Abstract
Pear is one of the most important temperate fruits, with high genetic diversity, but controversial classification for some genotypes or species. Our study evaluates the polymorphism of 385 pear resources belonging to five cultivated species or interspecies of Pyrus, based on a set of 134 core simple sequence repeat (SSR) markers. A total of 690 variant alleles were detected, from 2 to 12 per locus, with an average of 5.45, as well as 30 rare alleles. The clustering relationship divided the pear genotypes into three groups, with the primary division between occidental and oriental pears, revealing separate evolution processes, followed by division of Pyrus ussuriensis, Pyrus pyrifolia, and Pyrus bretschneideri. Population structure analysis with K values of 2 to 8 reflected a clear genetic composition within different genotypes, supporting Pyrus sinkiangensis as a hybrid of oriental and occidental pears and P. pyrifolia and P. bretschneideri sharing a common ancestor. However, the division of genetic components also revealed separate evolution at the different geographic and environmental conditions of South China and North China. The varieties “Pingguoli” and “Chaoxianyangli,” which currently have controversial classification, were classified into P. bretschneideri and Pyrus communis, respectively. A core collection of 88 accessions was chosen, covering all of the rare alleles and 95.54 % of all alleles. The high-quality and comprehensive evaluation of a wide range of pear cultivars by core SSR markers covering the whole genome demonstrated their excellent application for the study of genetic diversity, genetic relationships, and building a core collection for pear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pear (Pyrus spp.) is one of the most important temperate fruit trees, with the third largest growing area worldwide, after apple and grape, and has a long cultivation history (Oliveira et al. 1999). Pear belongs to the genus Pyrus, in the family Rosaceae, and originates from China, with three known secondary centers of origin: the Chinese center, the Central Asian center, and the Near Eastern center (Vavilov 1951). According to its origination and distribution, pear germplasm has mainly been divided into two categories, known as occidental pears (European pear) and oriental pears (Asian pear) (Bailey 1919). Occidental pears include more than 20 populations, mainly distributed in Europe, North Africa, Asia Minor, Iran, Central Asia, and Afghanistan. Oriental pears contain 13 populations with about a thousand cultivars (Rubtsov 1944). The division of pear into populations has been studied over years by many researchers but remains ambiguous, with at least 22 widely recognized primary species (Challice and Westwood 1973). However, the main cultivated species of pear are commonly divided into 5 populations: Pyrus ussuriensis, Pyrus pyrifolia, Pyrus bretschneideri, Pyrus communis, and Pyrus sinkiangensis (Pu 1988). With long-term natural selection and genetic flow, modern cross-breeding between different species, and self-incompatibility, many cultivars show highly complex genetic backgrounds, undoubtedly making it very difficult to classify pear resources.
Pear classification has been studied since the 1920s, when the methods mostly relied on were geographic distribution and pear morphological taxonomy (Bailey 1919; Rehder 1940). Results showed great differences between occidental and oriental pears. More accurate methods arose such as palynology and chemical components in the 1970s (Westwood and Challice 1978; Challice and Westwood 1973) and enzymatic analysis from the 1980s to the 1990s (Lin and Shen 1983), but still, all these methods are easily affected by the environment, especially when using morphology characteristics to study the diversity of pear. Additionally, with the increase in new cultivars and quantities of pears, those methods could not meet the requirement of identifying the diversity of cultivars with similar morphological traits. With the rapid development of science and technology, molecular markers were soon being used for many of species, including pear. The development of molecular techniques has allowed ready access to the information provided by DNA, which is stable regardless of environment, tissue, and growth stage. There are many types of molecular markers, such as AFLP (Teng et al. 2001), RFLP (Kim et al. 2002; Castillo et al. 2001; Takasaki et al. 2004), RAPD (Chen et al. 2005; Ahmed et al. 2012), ISSR (Monte-Corvo et al. 2000), IRAP (Sun et al. 2015), SRAP (Xu et al. 2011), and simple sequence repeat (SSR) (Fan et al. 2013; Song et al. 2014) which provide a good choice for genetic diversity analysis and resource classification. With the excellent characteristics of polymorphism, reproducibility, and codominant nature, the diversity of SSR markers are better for genetic diversity analysis, molecular marker development, marker-assisted selection (MAS), fingerprinting, map construction, and comparative studies in several plant families, including Poaceae (Huang et al. 2012), Moraceae (Saleh 2013), Rosaceae (Pluess and Stöcklin 2004), Brassicaceae (Chen et al. 2005), and pear. For example, six SSR loci were used to evaluate the diversity of 98 pear cultivars native to East Asia and occidental pears generally had low affinities to Asian pears (Bao et al. 2007). One hundred thirty-four SSR markers with high polymorphism were developed to study the genetic variability and relationships of 99 P. pyrifolia cultivars, and it has been shown that pears from Yangtze River Basin and Japan had a close relationship (Song et al. 2014). A high-density linkage map was firstly constructed by 98 SSR markers and other markers with a total of 32 potential QTLs for 11 traits identified and positioned on the genetic map (Wu et al. 2014). A high-density genetic linkage map consisting of 734 loci distributed along all 17 linkage groups was constructed, with a total length of 1661.4 cM and an average marker interval of 2.26 cM with 894 SSRs covering the whole genome of pear (Chen et al. 2014). Sixty-seven SSR markers selected from the pear genome with good transferability were applied to construct comparative mapping between seven other Rosaceae species (Fan et al. 2013). Recently, the genome data for many species has been released and the markers selected from genome-wide data can provide favorable access for evaluating genetic diversity; for example, after the release of apple genome, researchers such as Khan et al. (2012) and Costa et al. (2010) developed a number of SNP markers. The genome data for pear “Dangshansuli” (P. bretschneideri) was released in 2012 by our lab, providing an invaluable new resource for developing new SSR markers covering the whole genome. The SSR markers developed based on genome-wide SSR loci would be a favorable method to evaluate the diversity of pear germplasm and identify the relationships of pear.
Recently, genetic structure has been increasingly concerned on germplasm collection, protection, and utilization in some species, such as wheat (Hao et al. 2011), rice (Zhang et al. 2011), grape (Emanuelli et al. 2013), banana (de Jesus et al. 2013), rye (Bolibok-Brągoszewska et al. 2014), soybean (Dong et al. 2014), etc. Estimating population structure is important for avoiding false genetic trends and identifying good alleles and for identifying cultivars with specific or minor alleles that will be important for molecular breeding programs. However, as far as we know, not enough information on the population structure of pear collections is available or has been assessed with a large and comprehensive set of SSR markers, besides the smaller populations used by Song et al. (2014) with 99 P. pyrifolia and by Rana et al. (2015) with 48 pear accessions belonging to six species.
Therefore, despite the abundant research on pear germplasm by SSR markers, most relied on a small number of markers with few cultivars and were non-representative for all species, the genetic structure of many pears remained unknown. In this study, we used a set of 134 pairs of genome-wide core SSR markers covering all 17 chromosomes of pear to study 385 genotypes covering 5 populations and spread over all production regions, which contained 127 P. pyrifolia, 90 P. bretschneideri, 57 P. ussuriensis, 8 P. sinkiangensis, 61 P. communis, and 42 interspecific hybridization cultivars, in order to evaluate the diversity of pear germplasm, reveal the population structure, and construct a preliminary core collection. The results will be useful for making rational and scientific conservation and management strategies for pear germplasm.
