooting (left), callus development (middle), and no callus or roots (right) in cuttings from three individuals in the “Old Home”דLouise Bonne de Jersey” (OH × LBJ) segregating population, 6 weeks after cuttings had been treated with 5 g/L indolebutyric acid and bases placed in a sand bed held at 25 °C
Data analysis
R 3.3.2 (R Core Development Team 2013) and lme4 package version 1.1-7 (Bates et al. 2012) were used to account for the effect of the genotype and the year on vegetative propagation traits in a linear mixed effects analysis. Best linear unbiased prediction (BLUP) and best linear unbiased estimator (BLUE) analysis were employed to account for the year effect, taking year as a random and fixed effect, respectively. The genotype was added into both models as a further random effect. Residual plots were visually examined to reveal any obvious deviations from homoscedasticity or normality. Likelihood ratio tests of the full model with the year/genotype effect against the model without the year/genotype effect were used to obtain P values.
Linkage map construction and QTL analysis
A genetic map was constructed for both parents separately using a 9K Illumina SNP array, as previously reported in Montanari et al. (2013) and Knäbel et al. (2015). The female (OH) map consists of 399 markers located on 17 linkage groups (LGs) with a total distance of 913 cM. The male (LBJ) map comprises 446 markers spanning 1044 cM on 16 LGs.
QTL detection for the BLUP transformed data was carried out using the Kruskal-Wallis test, interval mapping (IM), multiple QTL mapping (MQM), and permutation test (1000 permutations) with MapQTL5 (van Ooijen 2004). ANOVA was used to calculate the percentage of the phenotypic variance explained by each QTL and the phenotypic variance explaint by all QTLs was calculated using multiple-regression analysis in R 3.3.2 (R Core Development Team 2013).
For non-transformed data, only the Kruskal-Wallis test was used for QTL detection
Minitab® version 16.1.1 was used for plotting phenotypic data and QTL results. Where QTLs were derived from the same loci in both parents, allelic effects were calculated using phenotypic means (μ) corresponding to the phenotypic classes ac, ad, bc, and bd, from the cross ab × cd. Female and male additivities were calculated with a formula developed by Guitton et al. (2012): A f = [(μ ac + μ ad) − (μ bc + μ bd)] / 4, male additivity with A m = [(μ ac + μ bc) − (μ ad + μ bd)] / 4 and dominance with D = [(μ ac + μ bd) − (μ ad + μ bc)] / 4.
Results
Phenotypic variation
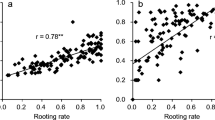
Of the 421 F1 individuals of the OH × LBJ population grafted onto P. calleryana, 18 individuals died and were not included in any analyses. In year 1, 55 individuals did not have suitable shoots for propagation and the remaining 348 individuals were phenotyped, of which 148 formed ARs. In year 2, 365 individuals were assessed for their propagation ability, with 107 showing success in rooting. In year 3, a proportion of the trees were removed from the orchard because of European canker (Neonectria ditissima) and only 299 individuals were screened, of which 210 exhibited the ability to root. In year 4, 229 of 303 individuals showed the ability to root. The variability of root development within the replicates of each individual was used as an indicator of the ease of AR development for each individual by evaluating the percentage of rooted cuttings. Fifty of the progeny had a consistent rooting response (rooting or no rooting) over all 4 years. Consistent results were observed for 84 individuals between year 1 and year 2, 127 trees between year 1 and year 3, 124 between year 1 and year 4, 128 between year 2 and year 4, 208 between year 3 and year 4, and 106 trees between year 2 and year 3. There was strong variability in the data for the rooting success of individual genotypes among the 4 years of assessment (Fig. 2A) and it was notable that in year 3 and year 4 rooting success was substantially higher than in years 1 and 2, with year 2 exhibiting the lowest percentage of individuals developing ARs. The percentage of individuals rooting in the total population was not normally distributed (Fig. 3), and square root transformation did not improve normality (data not presented).
The year effect was accounted for by considering it either as a random or fixed effect when calculating BLUE and BLUP values. As the values obtained by BLUE and BLUP analysis were almost identical (P value = 1.0; correlation coefficient = 1.0), only the predicted BLUP data were used for further analysis.
