Abstract
Sour jujube (Zizyphus jujuba Mill. var. spinosus (Bge.) Hu) has gained considerable attention for its adaptation to drought prone environments. To characterize the physiological and biochemical basis of this drought adaptation, the effects of drought stress on Sour jujube seedlings were investigated in a greenhouse. Two contrasting populations were employed in our study, which were from the wet (YL) and dry (SB) climatic regions in the Loess Plateau of China. Results showed that SB exhibited lower water consumption and growth inhibition, but higher water use efficiency than YL under drought stress, indicating that growth of the wet-climate population is more sensitive to drought stress. SB exhibited higher non-photochemical quenching (NPQ) during progressive soil drying, higher photochemical quenching (qP) during the sustained water supply stage, and higher ΔF/\(F_{\rm m}^\prime\) and qP during a re-watering period than YL. These results further indicate that the dry-climate population possesses better PSII efficiency under adverse conditions. YL showed larger increases in the production rate of superoxide anions, hydrogen peroxide, and hydroxyl radicals than SB during the progressive soil drying stage, indicating that SB suffered from less oxidative damage than YL. Antioxidant enzymes including catalase, ascorbate peroxidase, peroxidase and glutathione reductase, and antioxidants including carotenoids, flavonoids and proline; when these interact, they contribute greatly to the antioxidant capacity of the dry-climate population. Taken together, the better photosynthetic potential and antioxidant capacity contribute to the better performance of Sour jujube from the dry-climate, providing useful information for understanding the drought tolerance mechanisms of Sour jujube.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water shortage is a major limiting factor for vegetation restoration in the loess hilly region of northwest China (Chen et al. 2007). Here the characteristic drought-prone climate results in infrequent precipitation, low soil moisture, and high levels of soil erosion (Liu et al. 2012). To improve the environment, many plantations of plant communities have been developed on the loess hilly region in the last few decades. However, little successful vegetation rehabilitation and natural regeneration has occurred in this region because of the serious water shortage (Sun et al. 2006). In contrast, natural vegetation recovery by local native species after land closure has provided much more successful ecological restoration, and results in increased vegetation coverage and biodiversity, as well as decreased soil erosion (Chen et al. 2007). Thus, researchers have placed increasing attention on these native species because they are already adapted to dry environments (Wang 2006; Zheng and Shangguan 2007; Shan and Liang 2010; An et al. 2013; Xu et al. 2014).
Drought stress can inhibit plant growth (Skirycz and Inzé 2010) and decrease leaf relative water content (RWC) which may induce stomatal closure and thus result in a parallel decrease in photosynthesis (Reddy et al. 2004). Drought directly inhibits photosynthesis because of decreased CO2 availability or alterations of photosynthetic metabolism (Chaves et al. 2009). Additionally, like other multiple stress conditions, drought often increases the production of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide radical (·O2 −), singlet oxygen (1O2), and hydroxyl radical (·OH) (Mittler 2002). ROS function as signal molecules in response to stress, but excessive ROS can damage plant cells (Mittler 2002). Plants have developed multiple strategies to avoid or tolerate drought stress. Plants can minimize water loss by closing stomata, decreasing leaf size or inhibiting the growth of aboveground parts (Kozlowski and Pallardy 2002). Plants may adjust their growth rate, such as a reduction in shoot height, as an early morphological response to avoid drought stress (Lei et al. 2006; Skirycz and Inzé 2010). Plants use harmless thermal dissipation of excess excitation energy through the xanthophyll cycle function to maintain photosynthetic processes under water stress; this is one of the major photo-protective responses of plants, and is usually estimated by measuring non-photochemical quenching (NPQ) (Demmig-Adams et al. 1996). In addition, plants have employed multiple mechanisms to modulate ROS metabolites. These mechanisms include the production of antioxidants, such as ascorbate (ASC), reduced glutathione (GSH), α-tocopherol, and antioxidative enzymes, such as superoxide dismutase (SOD), peroxidase (POX), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) (Mittler 2002). Responses of plant antioxidants and antioxidative enzymes to drought depend on the plant species and genotypes, the length and severity of water deficit, as well as the age and stage of plant development (Türkan et al. 2005; Chugh et al. 2011; Cia et al. 2012). Certain metabolites, such as proline (Szabados and Savoure 2010) and flavonoids, also exhibit a strong antioxidative ability (Treutter 2006).