Materials and methods
Plant materials
A total of 385 accessions, collected from Chinese National Pear Germplasm Repository in Wuhan, Jiangpu, a pear resource depository in Nanjing Agricultural University, were used in this study, including 127 P. pyrifolia (sand pear), 90 P. bretschneideri (white pear), 57 P. ussuriensis, 8 P. sinkiangensis, 61 P. communis, and 42 interspecific hybridization cultivars (Table 1). These cultivars were chosen based on their economic value and geographical distribution. Young leaves of different accessions were collected in spring 2013.
DNA extraction and quality determination
DNA extraction from the young leaves was conducted using the improved CTAB method (Yan et al. 2008). The quality of DNA was tested on 0.8 % agarose gels (Invitrogen, China) and then stored in a freezer at −80 °C until use in subsequent experiments. NanoDrop 2000 (Thermo Scientific, USA) was used to detect the concentration, and then all DNA was diluted to 30–50 ng/μL for PCR amplification.
SSR markers and PCR amplification
Of the 134 pairs of SSR markers, 81 are from pear genome (http://peargenome.njau.edu.cn; Fan et al. 2013; Song et al. 2014; Chen et al. 2014), 46 are from apple genome, and 7 are from pear derived from expressed sequence tag (EST) (Yamamoto et al. 2002; Song et al. 2014). The marker selection steps were according to the article of Chen et al. (2014) and Song et al. (2014).
PCR reactions were carried out in a 10 μL volume containing 30 ng genomic DNA template, 1 μL of 10× PCR buffer (Mg2+ contained), 0.1 μL each of forward and reverse primer (10 pmol/L), 0.25 μL of 10 Mm dNTP, and 0.1 μL of 5 U/μL Taq polymerase (Takara Biotechnology Company, Dalian, China) with the protocol described by Zhang et al. (2014). PCR products (10 μL) were mixed with 2.0 μL formamide loading buffer (98 % formamide, 10 mM EDTA, 0.25 % bromophenol blue, 0.25 % xylene cyanol, pH 8.0) and 1.3 μL of each mixture (each PCR product (10 μL) mixed with formamide loading buffer (2.0 μL)), and two molecular size markers of 100 and 20 bp DNA ladders were loaded onto an 8 % non-denaturing polyacrylamide gel in 1× TBE buffer (Tris-borate, EDTA, pH 8.0) and then run at 200 V for 2–2.5 h and visualized using the silver staining protocol described by Bassam et al. (1991).
Data collection
The 100 and 20 bp DNA ladders (Dye Plus, DNA MW Standard Marker; Takara Biotechnology Co., Ltd., Dalian) were used as standard sizes to measure allele sizes, as SSR allelic composition was determined as codominant markers, and only clear and distinct bands were used for further analysis. Different putative alleles were represented by different letters in alphabetical order according to size.
Polymorphism and population structure analysis
To evaluate the genetic diversity of the cultivars, observed number of alleles (N a), effective number (N e), expected heterozygosity (H e), observed heterozygosity (H o), Shannon information index (I), and Wright’s fixation index (F is) were calculated with PopGene software (version 2.0) (Yeh et al. 1997) and pairwise differentiation among subpopulations (F ST) was calculated with Weir and Cockerham estimator (Antao et al. 2008) (Table 2).
The MEGA4.0 (Tamura et al. 2007) program was used to evaluate genetic relationships based on information from all polymorphic markers. The model-based Bayesian clustering method STRUCTURE 2.3 software (Pritchard et al. 2000) was used to identify the number of populations, capturing the major structure in data and for construction of a population structure. The parameters burn-in period and Markov chain Monte Carlo (MCMC) were set as 10,000 and 100,000, respectively. The average value of ln likelihood when K changed from 2 to 8 was calculated according to their genetic similarity, with each K value run at least ten times (Song et al. 2014), while genetic relationships between genotypes were analyzed by principal coordinate analysis (PCA) with NTSYSpc2.11 software (Nirgude et al. 2014) to determine the optimal number of genotypes in our study.
Construction of core collection
A core collection is based on the least number of accessions representing the most genetic diversity within the population. PowerCore v. 1.0 (Kim et al. 2007) was used to construct a core collection. In this study, two methods of random and nonrandom modes were chosen to analyze the genetic polymorphism of 385 genotypes. Both modes were used to construct the core collection, and then the better one was selected by comparing the preservation of alleles, particularly the rare alleles (Table 3). Jaccard’s coefficient value was used to visualize the relationships between core collection and original germplasm with the NTSYSpc program (version 2.10s) (Rohlf 1998), and the results calculated using the variance-covariance matrix of allele frequencies. The software SPSS 18.0 was used to calculate the parameters with the t value.
Results
High polymorphism of 385 pear accessions revealed by SSR markers
A set of 134 core SSR markers evenly distributed in 17 chromosomes was used to identify 385 pear accessions. All of the SSR markers produced clear bands and effectively analyzed the polymorphism of pear germplasm. In this study, 690 alleles, ranged from 2 (NAUpy07s) to 12 (NAUpy81c), were produced by the core SSR markers, with an average of 5.45 alleles per marker. The allele frequency was different from each SSR locus, strongly supporting the evidence explaining the effective number of alleles from different loci. A total of 30 (4.7 % of all 629 alleles) rare alleles with a frequency of less than 5 % were identified, indicating that many rare alleles were present in these pear collections and that the accessions in our research represented high pear germplasm biodiversity. The Shannon information index (I) varied from 0.83 of NAUpy23f to 1.96 of NAUpy16m, with an average of 1.52; the observed heterozygosity (H o) varied from 0.41 of NAUpy87s to 0.86 of NAUpy36E, with an average of 0.74; the expected heterozygosity (H e) varied from 0.41 of NAUpy87s to 0.83 of NAUpy36E, with an average of 0.73; Wright’s fixation index (F is) varied from −0.20 of NAUpy23F to 0.85 of NAUpy60E, with an average of 0.23, implying that inbreeding increased among populations (shown in Table 2). A higher value of H o than H e observed in one locus (NAUpy23f) resulted in negative Wright’s fixation index (F is) values, indicating a slight excess of heterozygosity for this locus. The pairwise differentiation among subpopulations (F ST) was estimated at each locus for all individuals, and the values varied from 0.0144 of CH02H11A to 0.2812 of NAUpy65d, with an average of 0.1368. The mean F ST values of the four populations (P. pyrifolia, P. bretschneideri, P. ussuriensis, and P. communis) were 0.1440, 0.1438, 0.1363, and 0.1229, respectively, indicating a slight genetic variation between populations.
Cluster analysis revealed three distinct groups of 385 accessions
The dendrogram of 385 pear cultivars was constructed with the software MEGA4.0. In Fig. 2, all the cultivars were divided into two clusters, with occidental pear separated out from oriental pear. The oriental pears were further divided into two subclusters, with P. ussuriensis separated out from P. pyrifolia and P. bretschneideri.
Cluster I contained 69 cultivars including all the 61 P. communis, 2 P. sinkiangensis (“Shayidongheisuanli” and “Linxiagadiaodan”), and 6 other cultivars (“Hongtaiyang,” “Zaosu,” “Jinxiangli,” “Wujiuxiang,” “Bayuehong,” and “Zaojinsu”) which are hybrid cultivars with one parent or grandparent belonging to P. communis. In this cluster, the cultivars of P. communis from America and Europe showed a mixed genetic background with no distinct genetic distance and having a better gene flow. “Bartlett” and its bud mutation “Red Bartlett” were clustered together, but “Doyenne du Comice” and the deduced bud mutation variety named “Early red Doyenne du Comice” were distinct from each other. The Early red Doyenne du Comice was clustered in the group of “Clapp’s Favorite,” indicating a close relationship of the two cultivars. The ambiguous cultivar “Chaoxianyangli” was clustered in this cluster, confirming that the cultivar has a high genetic component from occidental pear.
