Abstract
The non-expressor of pathogenesis-related genes 1 (NPR1) plays essential roles in the salicylic acid (SA) signal pathway and in systemic acquired resistance (SAR) responses. Although a genome-wide analysis of NPR1 gene family has been conducted in some plant species, little is known about these genes in apple (Malus spp.). In this study, eight NPR1 homologs were identified within the apple genome and 12 different transcripts were cloned by the reverse transcription-polymerase chain reaction. Based on these sequences, the gene structures and sequence alignment of the apple NPR1 homologs were analyzed. Phylogenetic analysis showed that apple NPR1 homologs could be classified into three groups as in Arabidopsis. Expression analysis demonstrated that NPR1 homologs showed different expression patterns in various tissues of apple. Under the induction of SA and MeJA, the transcription levels of some members were upregulated in leaves. Meantime, some NPR1 genes also showed significantly different expression levels between “Pacific Rose” and Malus baccata after inoculation with Marssonina coronaria. With the most similarity on amino acid sequence and expression pattern, MdNPR1 may function as a key regulator in SAR-like AtNPR1. These results suggested that the NPR1 genes may play an important role in plant immune responses, and their alternative splicing may contribute to disease resistance. Our study provides essential information about the NPR1 homologs in apple and contributes to the understanding of NPR1 homologs functions in other plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants are protected from a wide range of pathogens by specialized immune responses, including the hypersensitive resistance (HR), systemic acquired resistance (SAR), and induced systemic resistance (ISR). SAR is an important part of plant defenses, providing long-term, broad-spectrum resistance that is effective against a wide variety of fungal, viral, and bacterial pathogens in tissues distal to the initial site of infection (An and Mou 2011; Friedrich et al. 1996; Robert-Seilaniantz et al. 2011; Sticher et al. 1997). The accumulation of SA or its functional analogs, such as 2,6-dichloroisonicotinic acid, benzothiadiazole S-methyl ester, can activate the SAR response and pathogenesis-related (PR) gene expression (Metraux et al. 1991; Ward et al. 1991; Lawton et al. 1995). ISR, normally induced by Jasmonic acid (JA) and ethylene, always confers resistance to insects and some necrotrophic pathogens (Durrant and Dong 2004; Gfeller et al. 2006; Kessler and Baldwin 2002).
The NPR1 gene was discovered in Arabidopsis mutants with compromised resistance against pathogens (Cao et al. 1994). The AtNPR1 mutants lost or had reduced transcriptional expression of some PRs, including PR-1 and lost the capacity for SAR, suggesting that the AtNPR1 gene could prevent secondary infection of virulent pathogens. NPR1 is also involved in JA/ET-dependent ISR, which is triggered by certain Pseudomonas fluorescens strains. NPR1 has been shown to modulate cross talk between SA- and JA-dependent defense pathways (Dong 2004; Iavicoli et al. 2003; Pieterse and Van Loon 2004). The AtNPR1 gene encodes a protein that contains two domains, a BTB/BOZ domain and an ankyrin repeat domain (Cao et al. 1997; Ryals et al. 1997). These domains mediate protein-protein interactions and are present in a wide range of proteins with diverse functions (Aravind and Koonin 1999). NPR1 shares structural features with the transcriptional regulator IκB, which mediates innate immune responses in animals (Baldwin 1996). A recent review on NPR1 described its mechanisms of action and indicated that NPR1 functions as a bona fide SA receptor (Pajerowska-Mukhtar et al. 2013). During the induction of SAR, Arabidopsis NPR1 protein oligomers in the cytoplasm depolymerize and release active monomeric NPR1, which is translocated into the cell nucleus via a C-terminal bipartite nuclear localization signal (NLS) (Kinkema et al. 2000; Somssich 2003). In the nucleus, NPR1 protein interacts with the TGA (TGACG motif-binding factor) family of basic leucine zipper (bZIP) transcription factors (Despres et al. 2000; Fan and Dong 2002; Kim and Delaney 2002; Subramaniam et al. 2001; Zhang et al. 1999; Zhou et al. 2000). These bZIP transcription factors are involved in SAR and SA-dependent activation of PR genes (Niggeweg et al. 2000). In the absence of pathogen challenge, NPR1 is continuously cleared from the nucleus by the proteasome, preventing activation of SAR. SA accumulation promotes NPR1 phosphorylation, which resulted in its recruitment by a Cullin3-based ubiquitin ligase. Turnover of phosphorylated NPR1 is required for full induction of target PR genes and the establishment of SAR in plant defense (Mukhtar et al. 2009; Spoel et al. 2009).
The Arabidopsis NPR1 gene homologs have been extensively studied. Besides AtNPR1, five other homologs have been found in Arabidopsis. Phylogenetic analysis of these six homologs revealed three distinct groups (Hepworth et al. 2005; Zhang et al. 2006). AtNPR1 and AtNPR2 are in the first group. The function of AtNPR2 has not been reported, but it is supposed to participate in the SAR and SA signaling pathway as AtNPR1. Proteins encoded by the second group, which includes AtNPR3 and AtNPR4, are thought to directly bind to SA with different binding affinity (NPR3, K d = 981 nM; NPR4, K d = 46 nM) and play a role in mediating the degradation of NPR1 and decreasing or keeping its function in basal defense (Fu et al. 2012). The members in the third group, encoding Arabidopsis NPR5 and NPR6, also known as BLADE-ON-PETIOLE 1 (BOP1) and BOP2, are associated with the development of lateral organs (Hepworth et al. 2005). A recent report found that the Arabidopsis BOP proteins are responsible for abiotic resistance induced by methyl jasmonate (MeJA) in the wild type (Canet et al. 2012). With the development of genome sequencing, NPR1 gene homologs have been identified from the whole-genome sequences of many plants, such as Vitis vinifera (Le Henanff et al. 2009), Populus trichocarpa (Shao et al. 2013), Carica papaya (Peraza-Echeverria et al. 2012), and Persea americana (Backer et al. 2015). Their functions in disease resistance have been widely reported. Overexpression of the AtNPR1 gene has been shown to result in enhanced bacterial and fungal resistance in many plants. Furthermore, overexpression of an endogenous NPR1 orthologue (named MdNPR3 in our research) in apple increased resistance to fire blight and two other major fungal pathogens (Venturia inaequalis and Gymnosporangium juniperi-virginianae) of apple (Malnoy et al. 2007). A study on two banana NPR1-like genes, MNPR1A and MNPR1B, found that they could induce PR-1 gene expression and restore pathogen resistance as in the Arabidopsis npr1 mutant (Yocgo et al. 2012). As master regulatory genes in defense responses, the functions of NPR1 genes have been reported in many studies, but the full complement of gene members and their functions in disease resistance in apple have not been described.