Many studies have compared the different responses of plants to drought stress in dry- and wet-climates. In these comparisons dry-climate populations always exhibit reduced shoot height, growth rates, total biomass, leaf area, photosynthesis and transpiration while they have higher root/shoot ratios under drought stress conditions (Yin et al. 2004; Lei et al. 2006; Maatallah et al. 2010; Wang et al. 2012). In addition, dry-climate populations also exhibited higher abscisic acid (ABA) content and water use efficiency (WUE) than the wet-climate populations under drought stress condition (Yin et al. 2004; Lei et al. 2006; Maatallah et al. 2010). The morphological and physiological adaptations to water availability showed that wet-climate populations employ a prodigal water use strategy and have rapid growth, although a dry-climate population exhibits a conservative water use strategy and slow growth (Li 2000; Yin et al. 2004; Lei et al. 2006; Maatallah et al. 2010). These research studies indicate that mechanisms plants use to tolerate drought can be revealed by comparing the responses of different plant populations from contrasting water regions. However, little information is available related to the comparison between dry- and wet-climate populations that compare the aspects of photo-protective and antioxidant systems under drought conditions (Ren et al. 2007). Most studies of photo-protective and antioxidant systems have simply focused on desert plants (Pnueli et al. 2002; Xu et al. 2008; Bai et al. 2009), or on drought tolerant and sensitive crops (Türkan et al. 2005; Chugh et al. 2011; Cia et al. 2012) without comparing plants from mesic and xeric regions.
Sour jujube (Zizyphus jujuba Mill. var. spinosus (Bge.) Hu), a wild jujube species and a typical native shrub, is widely distributed on Loess Plateau in China (Fig. 1; Zheng and Shangguan 2007). In China, the seeds of Sour jujube are traditionally used as an herbal medicine with antitumor, sedative and hypnotic effects (Sun et al. 2011). The fruits and leaves are rich in triterpenoids, flavonoids, cerebrosides and phenolic acids (Yang et al. 2008; Sun et al. 2011). Furthermore, being a very cold and drought tolerant species as well as thriving in barren and saline-alkali soil, Sour jujube provides a good rootstock for cultivated jujube. Moreover, Sour jujube can perform an important function during ecological restoration as well as in soil and water conservation in loess hilly regions (Wang 2006; Wang et al. 2010). Thus, understanding the responses of Sour jujube to drought is of great importance and also provides fundamental data for the use of native species in drought-prone regions.
Photos of Sour jujube from the wet-climate region (a–c) show a high level of ground cover from these plants and from the dry-climate region (d–f) showing extensive bare loess soil with the plants. One photo (g) shows Sour jujube seedlings at the 6-leaf stage. Sour jujube plants are abundant in the loess hilly region (e)
Despite its ecological and economic value, the mechanism involved in the tolerance of Sour jujube to droughty environments has received relatively little attention. The loess hilly region of Shaanbei typically has a dry-climate (Table 1). Located in the northern part of the Loess Plateau, the conditions in this region mainly include drought, intense sunlight, low temperature, high elevation, and a lack of adequate soil nutrients (Chen et al. 2007). Unlike the northern hilly region, the southern has a wet-humid climate at low elevations with soil rich in mineral elements and with abundant water (Table 1; Li et al. 2010; Wang et al. 2010). Sour jujube can thrive in both wet and dry regions, but we believed that Sour jujube from Shaanbei was better adapted to drought than that from the southern region. To better understand the adaptive mechanisms of Sour jujube to drought-prone environments, we compared several physiological and biochemical traits of two Sour jujube populations from contrasting climates (Fig. 1) in this study, providing information on the use of this native species in drought-prone regions.