Cluster II included 127 P. pyrifolia, 90 P. bretschneideri, 57 P. ussuriensis, 6 P. sinkiangensis, and 36 interspecific hybridization cultivars. This cluster was divided into two subclusters, I and II. Subcluster I contained 57 P. ussuriensis and 11 interspecific hybridization cultivars that were hybrids of P. ussuriensis. At the same time, P. ussuriensis from Northwest China was clustered together with cultivars from North China, indicating that genetic communication occurred between cultivars originating from areas near to each other. Subcluster II contained 90 P. bretschneideri, 127 P. pyrifolia, 6 P. sinkiangensis, and 25 interspecific hybridization cultivars, confirming the previous hypothesis that P. pyrifolia and P. bretschneideri originated from the same ancestor and gene flow has occurred. Six P. sinkiangensis varieties, “Zhangyechangba,” “Guidechangba,” “Changbazi,” “Lanzhouchangba,” “Huachangba,” and “Kuerlexiangli,” were clustered with some varieties of P. bretschneideri and P. pyrifolia. In subcluster II, “Huanghua” and its bud mutation “Daguohuanghua” were clustered together but “Nanguoli” and “Dananguo” were separated. “Mili” and “Qiubaili” were clustered with “Pingguoli,” while “Huangguan” and “Zaokui” were clustered with “Xuehuali,” indicating that the filial generation has a close relationship with their parents. The controversial cultivar Pingguoli, which has been considered as either P. pyrifolia or P. bretschneideri in previous studies, was clustered together with most cultivars of P. bretschneideri, indicating a closer genetic relationship. At the same time, varieties from Korea such as “Whangkeumbae” and “Wonhwang” and varieties from Japan such as “Kousui” and “Choju” were all clustered together with Chinese P. pyrifolia, which is distributed in the Yangtze River Basin, indicating that P. pyrifolia originating from Japan and Korea might share an ancestor with the cultivars from the Yangtze River Basin.
The population structure declared the clear relationship of accessions and species of pear
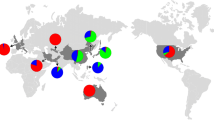
The codominant nature of the core SSR markers was used to analyze the structure of the populations using the model-based Bayesian clustering method. In order to analyze the population genetic structure of the pear germplasm, STRUCTURE (v. 2.3.4) was used with different K values from 2 to 8 to reveal the genetic components of the population (Fig. 1).
All the 61 cultivars in P. communis, 2 cultivars of “Shayidongheisuanli” and “Linxiagadiaodan” in P. sinkiangensis, and 6 hybrid cultivars with 1 parent or grandparent cultivar belong to P. communis. Subcluster 1 refers to 57 cultivars in P. ussuriensis and 11 interspecific hybridization cultivars. Subcluster 2 refers to 90 cultivars in P. bretschneideri, 127 cultivars in P. pyrifolia, 6 cultivars in P. sinkiangensis, and 25 interspecific hybridization cultivars
When K = 2, all accessions were divided into two populations, Pop1 and Pop2, with Pop1 containing all occidental pears and a few interspecific hybridization cultivars, all sharing the genetic background of occidental pear, and with Pop2 containing all cultivars of oriental pear. This structure indicated a great difference in genetic components between occidental and oriental pears. It was interesting to find out that occidental pears were all clustered together in Pop1 with no distinctive variation with increasing K values; however, the oriental pear was divided into different subpopulations. With the increase of K to 3, the accessions were divided into three populations, with oriental pear divided into two populations (Pop2 and Pop3). P. ussuriensis and closely related species were separated out from P. pyrifolia and P. bretschneideri, while Pop2 contained most of P. pyrifolia and P. bretschneideri, along with the cultivars of P. sinkiangensis and some interspecific hybrid cultivars. Pop3 contained all P. ussuriensis cultivars and some cultivars of P. pyrifolia, P. bretschneideri, and some interspecific hybrids that have a close relationship with P. ussuriensis. In this grouping, gene introgression in the three populations was clearly revealed and showed a great amount of gene flow within and among populations, especially the oriental pear species. When K = 4, all accessions were divided into four parts, which largely clustered with accessions within species range, except for P. sinkiangensis. In this population structure, P. pyrifolia was separated out from P. bretschneideri. Pop2 contained most of P. pyrifolia and several varieties of P. bretschneideri, such as “Daguochuanli,” “Yunnanhongxiangsu,” and “Jinchuanxueli,” originating from South China, and the varieties from Japan and Korea. Pop3 contained most P. bretschneideri and several P. pyrifolia such as “Nanpingli” and “Linxiaxiangshuili,” which mostly originated from North China. Numerous varieties in these two populations share similar genetic components, with some cultivars intersecting each other because of their distribution areas. The information indicated that over evolution, great gene flow occurred between P. pyrifolia and P. bretschneideri. Particularly, varieties belonging to P. pyrifolia but cultivated in North China communicated with P. bretschneideri and varieties belonging to P. bretschneideri but cultivated in South China communicated a lot with P. pyrifolia. These results confirmed that varieties distributed in the same area, with the same climate and soil nutrient status, might gradually evolve into having similar gene components, either due to natural hybridization or human intervention. When K = 5, P. pyrifolia was divided into two populations (Pop2 and Pop3), mainly reflecting their geographic distribution. Pop2 contained the cultivars of P. pyrifolia mostly originating from South China, while Pop3 contained the other cultivars of P. pyrifolia, mostly originating from the middle and lower reaches of the Changjiang River, Japan, and Korea, and their hybrid cultivars. The result indicated that geographic barriers might restrict gene flow of different cultivars, even if the cultivars came from the same species. When K = 6, P. bretschneideri was divided into two populations (Pop5 and Pop6) based on different areas of origination. Pop5 contained P. bretschneideri mainly originating from Northeast China, such as “Yali,” “Beifeng,” and Mili, while Pop6 contained P. bretschneideri mostly originating from the Yellow River Basin and the west, such as “Mianbaoli,” Zaosu, “Chili,” and “Jinli.” Most cultivars in these two populations had a complex genetic relationship with the other species, indicating an extensive gene exchange between different cultivars. When K = 7, the cultivars from North China were clustered together in a single population including some P. bretschneideri, P. pyrifolia, and interspecies hybrids, indicating great gene flow between different cultivars from the same area, and the environment and geographic traits are important factors in structure components. When K = 8, the cultivars originating from Japan and Korea were separated out from other P. pyrifolia from China, the differences among them also gave us a better understanding that geographical location would be an important factor affecting gene flow between different cultivars.
We saw that, at K = 2, two cultivars of P. sinkiangensis (Shayidongheisuanli and Linxiagadiaodan) were clustered with occidental pears and the other six (Lanzhouchangba, Zhangyechangba, Guidechangba, Changbazi, Huachangba, and Kuerlexiangli) with oriental pears and that the genetic background of all eight cultivars contained gene structures of both occidental and oriental pears, suggesting a close relationship of P. sinkiangensis with occidental as well as oriental pears. When K was 4, the cultivars Zhangyechangba and Kuerlexiangli were clustered together with most of P. bretschneideri; the cultivars Lanzhouchangba, Guidechangba, and Huachangba were clustered with P. pyrifolia; the cultivar Changbazi was clustered with P. ussuriensis; and the genetic components of each variety shared all four genetic backgrounds. Then, with the increase of K to 5, the cultivars Lanzhouchangba, Guidechangba, and Huachangba were all clustered together with the P. pyrifolia, originating from South China, and with the increase of K to 6, Zhangyechangba and Kuerlexiangli were clustered together with most cultivars of P. bretschneideri from North China. Genetic components of the eight varieties of P. sinkiangensis displayed in our study showed complicated genetic components including P. communis, P. bretschneideri, P. ussuriensis, and also P. pyrifolia, revealing that it had experienced much gene flow with occidental and oriental pears.