Alternative splicing (AS) is a process that generates two or more different splice variants from the same pre-mRNA molecule using different splice sites. These splice sites are recognized by a large RNA-protein complex, the spliceosome. Four main types of such splicing are known: exon skipping, alternative 5′ and alternative 3′ splice sites, and intron retention. AS not only contributes to proteome diversity but also generates truncated proteins that are potentially regulatory or detrimental to the cell. It also plays a role in gene expression by regulating transcript levels through the production of isoforms that are degraded by the nonsense-mediated decay pathway (Mastrangelo et al. 2012; Syed et al. 2012). In Citrus clementina, a defense-related gene, acidic chitinase II, can produce an additional transcript containing a premature termination codon after the first 135 amino acid that is inducible by pathogen infection and MeJA treatment (Del Carratore et al. 2011).
Apple (Malus domestica Borkh.) is one of the most important fruit crops cultivated in China and worldwide. Fungal diseases, such as Marssonina coronaria, Glomerella cingulata, and Valsa canker, pose a great threat to the growth and production of apple trees. In this study, eight NPR1 gene homologs were identified from the apple genome and their full complementary DNA (cDNA) sequences were cloned; furthermore, AS of some NPR1 homologs were found. The tissue- and organ-specific expression of the NPR1 homologs and their differential expression in response to M. coronaria, SA, and MeJA induction between resistant and susceptible cultivars were investigated. Some specific members and AS variants of NPR1 were only found in the resistant cultivar. This implies that these AS forms may be related to disease resistance responses in apple. Our findings provide an invaluable resource for further study and functional characterization of the NPR1 homologs in apple.
Materials and methods
Identification and annotation of apple NPR1 homologs
Six Arabidopsis NPR1 homolog protein sequences including AtNPR1–6 were downloaded from The Arabidopsis Information Resource (TAIR, http://www.arabidopsis.org/) with the accession numbers AT1G64280.1, AT4G26120.1, AT5G45110.1, AT4G19660.1, AT2G41370.1, and AT3G57130.1. Their amino acid sequences were used as queries for BLASTp searches at the Genome Database for Rosaceae (GDR, https://www.rosaceae.org/) with an E-value cutoff of 1e − 003. The results were submitted to the Simple Molecular Architecture Tool (SMART, http://smart.emblheidelberg.de) and Pfam protein family databases (http://pfam.xfam.org/) to detect the presence of BTB/POZ and ankyrin domains. Sequences containing both domains were selected as putative NPR1 homologs. Afterward, the coding sequences identified from the apple genome data were inspected and revised according to the M. domestica and M. pumila expressed sequence tags (ESTs), which are available from the NCBI web server (http://www.ncbi.nlm.nih.gov/).
Plant materials and treatments
Five-year-old apple trees of the “Qinguan” and “Pacific Rose” cultivars and a wild apple species Malus baccata Borkh were grown in the field under a rain-shelter at the experimental station of Northwest A&F University (Yangling, Shaanxi, China) and used as materials throughout the study. The apple cultivars Qinguan and Pacific Rose were shown to have different levels of resistance to M. coronaria, which causes apple brown spot. Pacific Rose was previously reported as susceptible, while Qinguan and M. baccata are resistant and highly resistant, respectively, to the disease (Yin et al. 2013).
Tissue- and organ-specific expression of the apple NPR1 homologs was investigated in Pacific Rose. New shoots (near the apices of newly-growing shoots and 3–4 mm in diameter), leaves (the third to fifth fully expanded young leaves beneath the shoot apices when newly-growing shoots were 40–60 cm in length, without petioles), flowers (with receptacle), pericarp, pulp (from mature fruits of about 7 cm in diameter), and seeds of mature fruit were collected for total RNA isolation and expression analysis.
The induction of NPR1 expression by SA and MeJA was examined in Qinguan and Pacific Rose, and by M. coronaria inoculation in Pacific Rose and M. baccata. SA and MeJA treatment was performed by spraying leaves with 100 mM SA or 50 mM MeJA solutions once at 8:00 a.m. on whole sample plants. Leaves sprayed with sterile distilled water were used as controls. Each treatment and control were performed on 15 trees. All treated plants were sampled at 0, 1, 3, 6, 12, and 24 h postinoculation (hpi) for subsequent RNA isolation and NPR1 homolog expression analysis. M. coronaria inoculation was conducted on 90~120 detached leaves according to Li et al. (2014) under a 24-h light/0-h dark photoperiod and 15~20 sample leaves were collected at 6, 24, 48, 72, 96, and 144 hpi. All treatments were performed in triplicate.
RNA extraction and cDNA synthesis
Total RNA was extracted using a modified SDS-LiCl method (Hou et al. 2011). All reagents were prepared with 1 ‰ DEPC treated water. Potassium acetate buffer (pH 5.0) was added to precipitate the SDS and macromolecule material. The chloroform/isoamyl alcohol extraction step was repeated three or four times until the protein phase was completely removed and the water phase became clean. RNA integrity was assessed on 2 % TAE agarose gel under non-denaturing conditions. The first-strand cDNA was synthesized from mature mRNA using the PrimeScriptII RT reagent Kit (Takara, Dalian, China) with Oligo(dT) primers according to the manufacturer’s guidelines. Intron-spanning primers were used to assess genomic DNA contamination.