The likelihood ratio test showed that both the year (x 2(1) = 83.34, P value = 0.0010) and the genotype affected rooting (x 2(1) = 108.16, P value = 0.0001). While the genotype and the year had a very similar variability (standard deviation (Std.Dev.): genotype = 10.4; year = 9.1), there was a strong variability (residual = 18.5) that was not due to genotype or year. The genotype effect on the growth of roots and/or callus (x 2(1) = 148.9, P value = 0.0001) was less than the year effect for root and/or callus growth (x 2(1) = 438.4, P value = 0.0001), and the variability of the year (Std.Dev. = 20.9) was stronger than the variability of the genotype (Std.Dev. = 12.2). However, the residual variability (Std.Dev. = 28.9) was higher than both genotype and year variability taken together. Taking the year and genotype into account as random effects increased normality in the data and these predicted data were used for QTL analysis (Fig. 2B).
QTL detection
QTLs associated with the percentage of cuttings that developed ARs (BLUP transformed data) were detected on LGs 7 (P value 0.0005), 15 (P value 0.001), and 16 (P value 0.005) of LBJ, and LGs 7 (P value 0.0001), 8 (P value 0.005), 10 (P value 0.0001), and 11 (P value 0.0001) of OH (Table 1; Fig. 4). An additional QTL controlling root and/or callus development was identified on LG4 (P value 0.0005) of LBJ. Logarithm of the odds (LOD) scores for markers at QTL peaks ranged from 5.29 to 2.74 and the explained from 8.4 to 4.4% of the variance. The highest LOD score and percentage variance explained was for the QTL on LG7 of OH. The LG7 QTLs associated with rooting in OH and LBJ were located in homologous regions at the bottom of the LG (Fig. 4). The total phenotypic variance explained by all QTLs associated with rooting was 22.01% for OH and 13.11% for LBJ.
Quantitative trait loci (QTLs) controlling adventitious rooting and alignment of linkage groups from “Louise Bonne de Jersey” (LBJ) and “Old Home” (OH) pear. The blue and red bars represent QTLs detected for rooting success (blue) and root or callus development (red), respectively. The markers are named using the NCBI dbSNP accessions (Montanari et al. 2013) and their positions are indicated in centimorgan (Color figure online)
Results for allelotype vs phenotype comparisons for all QTLs, for each year of assessment, are shown in Fig. 5. The AB genotype of the marker with the highest LOD score for the OH LG7 QTL (ss527788659) had a higher tendency to root in all years when compared with the AA genotype (Fig. 5A). For the LBJ LG7 QTL, the AA genotype of the marker with the highest LOD score (ss527789100) was associated with a higher tendency to root in all years of assessment than the AB genotype (Fig. 5B).
When the remaining QTLs from OH were considered, the heterozygous genotype (AB) of marker ss527787780 on the OH map (LG8, OH) was found to be associated with a higher percentage of cuttings that developed roots in all years than the homozygous AA genotype. Marker ss527787893 (LG10, OH) exhibited a heterozygous genotype (AB) linked to a slightly higher rooting rate than the AA genotype for all years of assessment. A higher percentage of rooted cuttings was associated with the heterozygous AB genotype of the marker ss52788422 (LG11, OH) in years 1, 3, and 4 than for the homozygous AA genotype. For LBJ, the homozygous (AA) genotype of marker ss52779764 (LG15, LBJ) was associated with a higher percentage of rooting in years 1, 3, and 4. Marker ss527788450 (LG16) exhibited a homozygous genotype (AA) associated with a higher percentage of root development in years 3 and 4 only, when rooting was most successful. Finally, the LBJ marker found to be associated with increased root and callus formation (ss475880644 (LG4, LBJ)) exhibited the homozygous (AA) genotype in all years. It should be noted that allelic associations for all QTLs identified were consistent over 3 years and sometimes 4.
The allelic effect for the LG7 marker from both parents was calculated and shown to exhibit male and female additive and dominance effects for all years and for the BLUP data (Table 2). These additive and dominance effects are visualized in Fig. 6, where the bc (AB/AA) genotype exhibited the highest propensity to root across years.