In genetic population structure, Pingguoli was clustered together with oriental pear in the first group of K = 2 and then, with the increase of K values, it was clustered with P. bretschneideri. The genetic components were similar with most P. bretschneideri and also contained a genetic background from P. pyrifolia, but little from P. ussuriensis, suggesting a close relationship of Pingguoli and P. bretschneideri, and also, a lot of gene communication has occurred in it. Chaoxianyangli was clustered together with most occidental pears and contained a genetic background from P. pyrifolia and P. bretschneideri, indicating a closer relationship between Chaoxianyangli and P. communis.
According to the results of our structure analysis, with the increase of K value, the pear accessions clustered with stratification and each increased population gave a better interpretation of the evolution of pear. However, with higher K values, these accessions were clustered into distinct clusters, better reflecting the genetic exchange between pears from different varieties. As with the increase of K value, the cultivars were divided into smaller populations based on geographical distribution, which was strong evidence for identifying the relationships between different pear cultivars and better analyzing the gene flow and evolution of pear germplasm.
Principal component analysis revealed a similar pattern with population structure and cluster analysis
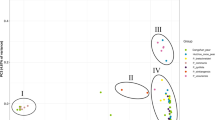
Principal component analysis (PCA) was also performed to analyze the genetic relationship and population structure. Most accessions were divided into three groups along the circles (Fig. 3), although there were some redundant or overlapping cultivars. The most aggregates among the three populations were P. pyrifolia, P. bretschneideri, P. ussuriensis, and P. communis, in accordance with STRUCTURE and cluster analysis. P1 (the yellow circle) contained most P. bretschneideri and P. pyrifolia, P2 (the red circle) contained most P. ussuriensis, and P3 (the blue circle) contained most P. communis. There were also some varieties of genotypes scattering out of the circles, giving us a better understanding of the higher diversity of the pear genome and the close genetic relationship between each population.
Finally, we found that the phylogenetic tree based on MEGA, population structure analysis, and PCA all strongly supported that pear varieties have high diversity but genes have been communicated between different clusters. All the results from the above support that pear has three well-differentiated genetic populations.
Eighty-eight genotypes selected as the core collection of 385 pear accessions
The main purpose of constructing a core collection is to have a small quantity of accessions to represent the maximum genetic diversity and avoid repetitiveness. The random method selected 88 accessions to represent all 385 pear collections and contained 10 P. communis, 30 P. bretschneideri, 30 P. pyrifolia, 1 P. sinkiangensis, 11 P. ussuriensis, and 6 interspecies. The alleles in those 88 accessions covered all rare alleles and 95.54 % of all alleles (Table 4). The nonrandom method was used to select 52 accessions, only including 6 P. communis, 14 P. bretschneideri, 20 P. pyrifolia, 6 P. ussuriensis, and 4 interspecies, covering 80.46 % of rare alleles and 86.23 % of all alleles; however, these cultivars did not comprehensively represent the alleles and rare alleles and did not give us an optimum result. A core collection of 88 cultivars representing all 385 pear collections covering all rare alleles was developed, and it has been indicated that the core collection can be used to identify the pear diversity (Table 5). Abundant genetic diversity was detected by 88 pear genotypes based on 134 SSR markers, with a total of 601 alleles detected ranging from 2 to 11 per locus. The average value of the Shannon information index (I) was 1.49, the average value of the observed heterozygosity (H o) was 0.73, the average value of the expected heterozygosity (H e) was 0.70, and the average value of Wright’s fixation index (F is) was 0.18. Then, the SPSS 18.0 software was used and the parameters of I, H o, H e, and F is were detected by t value, showing that no significant difference occurred between the 385 pear germplasm and 88 core collections. The results also indicated that the core collection of 88 can represent the 95.54 % of the total diversity of all pear germplasm in our study.
Identification of the molecular fingerprinting among different accessions
Besides the polymorphism of different accessions revealed by SSR markers, the specific or rare alleles identified could be used as markers to distinguish the different genotypes. The fingerprinting of 385 pear accessions was constructed based on the fragment diversity developed from 134 SSR markers. The result from 30 randomly selected pear accessions by eight SSR markers (Table 2) revealed that a combination of at least two markers could be used to identify the genotypes. For example, the marker ‘NH039a” could distinguish the genotypes “Baipisu,” “Hanfu,” Chili, “Daaoao,” “Youli,” “Fanshan,” Hongtaiyang, “Hongxiao,” “Jinhua No. 4,” Jinli, “Jinxing,” “Jinzhui,” “Qixiadaxiangshui,” and “Xiaobaixiao”; combined with the marker “NAUpy20b,” all other cultivars could be separated. Using the same method, a total of 23 selected markers (NAUpy97a, CH01D09, CH03E03, CH05F04, NAUpy81c, NAUpy45b, NAUpy58b, NAUpy27D, NAUpy45d, CH05A04, CN863717, CN875141, NAUpy53k, NAUpy16m, NAUpy17M, HI04A05, NAUpy25n, NAUpy47N, NAUpy63n, NAUpy52s, NAUpy26s, NAUpy10U, and NH39A) could clearly distinguish all 385 pear accessions. The fingerprinting of 385 pear cultivars with different SSR markers can act as individual allele patterns and has potential application for further cultivar identification.
Discussion
Polymorphism and heterozygosity of pear resources evaluated by a set of SSR markers
In previous studies, SSR markers used to evaluate pear resources were mainly developed from the apple genome and the available markers developed from pear were still few and usually only scattered in a few linkage groups (Bao et al. 2007; Fernandez-Fernandez et al. 2006; Terakami et al. 2009). However, with the increasing amount of new cultivars and some controversial pear varieties, these limited SSR markers did not meet the requirement of evaluating the diversity of pear resources. In this study, 134 high polymorphic core SSR markers distributed over the whole pear genome were chosen to analyze the diversity of 385 pear varieties and evaluate the polymorphism of SSR markers.
Marker polymorphism is an important factor for evaluating germplasm diversity, and high marker polymorphism is important. In previous studies of pear, 277 alleles were detected on 7 pear genotypes by 67 SSR primer pairs with an average of 4.13 alleles per locus (Fan et al. 2013) and 311 alleles were detected by 108 EST-SSR primers with dinucleotide and trinucleotide motifs, with an average of 2.88 and 2.86 alleles per locus, respectively (Zhang et al. 2014). Other studies found 65 putative alleles generated by 7 primer pairs with a mean of 9.2 (Yamamoto et al. 2002); 173 different alleles were detected based on 14 loci with an average of 12.4 alleles per locus (Zong et al. 2014), and 133 putative alleles were detected by 9 SSR markers with a mean of 14.8 per locus (Kimura et al. 2002). In our study, all 134 SSR markers amplified 629 alleles, with a mean of 5.45 per locus, and also detected 30 rare alleles.