Molecular cloning of NPR1 homologs in apple
To confirm the identified apple NPR1 homolog loci in the apple genome, eight pairs of gene-specific primers targeting the exons were designed based on the putative NPR1 homolog gene sequences from the apple genome database and the apple EST sequences (Supplementary Table S1). Putative full-length coding sequences of the apple NPR1 homolog genes were amplified by RT-PCR from both Qinguan and Pacific Rose leaves with cDNA templates instead of genomic DNA because some of the gene fragments were too long to amplify. PCR was performed with pfu Taq DNA polymerase (Thermo, Shanghai, China), using the manufacturer recommended reaction conditions. The PCR products were retrieved and purified with a DNA Fragment Purification Kit (Tiangen, Beijing, China). A tails were added to the PCR fragments using rTaq (Takara) at 72 °C for 35 min, and the fragments were inserted into the pMD-19T cloning vector (Takara). Positive clones were verified by blue/white colony assays and sequencing. The absence or presence of an AS variant was judged by agarose gel or sequencing of 10 independent clones from the RT-PCR product of NPR1s.
Sequence alignment, phylogenetic analysis, and exon-intron structure analysis of apple NPR1 homologs
Because the eight deduced apple NPR1 homolog cDNA sequences and most transcript variants were detected in Pacific Rose, the cDNA sequences of the apple homologs in Pacific Rose were used for subsequent sequence alignment analyses. The apple NPR1 protein sequences deduced from the cDNA fragments were aligned using the program ClustalX with BLOSUM30 as the protein weight matrix. A phylogenetic tree of apple NPR1 protein sequences was constructed with 28 other putative NPR1-like proteins sequences downloaded from the NCBI database from eight plant species (Table 1) except for CpNPR1–4 (the sequences were obtained from the supplementary material of Peraza-Echeverria et al. (2012)). Phylogenetic trees were constructed using the neighbor-joining method by the program MEGA5 (Molecular Evolutionary Genetics Analysis) with the p-distance and complete deletion option parameters selected (Saitou and Nei 1987; Tamura et al. 2011). A diagram of the exon/intron organization was generated by the online Gene Structure Display Server (GSDS, http://gsds.cbi.pku.edu.cn). A gene structure map was generated based on the coding sequence of the apple cultivar Pacific Rose and corresponding genome sequence of apple (Ver 1.0, https://www.rosaceae.org/).
Gene expression detection by qRT-PCR
Real-time quantitative PCR (qRT-PCR) was performed on a Bio-Rad iQ5.0 platform to detect the expression levels of apple NPR1 homologs in different tissues and in response to different treatments. The reactions were performed in 20-μL volumes containing 10 μL of SYBR® Premix ExTaq II (Takara), 2.0 μL of cDNA template (50 ng/μL), 1.6 μL of gene-specific primers (10 pmol/L), and 6.4 μL of sterile distilled water. The PCR amplification conditions were 95 °C for 1 min, and then 40 cycles of 95 °C for 10 s, 58 °C for 30 s, and 72 °C for 20 s, followed by melting curve analysis in which the products were heated from 58 to 95 °C by 0.5 °C per cycle. Fluorescence was measured during the extension step of each cycle. Specific primers were designed by primer premier 5.0 for each gene, and each pair was subjected to a local blast search to ensure its specificity (Supplementary Table S1). The apple actin gene (GQ339778) was selected as an internal standard (Fan et al. 2011). Three biological replicates were used for each assay. The relative expression level of each gene was calculated according to the 2−ΔΔCT method (Livak and Schmittgen 2001).
Results
The published apple genome contains eight NPR1 gene homologs
A total of eight NPR1-like genomic sequences containing both BTB/POZ and ankyrin repeat domains were identified from the apple genome database (Ver 1.0) and named MdNPR1 to MdNPR8. The locations of these apple NPR1 gene homologs in the apple genome were listed in Table 2. The eight genes were located in seven different chromosomes; two in chromosome 2 and one each in chromosomes 5, 9, 10, 12, 14, and 17. Their location was scattered over different chromosomes and did not show any cluster distribution clues to the expansion of the gene family. Blast analysis using the predicted amino acid sequence of the apple NPR1 homologs showed that MdNPR1 was most similar to AtNPR1 (49.7 % identity) (Table 3), MdNPR2, MdNPR3, MdNPR4, and MdNPR5 were most similar to AtNPR3 (54.7, 59.9, 59.7, and 53.8 % identity, respectively), and MdNPR6 was most similar to AtNPR4 (51.9 % identity). Furthermore, MdNPR7 and MdNPR8 were most similar to AtNPR5 (77.8 and 77.8 % identity, respectively). No homologs in apple were found to be similar to AtNPR2 or AtNPR6.
Different transcripts of the NPR1 homologs were found in resistant and susceptible apple cultivars
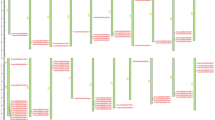
The full cDNA fragments of all eight NPR1 gene homologs were cloned and sequenced in the apple cultivars Qinguan and Pacific Rose. A comparison of all the NPR1 homolog sequences showed that the coding sequences of MdNPR1, 3, 5, and 6 were the same in both varieties (Supplementary Fig. S1~S8). The MdNPR4, 7, and 8 gene sequences of the two varieties differed in several bases, but the protein sequences were unchanged. The MdNPR2 gene sequences of the two cultivars had several different bases leading to two amino acid mutations in the conserved ankyrin domain and two outside the conserved domain region. Two different MdNPR2 transcripts with several base differences at different positions were cloned from Qinguan, implying that gene editing may have occurred. A total of 12 transcripts of the eight NPR1 homologs were found, showing differential splicing of NPR1 homologs. MdNPR1, 2, 4, and 5 had two different splicing variants each, while MdNPR3, 6, 7, and 8 had only one. The variation of spliced size varied from 77 bp which only could be seen by sequencing to 754 bp which could be seen by the agarose gel (Fig. 1). The sequences of the different splicing variants were submitted to GenBank, and their presence or absence in the resistant and susceptible apple cultivars is shown in Table 2. According to the sequences (Supplementary Fig. S1~S8), different forms of alternative splicing were found for the NPR1 homologs (Fig. 2). An alternative 3ʹ splicing site in the last exon was found in MdNPR1, an alternative 5ʹ splicing site in the second exon and an alternative 3ʹ splicing site in the last exon was found in MdNPR2, intron retention in the third intron was found in MdNPR4, and exon skipping in the second exon was found in MdNPR5. The splicing variants MdNPR1a and MdNPR4b were only found in Pacific Rose, but other splicing variants were found in both cultivars.