Materials and methods
Plant materials and treatments
To obtain the overall variation pattern of Sour jujube across two different environments on the Loess Plateau in Shaanxi Province, we selected two regions: Region I with a wet-climate population and Region II with a dry-climate population (Fig. 2), whose parameters are listed in Table 1. Three natural populations for each region were randomly selected, and ten individuals were sampled from each population. A minimum distance of at least 50 m was maintained between individuals to ensure different clones were analyzed. Finally, seeds from at least ten mature and healthy plants were collected for further experiments.
Annual precipitation (AP) and sampling point locations for both regions on the Loess Plateau of Shaanxi. Xi’an, the capital of Shaanxi province; Region I, the wet-climate region, three Sour jujube populations from Baoji, Yangling, and Zhouzhi; Region II, the dry-climate region, three populations from Ansai, Yan’an, and Zizhou
Seeds from Regions I and II are designed as YL and SB, respectively. Experiments were conducted in a greenhouse of the Institute of Soil and Water Conservation, Chinese Academy of Sciences and Ministry of Water Resources. The collected seeds were sowed in the field. When the seedlings grew to the 6-leaf stage, a total of 36 healthy and uniform seedlings of each population were selected and transplanted to 6 pots (27 cm in depth and 35 cm in diameter). That is, 108 seedlings were transplanted into 18 pots each region (SB or YL) after 13 kg of soil was filled into each pot. The soil had been obtained from native croplands, and was air-dried and sieved (0.5 mm), and evenly mixed. The soil contained total N of 0.97 g kg−1, Olsen-P of 23.3 mg kg−1, and exchangeable K of 217 mg kg−1. The field capacity water content (FC) was 28.2 %. Water was supplied through the bottom of the pots using a 1.5 cm diameter PVC pipe to control soil moisture. Each population of 6 pots was randomly separated into two groups for drought treatment experiments. Soil water was maintained at 75 % of FC for the control group (CK). The water shortage group (WS) experienced a three-stage continuous soil water treatment, progressive soil drying (T1, 0–12 days), sustained water supply (T2, 13–25 days, 40 % of FC), and complete re-watering (T3, 26–30 days, 75 % of FC). Figure 3 shows the duration of each stage. Each treatment included three independent replications using three pots per replication. To control soil water content in the pots, we re-watered the pots to 75 % FC in the control pots and to 40 % FC in the treatment group during T2 stage by replacing the amount of water that was transpired daily. An electronic scale (d = 0.001 kg) was used to determine the amount of water needed.
Changes of relative soil water content (SC/FC) in control and drought stress groups. Values are mean ± SE, N = 3. CK Control, FC field water capacity, SC soil water content, T1 progressive soil drying stage, T2 sustained water supply stage, T3 re-watering treatment stage in stress groups, WS drought stress groups
Mature functional leaves were collected on days 0, 3, 6, 9, 12, 25, and 30 for the assays of ROS, enzymes, and antioxidants.
Leaf morphological determination
To determine the differences between leaf morphological traits in Sour jujube from SB and YL, 18 mature, healthy leaves from each population were randomly sampled and photographed. Then, leaf length, width, and area were measured using a Yaxin-1241 portable leaf area meter (Beijing Yaxinliyi Science and Technology Co., Ltd., Beijing, China).
Growth parameters, relative water content (RWC), total water consumption and WUE assays
The shoot lengths were quantified every 5 days and recorded. Finally, biomass of shoots and roots of each plant were recorded after harvest on day 30. Samples were oven-dried at 70 °C for 72 h, and then weighed to determine the dry weight.
Leaf samples were collected on days 0, 5, 10, 15, 20, 25, and 30 for determination of relative water content. After weight determination of fresh leaves, the samples were floated on de-ionized water for 6 h under dark conditions to saturate the leaves, and then quickly blot dried prior to the determination of turgid weight. The dry weight of leaves was determined after being oven-dried at 70 °C for 72 h. RWC was calculated according to the following formula:
Individual water consumption during the experiment period was calculated by dividing the amount of plant transpiration per pot by the number of plants in the pot. The amount of water consumed per pot was calculated by subtracting the amount of transpired water in the blank pot from the total transpiration recorded daily. WUE was calculated as the ratio of biomass to the water consumption.