There was a higher average value of the detected alleles than previous studies of pear, possibly because our core SSR markers could comprehensively detect the distinctive bands distributed in each variety and thus find out a lot of alleles. Of many markers used in our study, some only detected a small number of alleles, bringing the average down, so some other studies have detected more alleles. All the results indicate that a core set of SSR markers distributed genome-wide is reasonable and necessary in comprehensive evaluation of germplasm genetic diversity. In addition, pear self-incompatibility, prior long cross-pollination history, and the different genotypes of pollination widespread among the pear cultivars caused high heterozygosity and the high heterozygosity was always measured by the observed heterozygosity (H o) in the previous studies; for example, 142 apple accessions were studied with the value of H o at 0.62 (Hokanson et al. 2001), 28 peach accessions were studied with the value of H o at 0.28 (Sosinski et al. 2000), 106 pear accessions were evaluated by 9 SSR markers with the value of H o at 0.6 (Cao et al. 2011), and 92 P. bretschneideri accessions were evaluated by 10 SSR markers derived from apple and pear with the average value of H o at 0.61 (Tian et al. 2012), and in our study, there were 385 pear accessions with H o of 0.72. The low value in peach might be because of self-pollination, while in apple, it might be because of the restricted materials or markers. Otherwise, the SSR markers derived from the pear genome could detect more polymorphic loci than the pear derived from apple and the amount of SSR markers would also be an important factor.
The inbreeding coefficient F is ranged from −0.23 to 0.85, with an average of 0.23. The high F is values greater than 0.5 were only detected in three loci, and the average F is value was also quite low, which were similar with the results reported by Liu et al. (2012), suggesting no loss of heterozygosity and also proving the validity of our results. In addition, the same phenomenon was also detected in Tibetan poplar (Shen et al. 2014). However, in this study, the positive values of F is for different alleles imply the increased inbreeding among populations, which was very surprising in pear, because it is self-incompatible and thus always experiences heterologous hybridization. Iketani et al. (2010) considered the increased inbreeding arisen from human activities, such as selection, transportation, and propagation. Liu et al. (2012) attributed the positive F is values to the Wahlund effect resulting from the subpopulation structure or the existence of null alleles, which could increase the inbreeding index.
Generally, insect-pollinated and outcrossing species would have relatively low genetic differentiation, which supports the view that genetic diversity mainly exists within populations of outcrossed and widespread species (Hamrick et al. 1992; Liu et al. 2012). In our study, the similar genetic differentiation of P. pyrifolia and P. bretschneideri was in accordance with cluster analysis and PCA, and they were clustered together. However, the relatively low level of genetic differentiation of P. communis indicates that wide gene flow existed in the population, which forced it to become less differentiated than P. pyrifolia and P. bretschneideri. From PCA, we found distant relationships between P. communis and P. pyrifolia and P. bretschneideri populations, in accordance with the F ST values.
Genetic relationships of 385 pears
Overall, pear is a complex population with no obvious genetic differentiation and great amount of gene flow between different species. Gene flow among accessions influences the capability of STRUCTURE to correctly evaluate the genetic diversity and components of germplasm (Hubisz et al. 2009). Also, pear varieties with uncertain relationships exist, as has been reported by a number of studies (Yamamoto et al. 2001; Teng et al. 2002; Bao et al. 2008; Erfani et al. 2012; Song et al. 2014). Understanding the population structure is essential for efficient germplasm classification, protection, and utilization. The software STRUCTURE might be the most popular method to detect the diversity of germplasm; furthermore, combined with cluster analysis and PCA, it is an effective method to analyze genetic relationships, population structure, and structure components. Previous studies have used these methods on germplasm of different species, such as poplar (Shen et al. 2014), grape (Emanuelli et al. 2013), rice (Zhang et al. 2011), soybean (Dong et al. 2014), apple (Hokanson et al. 2001), and smaller sets of pear (Song et al. 2014; Bao et al. 2008; Zhou et al. 2013).
Occidental pears displayed lower levels of diversity compared to Asian species and have been inferred to derive from oriental species based on geographical spread (Zheng et al. 2014). In our research based on MEGA, the first cluster divided all 385 pear accessions into two populations: oriental pear and occidental pear, indicating that occidental and oriental pears have evolved independently for a long time and a great difference exists between them. This result was consistent with Bailey (1919), Bao et al. (2008), Erfani et al. (2012), and Yue et al. (2014), which considered that the two categories of occidental and oriental pears have a little similarity in the pear germplasm, supporting their separate evolution. Meanwhile, occidental pears were all clustered together regardless of geographical distribution, consistent with the previous research of Liu et al. (2015), who evaluated 45 P. communis based on 134 SSR markers and found that pears from America and Europe were all clustered together. The possible reason might be that the occidental pear pollinated without restriction of geographic distribution through self-incompatibility, encouraging gene exchange. Meanwhile, with the increase of K values, the material of occidental pears preserved a smooth and steady variation and showed a relatively clear gene background, all of which demonstrate the limited gene background of occidental pears and great gene communication and recombination among them. Meanwhile, according to the simplex material components of occidental and oriental pears, we could conclude that with the evolution of pear germplasm, the two branches were geographically and ecologically isolated. The same conclusion was also reached for other species such as soybean. The genetic structure of 100 cultivars was constructed by 53 SSR markers based on the STRUCTURE method, revealing that soybean germplasm in each classified group showed great consistency with their origins, seed coat colors, and pedigrees (Dong et al. 2014).
In oriental populations, P. ussuriensis was separated out from P. pyrifolia and P. bretschneideri, indicating many natural barriers limiting gene flow between P. ussuriensis and other oriental pears. Then, when K = 4, P. pyrifolia was separated out from P. bretschneideri. Previous studies found that P. pyrifolia and P. bretschneideri clustered intercross, supporting a common ancestor of these two species (Teng et al. 2002; Bao et al. 2008), and Teng et al. (2002) considered P. bretschneideri as a cultivated group or an ecotype of P. pyrifolia. However, the genetic division found in our study revealed that the two species separately evolved specific genetic components at different geographic and environmental conditions in South China and North China. At K = 8, P. pyrifolia formed three populations with cultivars from Japan and Korea, cultivars originating from South China, and cultivars originating from North China. P. bretschneideri also formed three populations with cultivars from the Yellow River Basin and the western region, cultivars from North China, and cultivars originating from East China. All these indicate that geographical and ecological divisions affect gene communication between different varieties.
P. sinkiangensis was a controversial species, previous studies have evaluated that some cultivars have a close relationship with P. communis and some have a close relationship with oriental pear (Teng et al. 2001; Pan et al. 2001; Lu et al. 2011). In our research, two cultivars of P. sinkiangensis clustered with P. communis and the other six clustered with oriental pear (Fig. 2), suggesting a complex genetic background of this species. Alternatively, combined with population structure (Fig. 3), the steady variation of genetic materials identified that P. sinkiangensis might be a hybrid offspring of occidental and oriental pears.