Conserved domain and cysteine site mutations were found in some NPR1 homologs
Multiple sequence alignment was carried out to examine the conservation of the domains in the apple NPR1 proteins at each residue position. The results showed that the apple NPR1 proteins all contain the BTB/POZ and ankyrin repeat domains, except MdNPR5b, which had lost the ankyrin repeat domain (Fig. 3). The 12 apple NPR1 homolog proteins all harbored conserved cysteine residues at C82, C150, C155, and C160, but only the MdNPR1a and MdNPR1b proteins had a conserved cysteine at C216. A key cysteine at C521/529 in AtNPR1 was not conserved in any of the apple NPR1 proteins. These cysteine sites are thought to be important for the formation of functional oligomers, and the mutation or deletion of these cysteine sites may result in functional changes of the NPR1 proteins. The normal transcripts of the MdNPR1-MdNPR6 contained a NPR1_like_C region and NLS in the C-terminus of their protein. However, the alternative splicing variants MdNPR1b, MdNPR2b, MdNP4b, MdNPR5b, MdNPR7, and MdNPR8 did not have the NLS in their C-terminus; their function and localization need further investigation.
Gene structure and phylogenetic analysis of the apple NPR1 homologs
Gene structure analysis of the apple NPR1 homolog sequences indicated that they had similar exon-intron structures to the NPR1 genes of Arabidopsis. The homologs in group I and II had four exons, while those in group III had two. In addition, the lengths of corresponding exons in each group were almost the same (Fig. 2).
To study the evolutionary relationships among the NPR1 homologs, a phylogenetic tree was constructed with 28 putative NPR1-like proteins from eight plant species (Fig. 4). According to the results, all the selected NPR1 homologs were clustered into three groups, which was consistent with previous findings in Arabidopsis. In group I, only MdNPR1 was located in the same cluster as AtNPR1 and AtNPR2. Notably, Group II had the most numbers of apple NPR1 homologs (five homologs) compared with only two from Arabidopsis, two from Carica papaya, three from Populus trichocarpa, and one from Persea Americana. MdNPR2, MdNPR3, and MdNPR4 were closely clustered with PpNPR1.1, AtNPR3, and AtNPR4. MdNPR5 and MdNPR6 were clustered closely with CpNPR3, PtNPR3, and PtNPR4. Two members (MdNPR7 and MdNPR8) were classified into group III, which was called the BOP group in reference to Arabidopsis.
Differential expression levels of MdNPR1 homologs in apple tissue and organs
Investigating the specific expression of a gene in different tissues and organs can help infer its major function. The expression of all eight apple NPR1 homologs was examined in stems, flowers, leaves, pericarp, pulp, and seeds of Pacific Rose (Fig. 5). The results showed that their expression levels in different tissues were significantly different. The expression level of MdNPR1 was highest in pulp (2.09-fold), followed by leaves and relatively low in other tissues. MdNPR2, MdNPR3, and MdNPR4 were expressed at a relatively high level in pericarp compared with leaves, stems, pulp, and seeds. The expression levels of MdNPR2 and MdNPR3 were lowest in flowers, while the expression of MdNPR4 was highest in flowers. MdNPR5 was mainly expressed in pericarp and pulp. MdNPR6 expression was also highest in pulp (18.52-fold) like the MdNPR1 but was moderate in pericarp and seeds and low in leaves, flowers, and stems. Interestingly, the transcription levels of both MdNPR7 and MdNPR8 were relatively high in flowers and relatively low in stems, leaves, and seeds, but the expression of MdNPR7 in fruits was high, while the expression of MdNPR8 in fruits was very low.
The expression of apple NPR1 homolog responses to SA and MeJA inoculation
Since NPR1 homologs are thought to play important roles in plant defense responses, the expression of the apple genes under SA and MeJA inoculation was compared between the resistant cultivar Qinguan and susceptible cultivar Pacific Rose (Fig. 6).
Following SA induction, MdNPR2, MdNPR3, MdNPR5, and MdNPR6 were downregulated at 1 hpi in the resistant cultivar Qinguan, and then MdNPR2~7 expression was significantly upregulated at 6 hpi, while MdNPR8 was upregulated later at 12 hpi compared with controls. Similarly, in SA-induced susceptible Pacific Rose, the expression of MdNPR2–MdNPR8 was first downregulated at 1 hpi, and then some of the genes were upregulated and reached a peak (MdNPR2 at 6 hpi; MdNPR4 and MdNPR5 at 3 hpi; MdNPR3 and MdNPR6 at 12 hpi). Considering the average expression levels between the cultivars, the apple NPR1 gene homologs were more highly expressed in Qinguan than Pacific Rose, except for MdNPR5 at its peak point. However, the MdNPR4 and MdNPR5 genes responded earlier in Pacific Rose than in Qinguan.
Following MeJA induction, the expression level of MdNPR2 was significantly upregulated at 1 hpi in Pacific Rose and at 3 hpi in Qinguan. The expression of MdNPR3 in Qinguan was first downregulated at 1 hpi and then upregulated. MdNPR3 was upregulated at 1 hpi in Pacific Rose. MdNPR4 expression was sharply upregulated in Pacific Rose at 3 hpi but only slightly upregulated at 12 hpi in Qingquan. MdNPR5 was upregulated at 0–1 hpi in both varieties and then immediately downregulated until 12 hpi in Pacific Rose, while the expression began to drop at 6 hpi in Qinguan. The expression of both MdNPR6 and MdNPR7 was upregulated at 1 hpi in Pacific Rose, while only MdNPR7 was upregulated at 6 hpi in Qinguan. MdNPR8 expression was significantly downregulated at 3 hpi in Pacific Rose but upregulated in Qinguan at 12 hpi. The expression of the MdNPR1 gene was sharply upregulated at 6 and 12 hpi with SA and MeJA, respectively, in Qinguan, but no change was observed in Pacific Rose.