Bar charts of the allelotypes of the LG7 QTL markers controlling the percentage of rooted cuttings per individual within the crossing population derived from “Old Home” (OH) and “Louise Bonne de Jersey” (LBJ) pear, respectively. Bars show the mean and the error bars show the standard error of the mean
To confirm the QTLs detected with the transformed data, which takes the year effect into account, the Kruskal-Wallis test was conducted using the non-transformed data from all 4 years separately. The results are presented in Table 3. The Kruskal-Wallis significance ranged from 8 to 24 and the P value from 0.005 to 0.0001. For LBJ, the LG7 (years 1 and 3) and LG15 (years 2 and 3) QTLs controlling the percentage of rooted cuttings were also detected with data of 2 of the 4 years of assessment. Both QTLs were also found to control rooting and/or callus development in year 2. The QTL on LG16 controlling the percentage of rooted cuttings was confirmed with the data of year 2. The QTL on LG4, controlling rooting and/or callus development of the transformed data, was also present with rooting and/or callus development in year 2 and the percentage of rooted cuttings with year 3 and year 4 data.
For OH, the QTLs on LG7 (years 1 and 4) and LG10 (years 2 and 3) controlling the percentage of rooted cuttings in the transformed data were confirmed with the non-transformed data in 2 years, while QTLs on LG8 (year 3) and LG11 (year 4) confirm the results of the transformed data in 1 year of assessment. The transformed data did not reveal any QTL controlling the percentage of rooted and/or callused cuttings. With the Kruskal-Wallis test on the non-transformed data, QTLs, each unique to only 1 year, were found to control the percentage of rooted and/or callused cuttings on LGs 7 (year 4), 8 (year 2), 10 (year 3), and 11 (year 4).
Discussion
Genetic loci associated with the control of adventitious rooting of P. communis hardwood cuttings have been identified for the first time. Our study utilized QTL analysis in a segregating population developed from a cross between two pear cultivars and phenotyped in replicate for 4 years, enabling us to demonstrate that the loci controlling rooting were largely stable across years. The loci detected at the bottom of LG7 of OH and LBJ were demonstrated to overlap (Fig. 4) and explained 8.4 and 5.5% of the variance in ability to develop roots for the two parents, respectively (Table 1). We found that the additive and dominance effects of these QTLs resulted in a higher percentage of rooted cuttings when both loci were present with calculation of the allelic effect demonstrating both a male and a female additive effect and a dominance effect (Table 3). The results shown in Fig. 6 raise the possibility that a double recessive gene with recessive alleles inherited from both parents had a strong impact on the ability of hardwood pear cuttings to root.
Genetic control of this trait has been shown to be complex, as six additional small effect loci contributing to the ability to root were identified (LG15 and 16 of OH and LGs 8, 10, and 11 of LBJ), with variance explained ranging from 4.4 to 7.1%. This confirms previous findings in other species that oligogenic architecture controls adventitious rooting in some woody perennial plants. In oak, ten QTLs explained 4.4–13.8% of variance (Scotti-Saintagne et al. 2005), while in eucalyptus seven QTLs explained 3.1–6.4% of variance (Thumma et al. 2010). However, in some other species, vegetative propagation is controlled by major QTLs that explain a high percentage of the phenotypic variance. For instance, a single large effect QTL on LG17 controlled 57.1% of genetic variance in adventitious rooting in a species within the genus Malus (Moriya et al. 2015), which is closely related to Pyrus. This M. prunifolia locus is not syntenic to any of the QTLs we identified in P. communis. Han et al. (1994) reported a major QTL controlling adventitious rooting in poplar, with 51.1% genetic variance explained; while in Citrus, two QTLs controlling rooting traits (15.8 and 20.9% variance explained) were identified by Siviero et al. (2003). Similar results were obtained for Eucalyptus, where one QTL explained 66% of variance (Shepherd et al. 2006) and Pinus where a single QTL explained 40% of variance (Shepherd et al. 2006). It is noteworthy that the latter five studies that identified major effect QTLs utilized interspecific crosses of parents selected for high and low rooting ability, while our study and several others that identified small effect QTLs employed populations developed from intraspecific crosses between parents of similar rooting ability. Both Shepherd et al. (2008) and Moriya et al. (2015) suggested that a species effect was responsible for strong effect QTLs associated with rooting of hardwood cuttings from interspecific populations.