Population structure of 385 accessions with K of 2 to 8. Each individual was shown as a vertical line divided into segments representing the estimated membership proportion. Y-axis refers to the proportion of genetic background, and the height of each line with different colors represents the probability of varieties belonging to different genetic backgrounds
Principal component plots for 385 pear accessions based on 134 core SSR markers. Each integer point represents one cultivar, and dense integer points are circled. The yellow circle refers to most cultivars from P. pyrifolia and P. bretschneideri, the red circle refers to most cultivars from P. ussuriensis, and the blue circle refers to most cultivars from P. communis. X-axis and Y-axis refer to the first and second principal components, respectively
Chaoxianyangli has been a controversial variety, but clustered with P. communis in our study, agreeing with Cao and Qu (2006). Combined with the structural component showing that the cultivar also carries the genetic material of oriental pears, we conclude that Chaoxianyangli may be a hybrid of occidental and oriental pears. Pingguoli is a unique and desirable variety, as it looks like an apple, with morphological features similar to P. bretschneideri, such as the color of fruit and fresh branches, while other features such as the shape of fruit and leaves are just like P. pyrifolia, making its classification contentious. Previous studies on the taxonomic status of Pingguoli classified it to P. pyrifolia (Pu and Wang 1963), but the reverse conclusion also exists, classifying Pingguoli with P. bretschneideri (Song et al. 2014; Qu et al. 2001). In our study, Pingguoli clustered closely together with “Xinpingli” and other P. bretschneideri, and combined with the structure component, we found that most genome components of Pingguoli were in accordance with white pear. From this, we inferred that Pingguoli might be a variety of P. bretschneideri. Most of the cultivars share complicated genetic components, and classification is difficult; these results may solve the mystery of attribution of some controversial varieties but may not finally classify them into different species. Further studies including sequencing technology would be an effective method for analyzing controversial varieties. Some bud mutation cultivars did not cluster with their original cultivars, such as Huanghua and Daguohuanghua as well as Bartlett and Red Bartlett, consistent with a previous study (Lu et al. 2011), which indicates the limitation of simplex SSR markers, as they cannot detect any loci with a somatic mutation. Lu et al. (2011) also concluded that the SSR markers have little capacity to detect bud mutations, and the further studies with SRPA (Sun et al. 2015), SNP, or other markers would be better for identifying bud mutations. The cultivar Early red Doyenne du Comice, with an appearance like “Starkrimson,” a bud mutation of Clapp’s Favorite, was originally recognized as the bud mutation of Doyenne du Comice in China but did not cluster with Doyenne du Comice but with Clapp’s Favorite in our study, providing further evidence that the Early red Doyenne du Comice was not the bud mutation of Doyenne du Comice. The close relationship of Early red Doyenne du Comice and Clapp’s Favorite supported that the Early red Doyenne du Comice is a false name for Starkrimson or another bud mutant of Clapp’s Favorite.
A preliminary core collection of 385 pears
The essence of constructing a preliminary core collection is to use the minimum quantity of germplasm samples to represent the maximum genetic diversity of the species (Frankel 1984). Previous studies have confirmed that the number of alleles is a key factor for evaluating the genetic diversity, especially the rare alleles (Marshall and Brown 1975). A higher diversity of pear accessions would be more likely to capture the total genetic diversity with a small number of individuals, as was found for grape (Emanuelli et al. 2013). The 30 rare alleles retained in the core collection in our study indicated that our core collection is representative of most of the genetic polymorphism, especially as our accessions include all pear species, originating from China, Korea, Japan, Europe, and America. The core collection can help us efficiently select varieties with good properties, utilize and breed for further production, and then use an optimum method to select the representative varieties needed. In this study, two methods, random and nonrandom, were used for constructing a core collection, which were then compared for the ratio of covering rare alleles, covering all alleles, and polymorphism parameters. Finally, the random method was chosen to construct a core collection, although a previous study by Song et al. (2014) considered that the two methods were both appropriate for constructing a core collection and constructed two core collections of sand pear based on 99 cultivars of P. pyrifolia. Our larger core collection from the random method than nonrandom was in accordance with Song et al. (2014). The inconsistency between the two different methods might be ascribed to the different kinds and amounts of materials; with more materials and diversity, the comprehensive core collection should be constructed using the random method. The core collection of P. pyrifolia obtained in our study included 30 varieties, of which 6 originated from Japan, 2 originated from Korea, and 22 originated from China covering all of the production areas of sand pear, indicating the great genetic diversity of our collection. Comparing with the previous study of Song et al. (2014) who constructed a core collection of P. pyrifolia with 24 varieties using the nonrandom method and 32 varieties using the random method while the numbers of common varieties with our 30 varieties of core collection is 11 and 14, the reason might be that some different species studied in our study and their genetic diversity could be represented by other varieties. In the core collection, about 33 % (30 out of 90) of P. bretschneideri accessions and 24 % (30 out of 127) of P. pyrifolia accessions were in the core collection, larger than the P. communis of 16 % (10 out of 61), which revealed that the wide distribution of P. bretschneideri and P. pyrifolia determined their higher diversity than P. communis, which is distributed in a narrow area and had extensive genetic communication. Previous diversity studies of P. bretschneideri and P. pyrifolia have found that N a mainly varied from 9 to 20 (Zhang et al. 2007; Iketani et al. 2012) and the N a values of P. communis mostly varied from 5 to 7 (Erfani et al. 2012; Liu et al. 2015), clearly confirming the higher diversity of P. bretschneideri and P. pyrifolia.
Conclusion
This study constructs both a phylogenetic tree and builds a population structure to analyze the population diversity and phylogenetic relationships among the extensive collections of Pyrus species, based on a genome-wide core set of SSR markers. The results revealed that occidental and oriental pears are clearly distinguishable and cultivars from Japan and Korea might share an ancestor with P. pyrifolia, originating from China. Meanwhile, P. sinkiangensis had a genetic background of oriental and occidental pears and was determined to be a hybrid of both. Genetic structures of P. pyrifolia and P. bretschneideri supported a common ancestor for these two species; however, the division based on the increasing K value also revealed a separate evolution at different geographic and environmental conditions in South China and North China. The bud mutation Dananguo, from Nanguoli, was identified, and Early red Doyenne du Comice was not the bud mutant of Doyenne du Comice. The population structure gave us a better understanding of the genetic relationships and composition within different pear genotypes; meanwhile, a core collection was chosen to represent the composite diversity of pear germplasm. These conclusions give us evidence for further study of controversial pear cultivars. In addition, our genome-wide core SSR markers displayed high polymorphism, which is valuable for molecular breeding, investigation of population genetic diversity, and evolutionary studies among pears. The genotypes used and information obtained in this study can provide a guide for further exploration of pear genetic diversity and population structure as well as genome organization and evolution.