Apple NPR1 gene expression in response to M. coronaria in the susceptible apple cultivar and the resistant wild species
The expression changes of the apple NPR1 homologs were compared between the susceptible apple cultivar Pacific Rose and resistant wild species M. baccata after challenge with M. coronaria (Fig. 7). The expression of MdNPR1 was downregulated in M. baccata but upregulated and reached a peak in Pacific Rose at 48 hpi. There was also a big decrease in the expression of MdNPR5 in M. baccata at 24 hpi. The MdNPR2 gene was upregulated at 48 hpi in Pacific Rose but showed no change in M. baccata. Conversely, the expression of MdNPR6 was upregulated at 48 hpi in M. baccata but showed no change in Pacific Rose. The expression of MdNPR3 in the two varieties was significantly different at 6 hpi but almost the same at all other timepoints. MdNPR4 expression was significantly higher in Pacific Rose at 96 hpi. The expression of MdNPR7 and MdNPR8 showed the same expression trends in both varieties. Interestingly, MdNPR7 was upregulated at 24 hpi, while MdNPR8 were downregulated at the same timepoint; subsequently, MdNPR7 expression returned to a normal level, but the expression of MdNPR8 was upregulated.
Discussion
Identification of NPR1 homologs in apple
A large number of studies have revealed that NPR1 homologs play key roles in various biotic or abiotic responses. However, although NPR1 genes have been isolated and characterized in several plant species, there is little information about NPR1 homologs in apple. In this study, eight novel NPR1 homologs were identified in the apple genome. The NPR1 homolog in apple is the largest community compared with estimates for other plant species (six members in Arabidopsis, four in Carica papaya (Peraza-Echeverria et al. 2012), six in Populus trichocarpa (Shao et al. 2013), and five in Persea Americana (Backer et al. 2015)). The encoded protein sequences of all eight gene homologs included the BTB/POZ and ankyrin repeat domains; these two domains enable the apple NPR1 homologs to perform their functions, possibly by interacting with other proteins, like TGA and NIMIN1/2. The NPR1-like-C domain was found in the group I and II members, and is also thought to be an essential component of NPR1 proteins of Arabidopsis (Cao et al. 1997). The NLS in the C-terminus of the NPR1 homologs is essential for nucleus translocation (Kinkema et al. 2000; Maier et al. 2011). C-terminal deletion may have led to localization and functional changes in MdNPR1b, MdNPR2b, MdNPR4b, MdNPR5b, MdNPR7, and MdNPR8. The MdNPR1 and AtNPR1 proteins harbor the conserved cysteine residues Cys82 and Cys216 in the same position, indicating that the MdNPR1 protein may form an oligomer in apple-like AtNPR1. Additionally, Cys150, Cys155, or Cys160 is also highly conserved in all the apple NPR1 homologs. Mutations in these residues reduced the accumulation of NPR1 (Durrant and Dong 2004). A recent study reported that AtNPR1 could bind SA through the copper ion associated with Cys521/529 (Wu et al. 2012). However, no other NPR1 homolog has cysteine residues in the same site. Thus, the apple NPR1 may bind to SA with other domains, such as the novel NPR1 domain LENRV or NIMINs-binding sites (Canet et al. 2010; Pajerowska-Mukhtar et al. 2013).
Sequence alignment showed that putative proteins of MdNPR3 and MdNPR4 (90.6 % identity), MdNPR7 and MdNPR8 (94.4 % identity), and MdNPR5 and MdNPR6 (85 % identity) are highly similar in amino acid sequence (Table 3). These data indicate that the NPR1 homologs in apple may have undergone an extensive gene duplication and diversification process in comparison with other plants.
Interestingly, our phylogenetic analyses indicated that the numbers of homolog members in each of the three subgroups were significantly different. The MdNPR1 gene in the first group which is highly homologous to AtNPR1 has never been reported in apple, which means it may function as a key regulator in SAR as AtNPR1. The NPR1-like gene, which has been studied before in apple (Malnoy et al. 2007), named MdNPR3 is classified into the second group. This group also included another four apple NPR1 homologs, suggesting that their roles and interactions in plant immune responses are complicated. The two members in the third group, MdNPR7 and MdNPR8, share 94.4 % identity in protein sequence, which is higher than those in Arabidopsis (78.6 % identity), indicating that MdNPR7 and MdNPR8 are possibly newly evolved in apple.
Molecular cloning of the apple NPR1 cDNA homologs sequences showed the common existence of AS of NPR1, which has never been found in other species before. This AS made the transcription regulation patterns of NPR1 complicated in apple. The AS in MdNPR1 and MdNPR4 did not remove the two conserved domains but removed the NLS at the C-terminus, their effect on the functions need further study. In particular, MdNPR1b was found in both Qinguan and Pacific Rose. MdNPR1a and MdNPR1b may form oligomers individually or together in the cytoplasm. In SAR-induced cells, more MdNPR1a may be transcribed so that more functional NPR1 can be transported to the nucleus. In non-induced cells, more MdNPR1b may be transcribed to form oligomers with MdNPR1a in the cytoplasm. Thus, AS of the MdNPR1 transcript may make nuclear transport of NPR1 proteins faster and more convenient. In addition, MdNPR5 can lose its second exon during transcription, resulting in a small protein containing only the BTB/POZ domain. The transcription variant of the MdNPR2 gene can also be translated to a shorter protein but still harbors the two conserved domains. The different sequences of MdNPR2 in the two varieties indicate alleles or RNA editing of this gene. These proteins may lose their original functions or play other roles in plants. Because we could not isolate MdNPR1a or MdNPR4b from Qinguan, it is possible that some of the transcriptional variants are specifically expressed in different apple varieties. These transcriptional variants may respond to specific signals or pathogens. Alternative splicing may be an important form of regulation for these genes.