We observed a strong year effect on rooting phenotype, with rooting being particularly low in year 2 and high in years 3 and 4 and the variability of the year was stronger than the genotypic variation. Use of a BLUP analysis enabled us to account for year effect and increase normality in the data used for QTL analysis.
We used Kruskal-Wallis analysis on the non-transformed data to validate our QTLs found with the BLUP transformed data. All QTLs derived from both parents with the transformed data were also present in at least 1, mostly 2 years when using non-transformed data. The fact that they are not present in all years of assessment reflects the strong variability among years and confirms the need of multiple year phenotyping and data transformation taking the year effect into account.
A single QTL associated with callus and root development was detected, on LG4 of LBJ using the transformed data while the transformed data revealed QTLs controlling callus and root development on LG7, LG15, and LG16 of LBJ, indicating that the formation of callus and roots might be under the same genetic control. However, as the QTLs are associated with root as well as callus development, we cannot conclude that callus growth is genetically controlled in pear, although it has been shown to be under genetic control in other plant species, including wheat (Jia et al. 2007) and barley (Mano et al. 1996; Mano and Komatsuda 2002).
The strong variability in the data that was observed in our mixed linear analysis, and was not due to either genotype or year, suggests that additional factors affect the development of ARs. This may reflect the ongoing development of the mother plant’s physiological condition. Typically, severe pruning and subsequent “vegetative” growth responses are important to conferring better rootability of cuttings (Hartmann et al. 2011). The increase in response in year 3 and year 4 could be related to the mother plant condition becoming improved, providing better adapted cuttings for rooting.
A variation in adventitious rooting ability within species and individuals, which is influenced by the quality of the cuttings and environmental conditions, has been reported before. For example, a study in oak identified a significant year effect on AR when the experiment was repeated for 4 years under the same conditions (Scotti-Saintagne et al. 2005). To avoid the detection of unstable QTLs linked to rootability, we used a large mapping population of over 300 individuals and repeated our phenotyping over 4 years.
Our results underline the complexity of control of this trait and a strong genotype by environment (G × E) effect, with variation of the ability to root exhibited not only between individual genotypes but also for the same individual in different years and even among replicates. The relatively low proportion of individuals exhibiting the same phentoypes in all 4 years (17%) demonstrates the sensitivity of rooting in pear cuttings to environmental conditions. Even though the assessment was repeated under similar conditions across 4 years and using ten replicates per genotype, small changes in the time of harvest, condition of the mother plants, or handling of the samples may have influenced the results. Our findings suggest that there are improvements that might be made in future propagation assessments of pear. The cuttings should be randomized to account for variability in the planting bed, such as differences in temperature. Replicated populations in different regions with different environmental conditions could help to account for the G × E effect and may be used to calculate repeatability of the experiment within the same growing season and eliminate the year effect.
In addition, our choice of parents was not ideal for an experiment depending on segregation of rootability in a population. While pear has generally a low rooting ability compared to other species, both OH and LBJ have been reported as relatively easy to propagate by hardwood cuttings when compared to other cultivars (Hartmann and Hansen 1958; Webster 1998; Diack et al. 2016). It is best practice to utilize parents that differ for the trait of interest when developing a segregating population for genetic studies (Collard et al. 2005). Therefore, other cultivars that have been reported to be easier to propagate by cuttings, such as “Bartlett,” “Worden Sekel,” and “Beurré Bosc,” (Diack et al. 2016) may improve the strength of the QTL when crossed with a cultivar that has a low ability to produce AR. However, in this case the cross had been designed to detect QTLs for dwarfing pear rootstocks (Knäbel et al. 2015) and was available for this project. While our OH × LBJ population was not ideal for the identification of large effect QTLs controlling adventitious rooting, it has enabled the identification of small effect QTLs for rooting ability derived from both parents (OH and LBJ) and the determination of additive and dominance effects for a QTL on LG7 across all years and for the normalized data. The analysis of the most significant QTL markers with respect to phenotypic data from all 4 years demonstrates that the same genotype is linked to the rooting phenotype in at least 3 of the 4 years of assessment.