Chlorophyll fluorescence measurement
Chlorophyll (Chl) fluorescence was measured with a pulse amplitude modulated Chl fluorescence system (Imaging-PAM, Heinz Walz GmbH, Effeltrich, Germany). The measurements were conducted on days 1, 9, 25 and 30. In each pot, Chl fluorescence from three mature healthy leaves was measured from 08:00 to 09:30 am. The selected leaves were pre-dark adapted for 40 min, and then the minimal fluorescence yield (F o) was obtained with relatively weak measuring light pulses (0.5 µmol m−2 s−1) at a low frequency (1 Hz). Then the maximal fluorescence yield (F m) was determined after exposure to a saturating flash of light (1600 µmol m−2 s−1) for 0.8 s. Then, the actinic light (200 µmol m−2 s−1) was switched on. After the fluorescence yield (F s) was steady, a saturating pulse of white light for 0.8 s was added to the measured maximum fluorescence yield in the light-adapted state (\(F_{\rm m}^\prime\)), and then the minimum fluorescence yield (\(F_{\rm o}^\prime\)) was determined by exposing leaves to far-red light for 8 s. The data of Chl fluorescence were obtained automatically using Imaging Win software (Version 2.40, Heinz Walz GmbH). The two measured values for SB or YL per pot were averaged as one replicate, and the average values of six pots per treatment were used in the analysis. The Photosystem II (PSII) photochemical efficiency was calculated by the method of Genty et al. (1989) as follows, the maximum PSII photochemical efficiency: \(F_{\text{v}} /F_{\text{m}} = \left( {F_{\text{m}} {-}F_{\text{o}} } \right)/F_{\text{m}}\); effective PSII photochemical efficiency: \({\triangle}F/F_{\text{m}}^{{\prime }} = \left( {F_{\text{m}}^{{\prime }} {-}F_{\text{s}} } \right)/F_{\text{m}}^{{\prime }}\); photochemical quenching: \({\text{qP}} = \left( {F_{\text{m}}^{{\prime }} {-}F_{\text{s}} } \right)/\left( {F_{\text{m}}^{{\prime }} {-}F_{\text{o}}^{{\prime }} } \right)\) and non-photochemical quenching: \({\text{NPQ}} = \left( {F_{\text{m}} {-}F_{\text{m}}^{{\prime }} } \right)/F_{\text{m}}^{{\prime }}\).
Reactive oxygen species assays
The production rate of ·O2 − was measured according to the method of Elstner and Heupel (1976) which was based on monitoring nitrite formation from hydroxylamine in the presence of ·O2 −. Briefly, fresh leaf segments were homogenized in liquid N2 and dissolved in 65 mM potassium phosphate buffer (pH 7.8). The samples were centrifuged at 5000×g for 10 min. Then 0.9 mL of 65 mM phosphate buffer (pH 7.8) and 0.1 mL of 10 mM hydroxylamine hydrochloride were added to 1 mL of supernatant, and the mixture was incubated at 25 °C for 20 min. 2 mL of 17 mM sulfanilamide and 2 mL of 7 mM α-naphthylamine were added to the mixture for further incubation at 30 °C for 30 min, followed by adding 6 mL of ethyl ether. Finally, the mixture was centrifuged at 1500×g for 5 min and then quantified by a photospectrometry at 530 nm (UV-1700 spectrophotometer, SHIMADZU, Tokyo, Japan).
The H2O2 content was measured according to the methods described by Gay and Gebicki (2000). Leaf samples were homogenized in methanol at 0 °C and centrifuged at 10,000×g, at 4 °C for 10 min. An aliquot of 300 µL of the supernatant was added to 750 µL of 1 mM ammonium ferrous sulfate (Fe(NH4)2(SO4)2), 300 µL of 250 mM sulfuric acid, 300 µL of 1 mM xylenol orange and 750 µL of distilled water and incubated in the dark for 1 h at room temperature. The absorbance was read at 560 nm.