Expression of the apple NPR1 homologs
The Pacific Rose and Qinguan apple cultivars are generally considered to have different resistance to many diseases. The expression of the apple NPR1 homologs showed significant differences after induction with SA and MeJA. The apple NPR1 homologs in group II were more highly expressed in Qinguan than in Pacific Rose after SA induction. In banana cultivars that are resistant to Fusarium oxysporum, NPR1 (which is homologous to MdNPR1) is upregulated earlier and to a greater extent after SA treatment than in susceptible cultivars (Endah et al. 2008). The MdNPR3 gene was upregulated at 6 h after SA treatment in Qinguan. The result is the same as the previous findings that MhNPR1 (which is homologous to MdNPR3) and the SAR marker genes MhPR1 and MhPR5 can be induced by SA (Zhang et al. 2012). However, no significant expression change of MdNPR3 was found in the susceptible Pacific Rose after SA induction. Given that there was no difference in MdNPR3 sequence between the two varieties, the transcript levels of this gene may be regulated in different ways. The MdNPR4 and MdNPR5 genes responded earlier in susceptible Pacific Rose than in resistant Qinguan after SA treatment, and we speculate that these two genes may play a more important role in the susceptible cultivar Pacific Rose.
The specific expression of the NPR1 homologs was studied in six different apple tissues. The transcription level of the MdNPR1 gene was very low in all tissues, while relatively high in leaves and fruit, which were consistent with previous findings. MdNPR2, MdNPR3, MdNPR5, and MdNPR6 were highly expressed in the pericarp and pulp, with the expression of MdNPR6 in pulp being the highest. Thus, it seems that these four NPR1 homologs are more important in fruit. MdNPR4, MdNPR7, and MdNPR8 expression was highest in flowers, which may mean that these genes play important roles in flower development.
Conidia of M. coronaria are widely thought to germinate on the apple leaf surface at about 6 hpi and symptoms (disease spot) can be visually observed at 6 days after inoculation in Pacific Rose (Dang et al. 2011). MdNPR1, MdNPR3, MdNPR5, and MdNPR6 were highly expressed in M. baccata compared with Pacific Rose at 6 hpi. The expression of MdNPR2 and MdNPR4 genes fluctuated around only a little after inoculation in M. baccata. Interestingly, significant expression differences of MdNPR1, MdNPR2, and MdNPR6 were observed between Pacific Rose and M. baccata at 48 hpi. Thus, the different resistance of apple to M. coronaria could be explained in two ways. Firstly, one of genes may be the key gene affecting resistance, such as the MdNPR2 gene, which showed polymorphism in Qinguan and Pacific Rose. Secondly, the different transcription levels of the transcription variants after inoculation and their interaction may be a predominant factor in apple. The expression of MdNPR7 and MdNPR8 showed the same change trend after inoculation in both cultivars, suggesting that these two genes do not contribute to the differences in M. coronaria resistance in apple.
The functions of the NPR1 homologs are more complicated in apple than in Arabidopsis. At the protein level, it was speculated that two or more homologs may combine with each other or with signal molecules to regulate the balance of the MdNPR1. NPR1 homeostasis is controlled by SA binding to NPR3/NPR4 in a concentration-dependent manner, which have been proved in Arabidopsis (Moreau et al. 2012). At the transcription level, the apple NPR1 homolog genes can be regulated by transcription factors or transcribed to different transcripts. This complicated phenomenon should help to maintain the stability of the plant immune system.
In conclusion, this study provides evidence to help the preliminary NPR1 gene information and functional annotation of the eight newly discovered NPR1 homologs from apple. Sequence structure and homology as well as phylogenetic analysis suggest that six apple NPR1 homologs (MdNPR1–6) may be involved in defense responses, while the remaining two (MdNPR7 and 8) are most likely involved in tissue development. In particular, the expression level of MdNPR1 was very low but changed rapidly after SA and pathogen treatments. In addition to the most similarity to AtNPR1 on amino acid sequence, MdNPR1 gene may function as a key regulator of SAR. The hormone and pathogen inoculation treatment-induced expression, as well as specific expression in various tissues, of the apple NPR1 homologs support this and provide a foundation for future research. Future efforts will be focused on the intracellular interactions and localization of the NPR1 proteins. Additionally, the expression levels and functions of the different transcript variants of NPR1 homologs in apple need to be explored in depth.
References
An C, Mou Z (2011) Salicylic acid and its function in plant immunity. J Integr Plant Biol 53:412–428. doi:10.1111/j.1744-7909.2011.01043.x
Aravind L, Koonin EV (1999) Fold prediction and evolutionary analysis of the POZ domain: structural and evolutionary relationship with the potassium channel tetramerization domain. J Mol Biol 285:1353–1361. doi:10.1006/jmbi.1998.2394
Backer R, Mahomed W, Reeksting BJ, Engelbrecht J, Ibarra-Laciette E, van den Berg N (2015) Phylogenetic and expression analysis of the NPR1-like gene family from Persea americana (mill. Frontiers in plant science 6
Baldwin AS (1996) The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 14:649–683. doi:10.1146/annurev.immunol.14.1.649
Canet JV, Dobon A, Roig A, Tornero P (2010) Structure-function analysis of npr1 alleles in Arabidopsis reveals a role for its paralogs in the perception of salicylic acid. Plant Cell Environ 33:1911–1922. doi:10.1111/j.1365-3040.2010.02194.x