Conclusion
This study reports the first QTLs associated with vegetative propagation ability in pear using hardwood cuttings. It was conducted within the framework of a breeding program using a population constructed for the development of new rootstock cultivars for commercial production, as well as for genetic studies. Our results are the first step towards understanding the genetic control of this complex trait. We predict that MAS for AR formation, along with other important rootstock traits, such as disease resistance (Montanari et al. 2016) and vigor control (Knäbel et al. 2015), will substantially shorten the time required for breeding new pear rootstocks in the future. Old Home, already used as a parent in pear rootstock breeding, may also transfer the trait “ease of propagation” to its progeny.
Future research on the genetics of vegetative propagation should focus on identifying the candidate genes controlling adventitious rooting in the pear genome (Chagné et al. 2014), and on developing an understanding of the molecular and physiological mechanisms involved.
References
Bates D, Maechler M, Bolker B (2012) lme4: Linear mixed-effects models using S4 classes. R package version 1.1-7
Bell R, Zimmermann R (1990) Combining ability analysis of juvenile period in pear. Hort Sci 25(11):1425–1427
Butler E, Gallagher T (1999) Isolation and characterization of a cDNA encoding a novel 2-oxoacid-dependent dioxygenase which is up-regulated during adventitious root formation in apple (Malus domestica ‘Jork 9’) stem discs. J Exp Bot 50(333):551–552
Cameron R, Thomson G (1969) The vegetative propagation of Pinus radiata: root initiation in cuttings. Bot Gaz 130(4):242–251
Chagné D, Crowhurst R, Pindo M, Thrimawithana A, Deng C, Ireland H, Fiers M, Dzierzon H, Cestaro A, Fontana P, Bianco L, Lu A, Storey R, Knäbel M, Saeed M, Montanari S, Kim Y, Nicolini D, Larger S, Stefani E, Allan A, Bowen J, Harvey I, Johnston J, Malnoy M, Troggio M, Perchepied L, Sawyer G, Wiedow C, Won K, Viola R, Hellens R, Brewer L, Bus V, Schaffer R, Gardiner S, Velasco R (2014) The draft genome sequence of European pear (Pyrus communis L. ‘Bartlett’). PLoS One 9(4):e92644. doi:10.1371/journal.pone.0092644
Collard B, Jahufer M, Brouwer J, Pang E (2005) An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts. Euphytica 142(1):169–196
De Klerk G-J, Keppel M, Brugge J, Meekes H (1995) Timing of the phases in adventitous root formation in apple microcuttings. J Exp Bot 46(8):965–972
De Klerk G-J, von der Krieken W, De Jong J (1999) The formation of adventitious roots: new concepts, new posibilities. In Vitro Cell Dev Biol 35:189–199
Diack R, Friend A, Knäbel M, Palmer J, Tustin D (2016) Assessing ease of propagation of European pear cultivars and a Pyrus communis rootstock segregating population. XI Orchard Systems 2016, Bologna, Italy
Diaz-Sala C, Hutchison K, Goldfarb B, Greenwood M (1996) Maturation-related loss in rooting competence by loblolly pine stem cuttings: the role of auxin transport, metabolism and tissue sensitivity. Physiol Plant 97:481–490
Fett-Neto A, Fett J, Vieira Goulart L, Pasquali G, Termignoni R, Ferreira A (2001) Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiol 21:457–464
Girouard R (1967) Initiation and development of adventitious roots in stem cuttings of Hedera helix. Can J Bot 45:289–302
Guitton B, Kelner J, Velasco R, Gardiner S, Chagné D, Costes E (2012) Genetic control of biennial bearing in apple. J Exp Bot 63(1):131–149. doi:10.1093/jxb/err261
Han K-H, Bradshaw H, Gordon M (1994) Adventitious root and shoot regeneration in vitro is under major gene control in an F2, family of hybrid poplar (Populus trichocarpa × P. deltozdes). For Genet 1:139–146