The comparative content of ·OH was measured according to Schopfer et al. (2001) with slight modifications. Fresh samples weighing 0.5 g were homogenized with 3 mL of 50 mM potassium phosphate buffer (pH 7.0) and centrifuged at 10,000×g at 4 °C for 10 min. Then 1 mL of 25 mM potassium phosphate buffer (pH 7.0) containing 2.5 mM 2-deoxy-D-Rib was added to 1 mL of supernatant. The formation of the breakdown product malondialdehyde was determined to indicate the ·OH levels.
Enzyme activity assays
Fresh leaf samples were frozen in liquid N2 immediately after being harvested, and then stored at −80 °C until enzyme activity assays. Enzymes were extracted as described by An et al. (2013). Total soluble protein content of enzyme extracts were measured according to the method of Bradford (1976). SOD (EC 1.15.1.1) activity was determined according to Giannopolitis and Ries (1977). One unit of SOD activity was defined as the quantity of SOD required to produce a 50 % inhibition of nitroblue tetrazolium (NBT), and the specific enzyme activity was expressed as units of mg−1 protein h−1. CAT (EC 1.11.1.6) activity was measured according to Aebi (1984), which measures the decline of the extinction of H2O2 (extinction coefficient 0.04 mM−1 cm−1) at the maximum absorption (240 nm) for 3 min. POX (EC 1.11.1.7) activity was performed according to the method of Hendriks et al. (1985). The assay depends on the increase in absorbance at 470 nm, by the rate of formation of the oxidized guaiacol. One unit of POX activity was defined as the amount of enzyme that made absorbance at 470 nm increase 0.01 per min mg−1 protein. The enzyme activity was expressed in terms of unit mg−1 protein min−1. APX (EC 1.11.1.11) assay was based upon the method of Chen and Asada (1989), which was recorded as the decrease in absorbance at 290 nm for 3 min as ascorbate was oxidized and calculated from the extinction coefficient (ε) 2.8 mM−1 cm−1. GR (EC 1.6.4.2) activity was measured according to the method of Foyer and Halliwell (1976) which depends on the oxidation of NADPH at 340 nm (extinction coefficient 6.2 mM−1 cm−1) for 3 min.
Non-enzymatic antioxidants assays
ASC and dehydroascorbate, GSH and glutathione disulfide were extracted and measured according to the method described by Queval and Noctor (2007). Briefly, 200 mg of samples were ground in liquid N2 and then extracted with 2 mL of 0.2 M HCl followed by centrifuging at 16,000×g for 10 min at 4 °C. Then 50 µL of 0.2 M NaH2PO4 (pH 5.6) was added to 0.5 mL of supernatant, followed by adding 0.4 mL of 0.2 M NaOH. Finally, the pH was adjusted to between 5 and 6. The resulting supernatant was used for ASC and GSH assays. The experiment was performed with in at least triplicate with biological replicates.
To quantify ASC, 20 µL of supernatant were added to 96 well plates containing 0.1 mL of 0.2 M NaH2PO4 (pH 5.6) and 75 µL of water; then absorption at 265 nm was recorded (Microplate Readers, Spectra Max M2, Molecular Device, Sunnyvale, CA, USA). Next, 5 µL of ascorbate oxidase (40 U mL−1) was added to 96 well plates by mixing for another record at 265 nm. Solutions were remixed by shaking, and the decrease at 265 nm value was monitored and recorded after 5 min. To assay total ascorbate acid, 0.1 mL of neutralized supernatant was first added to 0.14 mL of 0.12 M NaH2PO4 (pH 7.5) and 10 µL of 25 mM dithiothreitol, and solutions were incubated for 30 min at room temperature. Triplicate aliquots of this solution were then assayed as described for ASC.