References
Ahmed N, Mir JI, Mir RR, Rather NA, Rashid R, Wani SH, Shafi W, Mir H, Sheikh MA (2012) SSR and RAPD analysis of genetic diversity in walnut (Juglans regia L.) genotypes from Jammu and Kashmir, India. Physiol and Mol Biol of Plants 18(2):149–160
Antao T, Lopes A, Lopes RJ, Beja-Pereira A, Luikart G (2008) LOSITAN: a workbench to detect molecular adaptation based on a F ST -outlier method. BMC bioinformatics 9(1):323
Bailey LH (1919) The standard cyclopedia of horticulture. Macmillan
Bao L, Chen K, Zhang D, Cao Y, Yamamoto T, Teng Y (2007) Genetic diversity and similarity of pear (Pyrus L.) cultivars native to East Asia revealed by SSR (simple sequence repeat) markers. Genet Resour and Crop Evol 54(5):959–971
Bao L, Chen K, Zhang D, Li X, Teng Y (2008) An assessment of genetic variability and relationships within Asian pears based on AFLP (amplified fragment length polymorphism) markers. Sci Hortic 116(4):374–380
Bassam BJ, Caetano-Anollés G, Gresshoff PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196(1):80–83
Bolibok-Brągoszewska H, Targońska M, Bolibok L, Kilian A, Rakoczy-Trojanowska M (2014) Genome-wide characterization of genetic diversity and population structure in Secale. BMC Plant Biol 14(1):184
Cao L, Qu B (2006) Study on the classification of four pear varieties by peroxidase isoenzyme. Hubei Agricultural Sciences 45(1):89–91 (in Chinese)
Cao Y, Tian L, Gao Y, Yang J (2011) Evaluation of genetic identity and variation in cultivars of Pyrus pyrifolia (Burm.f.) Nakai from China using microsatellite markers. J HorticScienc & Biotechnol 86(4):331–336
Castillo C, Takasaki T, Saito T, Yoshimura Y, Norioka S, Nakanishi T (2001) Reconsideration of S-genotype assignments, and discovery of a new allele based on S-RNase PCR-RFLPs in Japanese pear cultivars. Breed Sci 51(1):5–11
Challice JS, Westwood MN (1973) Numerical taxonomic studies of the genus Pyrus using both chemical and botanical characters. Bot J Linn Soc 67:121–148
Chen H, Song Y, Li L, Khan MA, Li X, Schuyler S, Korban SS, Wu J, Zhang S (2014) Construction of a high-density simple sequence repeat consensus genetic map for pear (Pyrus spp.). Plant Mol Biol Report 33:316–325
Chen S, Xia T, Chen S, Zhou Y (2005) RAPD profiling in detecting genetic variation in endemic Coelonema (Brassicaceae) of Qinghai-Tibet Plateau of China. Biochem Genet 43(3-4):189–201
Costa F, Peace CP, Stella S, Serra S, Musacchi S, Bazzani M, Sansavini S, de Weg WEV (2010) QTL dynamics for fruit firmness and softening around an ethylene-dependent polygalacturonase gene in apple (Malus × domestica Borkh.). J Exp Bot 61(11):3029–3039
de Jesus ON, Silva SO e, Amorim EP, Ferreira CF, de Campos JMS, de Gaspari Silva G, Figueira A (2013) Genetic diversity and population structure of Musa accessions in ex situ conservation. BMC Plant Biol 13(1):41
Dong D, Fu X, Yuan F, Chen P, Zhu S, Li B, Yang Q, Yu X, Zhu D (2014) Genetic diversity and population structure of vegetable soybean (Glycine max (L.) Merr.) in China as revealed by SSR markers. Genet Resour Crop Evol 61(1):173–183
Emanuelli F, Lorenzi S, Grzeskowiak L, Catalano V, Stefanini M, Troggio M, Myles S, Martinez-Zapater JM, Zyprian E, Moreira FM, Grando SM (2013) Genetic diversity and population structure assessed by SSR and SNP markers in a large germplasm collection of grape. BMC Plant Biol 13(1):39
Erfani J, Ebadi A, Abdollahi H, Fatahi R (2012) Genetic diversity of some pear cultivars and genotypes using simple sequence repeat (SSR) markers. Plant Molecular Biology Reporter 30(5):1065–1072
Fan L, Zhang M, Liu Q, Li L, Song Y, Wang L, Zhang S, Wu J (2013) Transferability of newly developed pear SSR markers to other Rosaceae species. Plant Mol Biol Reporte 31(6):1271–1282
Fernandez-Fernandez F, Harvey NG., James, CM (2006) Isolation and characterization of polymorphic microsatellite markers from European pear (Pyrus communis L.). Molecular Ecology Notes 1039–1041
Frankel OH (1984) Genetic perspectives of germplasm conservation. Genetic manipulation: impact on man and society. Cambridge University Press, Cambridge, 161-170
Hamrick JL, Godt MJW, Sherman-Broyles SL (1992) Factors influencing levels of genetic diversity in woody plant species. Population genetics of forest trees. Springer, Netherlands, 95-124
Hao C, Wang L, Ge H, Dong Y, Zhang X (2011) Genetic diversity and linkage disequilibrium in Chinese bread wheat (Triticum aestivum L.) revealed by SSR markers. PLoS One 6(2), e17279
Hokanson SC, Lamboy WF, Szewc-McFadden AK, McFerson JR (2001) Microsatellite (SSR) variation in a collection of Malus (apple) species and hybrids. Euphytica 118(3):281–294
Huang L, Zhang X, Xie W, Zhang J, Cheng L, Yan H (2012) Molecular diversity and population structure of the forage grass Hemarthria compressa (Poaceae) in south China based on SRAP markers. Genet Mol Res 11:2441–2450
Hubisz MJ, Falush D, Stephens M, Pritchard JK (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour 9:1322–1332
Iketani H, Yamamoto T, Katayama H, Uematsu C, Mase N, Ssto Y (2010) Introgression between native and prehistorically naturalized (archaeophytic) wild pear (Pyrus spp.) populations in Northern Tohoku, Northeast Japan. Conserv Genet 11(1):115–126
Iketani H, Katayama H, Uematsu C, Mase N, Sato Y, Yamamoto T (2012) Genetic structure of East Asian cultivated pears (Pyrus spp.) and their reclassification in accordance with the nomenclature of cultivated plants. Plant Syst Evol 298(9):1689–1700
Khan M, Han Y, Zhao Y, Korban S (2012) A high-throughput apple SNP genotyping platform using the GoldenGate™ assay. Gene 494(2):196–201
Kim HT, Hirata Y, Nou IS (2002) Determination of S-genotypes of pear (Pyrus pyrifolia) cultivars by S-RNase sequencing and PCR-RFLP analyses. Mol Cells 13(3):444–451
Kim KW, Chung HK, Cho GT, Ma KH, Chandrabalan D, Gwag JG, Kim TS, Cho EG, Park YJ (2007) PowerCore: a program applying the advanced M strategy with a heuristic search for establishing core sets. Bioinformatics 23(16):2155–2162
Kimura T, Shi YZ, Shoda M, Shoda M, Kotobuki K, Matsuta N, Hayashi T, Ban Y, Yamamoto T (2002) Identification of Asian pear varieties by SSR analysis. Breed Sci 52(2):115–121
Lin B, Shen D (1983) Studies on the germplasmic characteristic of Pyrus by use of isozymic patterns. Acta Agriculturae Universities Zhejiangensis 9(3):235–242 (in Chinese)
Liu J, Zheng X, Potter D, Hu C, Teng Y (2012) Genetic diversity and population structure of Pyrus calleryana (Rosaceae) in Zhejiang province, China. Biochem Syst Ecol 45:69–78
Liu Q, Song Y, Li J, Zhang M, Qi K, Zhang S, Wu J (2015) Analysis of genetic diversity of European pear (Pyrus communis L.) cultivars using core simple sequence repeat (SSR) markers. J Agric Bio 23(5):579–587 (in Chinese)
Lu J, Wu J, Zhang S, Wu H, Zhang Y (2011) Genetic diversity and polygentic relationship among pears revealed by SSR markers. Journal of Nanjing Agriculture University 34(2):38–46
Marshall DR, Brown AHD (1975) Optimum sampling strategies in genetic conservation. Crop genetic resources for today and tomorrow. 53-80
Monte-Corvo L, Goulao L, Oliveira C (2000) Discrimination of pear cultivars with RAPD, AFLPTM and ISSR. VIII International Symposium on Pear 596:187–191
Nirgude M, Babu BK, Shambhavi Y, Singh UM, Upadhyaya HD, Kumar A (2014) Development and molecular characterization of genic molecular markers for grain protein and calcium content in finger millet (Eleusine coracana (L.) Gaertn.). Mol Biol Rep 41(3):1189–1200