Hancock J, Luby J, Brown S, Lobos G (2008) Pears. In: Hancock J (ed) Temperate fruit crop breeding. Springer Science and Business, USA, pp 1–38
Hartmann H, Hansen C (1958) Rooting pear, plum rootstocks. Calif Agric 12(10):14–15
Hartmann H, Kester D, Davies FJ, Geneve R (2011) Hartmann & Kester’s plant propagation: principles and practices. Prentice Hall, New Jersey
Itai A (2007) Pear. In: Kole C (ed) Genome mapping and molecular breeding in plants, fruits and nuts, vol 4. Springer, Heidelberg
Jacob H (2002) New pear rootstocks from Geisenheim, Germany. Acta Hortic 596:337–344
Jayawickrama K, Jett J, Mckeand S (1991) Rootstock effects in grafted conifers: a review. New For 5:157–173
Jia H, Yi D, Yu J, Xue S, Xiang Y, Zhang C, Zhang Z, Zhang L, Ma Z (2007) Mapping QTLs for tissue culture response of mature wheat embryos. Mol Cells 23(3):323–330
Knäbel M, Friend A, Palmer J, Diack R, Wiedow C, Alspach P, Deng C, Gardiner S, Tustin D, Schaffer R, Foster T, Chagné D (2015) Genetic control of pear rootstock-induced dwarfing and precocity is linked to a chromosomal region syntenic to the apple Dw1 loci. BMC Plant Biol 15(1):230
Legue V, Rigal A, Bhalerao R (2014) Adventitious root formation in tree species: involvement of transcription factors. Physiol Plant 151(2):192–198. doi:10.1111/ppl.12197
Li T-Y, Wang Y, Zhang X-Z, Han Z-H (2012) Isolation and characterization of ARRO-1 genes from apple rootstocks in response to auxin treatment. Plant Mol Biol Report 30(6):1408–1414. doi:10.1007/s11105-012-0457-z
Lockard R, Schneider G (1981) Stock and scion growth relationships and the dwarfing mechanism in apple. Hortic Rev 3:315–375
Mano Y, Komatsuda T (2002) Identification of QTLs controlling tissue-culture traits in barley (Hordeum vulgare L.) Theor Appl Genet 105(5):708–715. doi:10.1007/s00122-002-0992-3
Mano Y, Takahashi H, Sato K, Takeda K (1996) Mapping genes for callus growth and shoot regenetartion in barley (Hordeum vulgare L.) Breed Sci 46:137–142
Marques C, Carocha V, Pereira de Sá A, Oliveira M, Pires A, Sederoff R, Borralho N (2005) Verification of QTL linked markers for propagation traits in Eucalyptus. Tree Genet Genomes 1(3):103–108. doi:10.1007/s11295-005-0013-1
Marques C, Vasquez-Kool J, Carocha V, Ferreira J, O’Malley D, Liu B, Sederoff R (1999) Genetic dissection of vegetative propagation traits in Eucalyptus tereticornis and E. globulus. Theor Appl Genet 99:936–946
Montanari S, Perchepied L, Renault D, Frijters L, Velasco R, Horner M, Gardiner SE, Chagné D, Bus VGM, Durel C-E, Malnoy M (2016) A QTL detected in an interspecific pear population confers stable fire blight resistance across different environments and genetic backgrounds. Mol Breed 36(4):47. doi:10.1007/s11032-016-0473-z
Montanari S, Saeed M, Knäbel M, Kim Y, Troggio M, Malnoy M, Velasco R, Fontana P, Won K, Durel C-E, Perchepied L, Schaffer R, Wiedow C, Bus V, Brewer L, Gardiner S, Crowhurst R, Chagné D (2013) Identification of Pyrus single nucleotide polymorphisms (SNPs) and evaluation for genetic mapping in European pear and inter-specific Pyrus hybrids. PLoS One 8(10):e77022. doi:10.1371/journal.pone.0077022
Moriya S, Iwanami H, Haji T, Okada K, Yamada M, Yamamoto T, Abe K (2015) Identification and genetic characterization of a quantitative trait locus for adventitious rooting from apple hardwood cuttings. Tree Genet Genomes 11(3). doi:10.1007/s11295-015-0883-9
Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19(1):118–130. doi:10.1105/tpc.106.047761
R Core Development Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Scotti-Saintagne C, Bertocchi E, Barreneche T, Kremer A, Plomion C (2005) Quantitative trait loci mapping for vegetative propagation in pedunculate oak. Ann For Sci 62(4):369–374. doi:10.1051/forest:2005032