Canet JV, Dobon A, Fajmonova J, Tornero P (2012) The BLADE-ON-PETIOLE genes of Arabidopsis are essential for resistance induced by methyl jasmonate. BMC Plant Biol 12. doi:10.1186/1471-2229-12-199
Cao H, Bowling SA, Gordon AS, Dong XN (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired-resistance. Plant Cell 6:1583–1592
Cao H, Glazebrook J, Clarke JD, Volko S, Dong XN (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88:57–63
Dang ZG, Gao H, Wang LC, YM L, Zhao ZY (2011) Evaluation and physiological analysis of resistance of apple (Malus domestica Borkh.) cultivars to apple brown spot [Marssonina mali (P. Henn.) Ito]. Plant Physiology Journal 47(7):691–698
Del Carratore R, Magaldi E, Podda A, Beffy P, Migheli Q, Maserti BE (2011) A stress responsive alternative splicing mechanism in Citrus Clementina leaves. J Plant Physiol 168:952–959. doi:10.1016/j.jplph.2010.11.016
Despres C, DeLong C, Glaze S, Liu E, Fobert PR (2000) The arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 12:279–290. doi:10.1105/tpc.12.2.279
Dong XN (2004) NPR1, all things considered. Curr Opin Plant Biol 7:547–552. doi:10.1016/j.pbi.2004.07.005
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209. doi:10.1146/annurev.phyto.42.040803.140421
Endah R, Beyene G, Kiggundu A, van den Berg N, Schluter U, Kunert K, Chikwamba R (2008) Elicitor and Fusarium-induced expression of NPR1-like genes in banana. Plant Physiol Bioch 46:1007–1014. doi:10.1016/j.plaphy.2008.06.007
Fan WH, Dong XN (2002) In vivo interaction between NPR1 and transcription factor TGA2 leads to salicylic acid-mediated gene activation in Arabidopsis. Plant Cell 14:1377–1389. doi:10.1105/tpc.001628
Fan H, Wang F, Gao H, Wang L, Xu J, Zhao Z (2011) Pathogen-induced MdWRKY1 in ‘Qinguan’ apple enhances disease resistance. Journal of Plant Biology 54:150–158. doi:10.1007/s12374-011-9151-1
Friedrich L, Lawton K, Ruess W, Masner P, Specker N, Rella GM, Meier B, Dincher S, Staub T, Uknes S, Metraux JP, Kessmann M, Ryals J (1996) A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J 10:61–70. doi:10.1046/j.1365-313X.1996.10010061.x
Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, Dong X (2012) NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486:228–232. doi:10.1038/nature11162
Gaëlle LH, Sibylle F, Flore K-M, Miclot A-S, Heitz T, Pere M, Bertsch C, Chong J (2011) Vitis vinifera VvNPR1.1 is the functional ortholog of AtNPR1 and its overexpression in grapevine triggers constitutive activation of PR genes and enhanced resistance to powdery mildew. Planta 234(2):405--417
Gfeller A, Liechti R, Farmer EE (2006) Arabidopsis jasmonate signaling pathway Science’s STKE: signal transduction knowledge. Environment 2006:cm1-cm1. doi:10.1126/stke.3222006cm1
Hepworth SR, Zhang Y, McKim S, Li X, Haughn GW (2005) BLADE-ON-PETIOLE-dependent signaling controls leaf and floral patterning in Arabidopsis. Plant Cell 17:1434–1448. doi:10.1105/tpc.104.030536
Hou P, Xie ZY, Zhang L, Song Z, Mi J, He Y, Li YF (2011) Comparison of three different methods for total RNA extraction from Fritillaria unibracteata: a rare Chinese medicinal plant. J Med Plants Res 5:2834–2838
Iavicoli A, Boutet E, Buchala A, Metraux JP (2003) Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Mol Plant Microbe In 16:851–858. doi:10.1094/mpmi.2003.16.10.851
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328. doi:10.1146/annurev.arplant.53.100301.135207
Kim HS, Delaney TP (2002) Over-expression of TGA5, which encodes a bZIP transcription factor that interacts with NIM1/NPR1, confers SAR-independent resistance in Arabidopsis thaliana to Peronospora parasitica. Plant J 32:151–163. doi:10.1046/j.1365-313X.2001.01411.x
Kinkema M, Fan WH, Dong XN (2000) Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 12:2339–2350. doi:10.1105/tpc.12.12.2339
Lawton K, Weymann K, Friedrich L, Vernooij B, Uknes S, Ryals J (1995) Systemic acquired resistance in Arabidopsis requires salicylic acid but not ethylene. Molecular plant-microbe interactions: MPMI 8(6):863–870
Le Henanff G, Heitz T, Mestre P, Mutterer J, Walter B, Chong J (2009) Characterization of Vitis vinifera NPR1 homologs involved in the regulation of pathogenesis-related gene expression. BMC Plant Biol 9. doi:10.1186/1471-2229-9-54
Li MM, Xu JH, Qiu ZH, Zhang J, Ma FW, Zhang JK (2014) Screening and identification of resistance related proteins from apple leaves inoculated with Marssonina coronaria (EII. & J. J. Davis). Proteome Sci 12. doi:10.1186/1477-5956-12-7
Liu G, Holub EB, Alonso JM, Ecker JR, Fobert PR (2005) An Arabidopsis NPR1-like gene, NPR4, is required for disease resistance. Plant J 41(2):304--318
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods, 25, 402--408. doi:10.1006/meth.2001.1262
Maier F, Zwicker S, Huckelhoven A, Meissner M, Funk J, Pfitzner AJP, Pfitzner UM (2011) Nonexprssor of pathogensis-related proteins1 (NPR1) and some NPR1-related proteins are sensitive to salicylic acid. Mol Plant Pathol 12:73–91. doi:10.1111/j.1364-3703.2010.00653.x
Malnoy M, Jin Q, Borejsza-Wysocka EE, He SY, Aldwinckle HS (2007) Overexpression of the apple MpNPR1 gene confers increased disease resistance in Malus x domestica. Mol Plant-Microbe Interact 20:1568–1580. doi:10.1094/mpmi-20-12-1568
Mastrangelo AM, Marone D, Laido G, De Leonardis AM, De Vita P (2012) Alternative splicing: enhancing ability to cope with stress via transcriptome plasticity. Plant science: an international journal of experimental plant biology 185-186:40–49. doi:10.1016/j.plantsci.2011.09.006
Metraux JP, Ahlgoy P, Staub T, Speich J, Steinemann A, Ryals J, Ward E (1991) Induced systemic resistance in cucumber in response to 2,6-dichloro-isonicotinic acid and pathogens. Advances in Molecular Genetics of Plant-Microbe Interactions 1:432–439