Oliveira CM, Mota M, Monte-Corvo L, Goulao L, Silva DM (1999) Molecular typing of Pyrus based on RAPD markers. Sci Hortic 79:163–174
Pan Z, Kawabata S, Sugiyama N, Sakiyama R, Cao Y (2001) Genetic diversity of cultivated resources of pear in north China. International Symposium on Asian Pears, Commemorating the 100th Anniversary of Nijisseiki Pear 587: 187-194
Pluess AR, Stöcklin J (2004) Population genetic diversity of the clonal plant Geum reptans (Rosaceae) in the Swiss Alps. Am J Bot 91(12):2013–2021
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Pu F (1988) Pear germplasm resource and its research. China fruit. (2):42-46 (in Chinese)
Pu F and Wang Y (1963) China fruit flora, vol. III (pear). Shanghai Scientific and Technical, Shanghai (in Chinese)
Qu B, Jin X, Chen Y, Liu H, Wang P (2001) RAPD analysis of germplasm resources in Pyrus. Acta Horticulturae Sinica 28(5):460–462
Rana JC, Chahota RK, Sharma V, Rana M, Verma N, Verma B, Sharma TR (2015) Genetic diversity and structure of Pyrus accessions of Indian Himalayan region based on morphological and SSR markers. Tree Genetics & Genomes 11(1):1–14
Rehder A (1940) Manual of cultivated trees and shrubs. 2nd. New York: Macmillan
Rohlf FJ (1998) NTSYS-pc version 2.0. Numerical taxonomy and multivariate analysis system. Exeter software, Setauket, New York
Rubtsov GA (1944) Geographical distribution of the genus Pyrus and trends and factors in its evolution. Amer, Naturalist 78:358–366
Saleh B (2013) Genetic diversity in Ficus sycomorus L. species (Moraceae) using RAPD and IRAP markers. Agriculture 59(3):120–130
Shen D, Bo W, Xu F, Wu R (2014) Genetic diversity and population structure of the Tibetan poplar (Populus szechuanica var. tibetica) along an altitude gradient. BMC Genet 15(Suppl 1):S11
Song Y, Fan L, Chen H, Zhang M, Ma Q, Zhang S, Wu J (2014) Identifying genetic diversity and a preliminary core collection of Pyrus pyrifolia cultivars by a genome-wide set of SSR markers. Sci Hortic 167:5–16
Sosinski B, Gannavarapu M, Hager LD, Beck LE, King GJ, Ryder CD, Rajapakse S, Baird WV, Ballard RE, Abbott AG (2000) Characterization of microsatellite markers in peach [Prunus persica (L.) Batsch]. Theor Appl Genet 101(3):421–428
Sun J, Yin H, Li L, Song Y, Fan L, Zhang S, Wu J (2015) Evaluation of new IRAP markers of pear and their potential application in differentiating bud sports and other Rosaceae species. Tree Genetics & Genomes 11(2):1–13
Takasaki T, Okada K, Castillo C, Moriya Y, Saito T, Sawamura Y, Norioka N, Norioka S, Nakanishi T (2004) Sequence of the S 9-RNase cDNA and PCR-RFLP system for discriminating S 1- to S 9-allele in Japanese pear. Euphytica 135(2):157–167
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24(8):1596–1599
Teng Y, Kenji T, Fumio T, Akihiro I (2002) Genetic relationships of Pyrus species and cultivars native to East Asia revealed by randomly amplified polymorphic DNA markers. J A MER Soc Hort Sci 127(2):262–270
Teng Y, Tanabe K, Tamura F, ITAI A (2001) Genetic relationships of pear cultivars in Xinjiang, China, as measured by RAPD markers. J Hortic Sci Biotechnol 76(6):771–779
Terakami S, Kimura T, Nishitani C, Sawamura Y, Saito T, Hirabayashi T, Yamamoto T (2009) Genetic linkage map of the Japanese pear ‘Housui’ identifying three homozygous genomic regions. Journal of the Japanese Society for Horticultural Science 78:417–424
Tian L, Gao Y, Cao Y, Liu F, Yang J (2012) Identification of Chinese white pear cultivars using SSR markers. Genet Resour Crop Evol 59(3):317–326
Vavilov NI (1951) The origin, variation, immunity and breeding of cultivated plants. Soil Sci 72(6):482
Westwood MN, Challice JS (1978) Morphology and surface topography of pollen and anthers of Pyrus species. J Amer Soc Hort Sci 103:28–37
Wu J, Li LT, Li M, Khan MA, Li X, Chen H, Yin H, Zhang S (2014) High-density genetic linkage map construction and identification of fruit-related QTLs in pear using SNP and SSR markers. Journal of Experimental Botany Eru 311
Xu A, Zhu L, Jia B, Heng W, Ye Z (2011) Primarily identification of pear variants and analysis of their genetic relationship by SRAP markers. Journal of Anhui Agricultural University 3:031
Yamamoto T, Kimura T, Sawamura Y, Kotobuki K, Ban Y, Hayashi T, Matsuta N (2001) SSRs isolated from apple can identify polymorphism and genetic diversity in pear. Theor APPl Genet 102:865–870
Yamamoto T, Kimura T, Sawamura Y, Manabe T, Kotobuki K, Hayashi T, Ban Y, Matsuta N (2002) Simple sequence repeats for genetic analysis in pear. Euphytica 124(1):129–137
Yan M, Guang C, Pan X, Ma H, Li W (2008) A method suitable for extracting genomic DNA from animal and plant modified CTAB method. Agricultural Science &Technology 9(2):39–41
Yeh FC, Yang RC, Boyle TBJ, Ye ZH, Mao JX (1997) POPGENE, the user-friendly shareware for population genetic analysis. Molecular Biology and Biotechnology Centre, University of Alberta, Canada, 10
Yue X, Liu G, Zong Y, Teng Y, Cai D (2014) Development of genic SSR markers from transcriptome sequencing of pear buds. J Zhejiang Univ Sci B 15(4):303–312
Zhang D, Shu Q, Teng Y, Chou M, Bao L, Hu H (2007) Simple sequence repeat analysis on genetic assessment of Chinese red skinned sand pear cultivars. Acta Horticulturae Sinica 34(1):47–52 (in Chinese)
Zhang M, Fan L, Liu Q, Song Y, Wei S, Zhang S, Wu J (2014) A novel set of EST-derived SSR markers for pear and cross-species transferability in Rosaceae. Plant Mol Biol Report 32(1):290–302
Zhang P, Li J, Li X, Liu X, Zhao X, Lu Y (2011) Population structure and genetic diversity in a rice core collection (Oryza sativa L.) investigated with SSR markers. PLoS One 6(12), e27565
Zheng X, Cai D, Potter D, Postman J, Liu J, Teng Y (2014) Phylogeny and evolutionary histories of Pyrus L. revealed by phylogenetic trees and networks based on data from multiple DNA sequences. Mol Phylogenet Evol 80:54–65
Zhou H, Liao J, Xia Y, Teng Y (2013) Determination of genetic relationships between evergreen azalea cultivars in China using AFLP markers. Journal of Zhejiang University Science B 14(4):299–308 (in Chinese)
Zong Y, Sun P, Liu J, Yue X, Li K, Teng Y (2014) Genetic diversity and population structure of seedling populations of Pyrus pashia. Plant Mol Biol Report 32(3):644–651
Acknowledgments
The work was financially supported by the Earmarked Fund for China Agriculture Research System (CARS-29), the Science Foundation for Distinguished Young Scientists in Jiangsu Province (BK20150025), the Ministry of Education Program for New Century Excellent Talents in University (NCET-13-0864), and the Six Talent Peaks Project in Jiangsu Province (2014-NY-025)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data archiving statement
The authors declare that all the work described in this manuscript followed the standard Tree Genetics and Genomes policy. All the primers used were in accordance with the article of Song et al. (2014).
Additional information
Communicated by D. Chagné
This article is part of the Topical Collection on Germplasm Diversity
Rights and permissions
About this article
Cite this article
Liu, Q., Song, Y., Liu, L. et al. Genetic diversity and population structure of pear (Pyrus spp.) collections revealed by a set of core genome-wide SSR markers. Tree Genetics & Genomes 11, 128 (2015). https://doi.org/10.1007/s11295-015-0953-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-015-0953-z