Shepherd M, Huang S, Eggler P, Cross M, Dale G, Dieters M, Henry R (2006) Congruence in QTL for adventitious rooting in Pinus elliottii × Pinus caribaea hybrids resolves between and within-species effects. Mol Breed 18(1):11–28. doi:10.1007/s11032-006-9006-5
Shepherd M, Kasem S, Lee D, Henry R (2008) Mapping species differences for adventitious rooting in a Corymbia torelliana × Corymbia citriodora subspecies variegata hybrid. Tree Genet Genomes 4(4):715–725. doi:10.1007/s11295-008-0145-1
Siviero A, Cristofani M, Machado M (2003) QTL mapping associated with rooting stem cuttings from Citrus sunki vs. Poncirus trifoliata hybrids. Crop Breed Appl Biotechnol 3:83–88
Smolka A, Welander M, Olsson P, Holefors A, Zhu L-H (2009) Involvement of the ARRO-1 gene in adventitious root formation in apple. Plant Sci 177(6):710–715. doi:10.1016/j.plantsci.2009.09.009
Sorin C, Bussell J, Camus I, Ljung K, Kowalczyk M, Geiss G, McKhann H, Garcion C, Vaucheret H, Sandberg G, Bellini C (2005) Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell 17(5):1343–1359. doi:10.1105/tpc.105.031625
Sorin C, Negroni L, Balliau T, Corti H, Jacquemot M-P, Davanture M, Sandberg G, Zivy M, Bellini C (2006) Proteomic analysis of different mutant genotypes of Arabidopsis led to the identification of 11 proteins correlating with adventitious root development. Plant Physiol 140(1):349–364. doi:10.1104/pp.105.067868
Thumma B, Baltunis B, Bell J, Emebiri L, Moran G, Southerton S (2010) Quantitative trait locus (QTL) analysis of growth and vegetative propagation traits in Eucalyptus nitens full-sib families. Tree Genet Genomes 6(6):877–889. doi:10.1007/s11295-010-0298-6
Van Huyssteen P (1992) Guidelines for successful propagation of pear rootstocks from hardwood cuttings. FFTRI Information Bull 617:1–6
van Ooijen J (2004) MapQTL® 5, software for the mapping of quantitative trait loci in experimental populations. Kyazma, B.V, Wageningen
Webster A (1995) Temperate fruit tree rootstock propagation. N Z J Crop Hortic Sci 23(4):355–372. doi:10.1080/01140671.1995.9513912
Webster A (1998) A brief review of pear rootstock development. Acta Hortic 475:135–141
Webster A (2003) Breeding and selection of apple and pear rootstocks. Acta Hort 622:499–512
Webster A (2004) Vigour mechanisms in dwarfing rootstocks for temperate fruit trees. Acta Hortic 658:29–41
Wertheim S (2002) Rootstocks for European pear: a review. Acta Hortic 596:299–309
Westwood MN, Chestnut NE (1963) How to propagate Old Home pear rootstocks. Oregon Ornamental and Nursery Digest 7(1):3–4
Willemsen V, Wolkenfelt H, de Vrieze G, Weisbeek P, Scheres B (1998) The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development (Cambridge, England) 125(3):521–531
Acknowledgements
This work was funded by the New Zealand Ministry of Business Innovation and Employment grant “Pipfruit: a Juicy Future” [contract number: 27744] and “Future Orchard Planting Systems” [contract number: 30467]. We want to thank Lester Brewer for making the cross, Sara Montanari for providing the reference map, Shona Seymour and Angela Shirtliff for help with phenotyping, and Robert Lamberts for providing the photographs of the cuttings.
Data archiving statement
The genetic map data used in this study can be found in Montanari et al. (2013).
SNP accessions are available in dbSNP (http://www.ncbi.nlm.nih.gov/projects/SNP/) under accessions ss527787751 to ss527789916. Phenotypic data is available on request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Dardick
Rights and permissions
About this article
Cite this article
Knäbel, M., Friend, A.P., Palmer, J.W. et al. Quantitative trait loci controlling vegetative propagation traits mapped in European pear (Pyrus communis L.). Tree Genetics & Genomes 13, 55 (2017). https://doi.org/10.1007/s11295-017-1141-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-017-1141-0