To measure total glutathione, triplicate aliquots of 10 µL of neutralized extract were added to plate wells containing 0.1 mL of 0.2 M NaH2PO4 (pH 7.5), 10 mM EDTA, 10 µL of 10 mM NADPH, 10 µL of 12 mM 5,5′-dithiobis(2-nitro-benzoic acid) (DTNB), and 60 µL of water. The reaction was started by the addition of 10 µL of GR (20 U mL−1), and the increase in 412 nm (Microplate Readers, Spectra Max M2, Molecular Device) was monitored for 5 min. Standards were run concurrently in the same plates as triplicate assays of 0–1 µM GSH. Glutathione disulfide was measured by the same principle after incubation of 0.2 mL of neutralized extract with 1 µL of 2-vinylpyridine for 30 min at room temperature to complex GSH. The experiment was performed with in at least triplicate biological replicates.
The total flavonoid content was measured by the method of Medaa et al. (2005) with minor modifications. The dried samples were ground with a mortar and pestle and sieved through a 0.45-mm screen. Each sample (50 mg) was extracted ultrasonically with 5 mL of methanol–water solution (8:2 v/v) for 30 min. The extract was centrifuged at 10,000×g for 15 min and assayed for total flavonoids by following this method. 2 mL of 2 % aluminum trichloride (AlCl3) in methanol was mixed with the same volume of extracting solution. Absorption readings at 415 nm were taken after 10 min against a blank sample consisting of 2 mL of extracting solution with 2 mL of methanol without AlCl3. The total flavonoids content was determined using a standard curve with quercetin (Sigma, Deisenhofen, Germany; 0–50 mg L−1) as the standard. The mean of three readings was used and expressed as mg of quercetin equivalents (QE)/g dry weight sample. Carotenoids (Car) and Chl were spectrophotometrically measured in 80 % acetone extract, as described by Lichtenthaler (1987).
Proline content
Free proline was extracted from 0.2 g of dry leaf samples in 3 % (w/v) aqueous sulfosalicylic acid and measured using a ninhydrin reagent according to the method of Bates et al. (1973). The absorbance of fraction with toluene aspired from liquid phase was read at 520 nm.
Statistical analysis
All analyses were performed using a completely randomized design, and data were subjected to two- or three-factor analysis of variance. The “Tukey HSD” post hoc multiple comparison tests were used for comparison of treatment means at P < 0.05. Data were analyzed using SPSS software (SPSS 17.0, SPSS Inc., Chicago, IL, USA). In all the figures the spread of values is shown as error bars representing standard errors of the means.
Results
Growth and leaf relative water content (RWC)
To reveal the growth strategy of Sour jujube to drought-prone environments, we first compared leaf morphological traits of the dry-climate populations (SB) and wet-climate populations (YL). Leaves of SB were significantly smaller than those of YL (Fig. 4a; Tables S1, S2 and S3). Leaf length and area of SB were 25 and 42 % smaller, respectively, than those of YL (Fig. 4b; P < 0.01). Then, we compared growth responses of SB and YL to drought stress. Drought treatment significantly inhibited shoot length (Fig. 5a; Table S4; P < 0.01). However, the shoot length of YL was inhibited more severely than that of SB. Additionally, YL plants showed significantly higher shoot length than SB under control conditions after the 10th day (P < 0.05). However, no significant differences were found between shoot lengths of two populations under drought conditions (Fig. 5a).
Effects of progressive soil drying (T1, on days 0, 5 and 10), sustained water supply (T2, on days 15, 20 and 25) and re-watering treatment (T3, on day 30) on shoot growth (a) and leaf relative water content (b) in two Sour jujube populations. Values are mean ± SE, N = 3. Acronyms for treatment groups and populations: SB-CK control group/dry-climate population, SB-WS drought treatment/dry-climate, YL-CK control/wet-climate, YL-WS drought treatment/wet-climate. Different small letters indicate significant differences in two populations at all points in time of control and drought periods (P < 0.05)
Leaf RWC in Sour jujube decreased significantly under progressive drought stress (T1 stage; Fig. 5b; Table S5; P < 0.01). After withholding water for 10 days, leaf RWC in SB was significantly higher than that in YL (P < 0.05). During the T2 stage, leaf RWC in both populations partially recovered, but was still significantly lower than the control groups (P < 0.05). When re-watered, leaf RWC in both populations recovered to the control level (Fig. 5b).