Moreau M, Tian M, Klessig DF (2012) Salicylic acid binds NPR3 and NPR4 to regulate NPR1-dependent defense responses. Cell Res 22:1631–1633. doi:10.1038/cr.2012.100
Mukhtar MS, Nishimura MT, Dangl J (2009) NPR1 in plant defense: it’s not over till it’s turned over. Cell 137:804–806. doi:10.1016/j.cell.2009.05.010
Niggeweg R, Thurow C, Kegler C, Gatz C (2000) Tobacco transcription factor TGA2.2 is the main component of as-1-binding factor ASF-1 and is involved in salicylic acid- and auxin-inducible expression of as-1-containing target promoters. J Biol Chem 275:19897–19905. doi:10.1074/jbc.M909267199
Pajerowska-Mukhtar KM, Emerine DK, Mukhtar MS (2013) Tell me more: roles of NPRs in plant immunity. Trends Plant Sci 18:402–411. doi:10.1016/j.tplants.2013.04.004
Peraza-Echeverria S, Santamaría JM, Fuentes G, de los Ángeles Menéndez-Cerón M, Vallejo-Reyna MÁ, Herrera-Valencia VA (2012) The NPR1 family of transcription cofactors in papaya: insights into its structure, phylogeny and expression. Genes & Genomics 34:379–390. doi:10.1007/s13258-011-0218-7
Pieterse CM, Van Loon L (2004) NPR1: the spider in the web of induced resistance signaling pathways. Curr Opin Plant Biol 7:456–464. doi:10.1016/j.pbi.2004.05.006
Robert-Seilaniantz A, Grant M, Jones JDG (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. In: VanAlfen NK, Bruening G, Leach JE (eds) Annual review of phytopathology 49. Annual review of phytopathology, pp. 317–343. doi:10.1146/annurev-phyto-073009-114447
Ryals J et al. (1997) The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor I kappa B. Plant Cell 9:425–439. doi:10.1105/tpc.9.3.425
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Shao Y, Zhang H, He H, Cheng B, Xiang Y (2013) Molecular cloning and characterization of orthologues of NPR1 gene from poplar. J Phytopathol 161:35–42. doi:10.1111/jph.12002
Somssich IE (2003) Closing another gap in the plant SAR puzzle. Cell 113:815–816. doi:10.1016/s0092-8674(03)00473-2
Spoel SH, Mou Z, Tada Y, Spivey NW, Genschik P, Dong X (2009) Proteasome-mediated turnover of the transcription coactivator NPR1 plays dual roles in regulating plant immunity. Cell 137:860–872. doi:10.1016/j.cell.2009.03.038
Sticher L, MauchMani B, Metraux JP (1997) Systemic acquired resistance. Annu Rev Phytopathol 35:235–270. doi:10.1146/annurev.phyto.35.1.235
Subramaniam R, Desveaux D, Spickler C, Michnick SW, Brisson N (2001) Direct visualization of protein interactions in plant cells. Nat Biotechnol 19:769–772. doi:10.1038/90831
Syed NH, Kalyna M, Marquez Y, Barta A, Brown JW (2012) Alternative splicing in plants--coming of age. Trends Plant Sci 17:616–623. doi:10.1016/j.tplants.2012.06.001
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Ward ER, Uknes SJ, Williams SC, Dincher SS, Wiederhold DL, Alexander DC, Ahlgoy P, Metraux JP, Ryals JA (1991) Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell 3:1085–1094. doi:10.1105/tpc.3.10.1085
Wu Y et al. (2012) The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep 1:639–647. doi:10.1016/j.celrep.2012.05.008
Yin L, Li M, Ke X, Li C, Zou Y, Liang D, Ma F (2013) Evaluation of Malus germplasm resistance to marssonina apple blotch. Eur J Plant Pathol 136:597–602. doi:10.1007/s10658-013-0190-y
Yocgo RE, Creissen G, Kunert K, Chikwamba R (2012) Two different banana NPR1-like coding sequences confer similar protection against pathogens in Arabidopsis. Trop Plant Biol 5:309–316. doi:10.1007/s12042-012-9112-y
Zhang YL, Fan WH, Kinkema M, Li X, Dong XN (1999) Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. P Natl Acad Sci USA 96:6523–6528. doi:10.1073/pnas.96.11.6523
Zhang Y, Cheng YT, Qu N, Zhao Q, Bi D, Li X (2006) Negative regulation of defense responses in Arabidopsis by two NPR1 paralogs. Plant J 48:647–656. doi:10.1111/j.1365-313X.2006.02903.x
Zhang JY, Qiao YS, Lv D, Gao ZH, Qu SC, Zhang Z (2012) Malus hupehensis NPR1 induces pathogenesis-related protein gene expression in transgenic tobacco. Plant Biol 14(Suppl 1):46–56. doi:10.1111/j.1438-8677.2011.00483.x
Zhou JM, Trifa Y, Silva H, Pontier D, Lam E, Shah J, Klessig DF (2000) NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol Plant Microbe In 13:191–202. doi:10.1094/mpmi.2000.13.2.191
Acknowledgments
The research was supported by the earmarked fund for China Agriculture Research System (CARS-28). We also thank Prof. Pengmin Li and Dr. Mingjun Li for their comments and suggestions on improving the manuscript.
Data archiving statement
All the apple NPR1 gene homologs cDNA sequence cloned from apple cultivar ‘Pacific Rose’ were submitted to GenBank. Their gene ID and GenBank accession numbers were list as follows: MdNPR1a (KU166911), MdNPR1b (KU166912), MdNPR2a (KU166913), MdNPR2b (KU166914), MdNPR3 (KU166915), MdNPR4a (KU166916), MdNPR4b (KU166917), MdNPR5a (KU166921), MdNPR5b (KU166920), MdNPR6 (KU166922), MdNPR7 (KU166918), and MdNPR8 (KU166919). The apple NPR1 homolog gene sequences from ‘Qinguan’ were displayed in the supplementary Fig. S1~S8.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by A.M. Dandekar
Electronic supplementary material
Supplementary Table S1
Primer sequences used for NPR1 homologs gene cloning and qRT-PCR (XLSX 10 kb)
Supplementary Fig. S1-S8
Alignment of the MdNPR1–NPR8 full-length coding sequences from ‘Qinguan’ and ‘Pacific rose’ and their corresponding genome sequences. The prefix ‘Pr′ or ‘Qg’ before the gene ID means the sequence cloned from apple cultivar ‘Pacific rose’ or ‘Qinguan’. (DOC 10969 kb)
Rights and permissions
About this article
Cite this article
Zhang, J., Jiao, P., Zhang, C. et al. Apple NPR1 homologs and their alternative splicing forms may contribute to SA and disease responses. Tree Genetics & Genomes 12, 92 (2016). https://doi.org/10.1007/s11295-016-1050-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-016-1050-7