Biomass accumulation, water consumption and WUE
The biomass of Sour jujube showed that drought treatment significantly decreased shoot, root and total biomass accumulation in both populations (Table 2; Tables S25, S26, S27; P < 0.01). Similar to the shoot length responses, the shoot and total biomass accumulation in YL were notably higher than those in SB under control conditions (P < 0.05), but no significant differences were found between them under drought stress.
YL plants showed significantly higher water consumption than SB under control conditions (Table 2; P < 0.01). Drought treatment significantly decreased water consumption in both populations (Table S28; P < 0.01). WUE of SB was significantly higher than that of YL under control conditions. When exposed to drought stress, WUE of SB changed slightly, although WUE of YL decreased significantly (Table S29).
Chlorophyll fluorescence
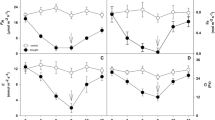
Drought treatment decreased F v/F m in both populations on days 9 and 25 (Fig. 6a; Table S6; P < 0.05). Although F v/F m in SB and YL were enhanced after 4 days of re-watering, they were still significantly lower than the control level (P < 0.05). The ΔF/\(F_{\rm m}^\prime\) and qP deceased significantly under drought condition in both populations on day 9 (Fig. 6b, c; Tables S7, S8; P < 0.05). They recovered to control levels in SB, but were still significantly lower than the control in YL during the T2 stage (on day 25; P < 0.05). When re-watered for 4 days, ΔF/\(F_{\rm m}^\prime\) and qP in SB were significantly enhanced (P < 0.05), although those in YL were still lower than the control (qP: P < 0.05). Drought treatment did not influence NPQ in YL on day 9, but significantly increased NPQ in SB (Fig. 5d; Table S9; P < 0.01).
Effects of progressive soil drying (T1, on days 1 and 9), sustained water supply (T2, on day 25) and re-watering treatment (T3, on day 30) on the maximum PSII photochemical efficiency (F v/F m) (a), effective PSII photochemical efficiency (ΔF/\(F_{\rm m}^\prime\)) (b), photochemical quenching (qP) (c) and non-photochemical quenching (NPQ) (d) in two Sour jujube populations. Values are mean ± SE, N = 6. Acronyms for treatment groups and populations: SB-CK control group/dry-climate population, SB-WS drought treatment/dry-climate, YL-CK control/wet-climate, YL-WS drought treatment/wet-climate. Different small letters indicate significant differences in two populations at all points in time of the control and drought periods (P < 0.05)
Reactive oxygen species
YL showed a significantly higher ·O2 − production rate than SB under control conditions (Fig. 7a; Table S10; P < 0.01). When exposed to drought stress, the ·O2 − production rate peaked on day 3 in YL, and on day 6 in SB. Then, the ·O2 − production rate in YL significantly decreased to 30 % of the control on day 6, and remained at low levels until re-watering treatment. The production rate of ·O2 − in SB decreased to the control level after 9 days of drought stress and remained that level. H2O2 and ·OH production in YL changed similarly to ·O2 − (Fig. 7b, c). They all increased at the beginning of the T1 phase, decreased to lower levels than the control during T2 stage, and recovered to control levels when re-watered. Compared with the control, H2O2 in SB significantly decreased on days 9, 12 and 30 (Table S11; P < 0.05), and ·OH in SB remained at a significantly lower level during the entire drought treatment (Fig. 7b, c; Table S12; P < 0.05).
Effects of progressive soil drying (T1, on days 0, 3, 6, 9 and 12), sustained water supply (T2, on day 25) and re-watering treatment (T3, on day 30) on superoxide radical (·O2−) (a), hydrogen peroxide (H2O2) (b) and hydroxyl radical (·OH) (c) production in two Sour jujube populations. Values are mean ± SE, N = 3. Acronyms for treatment groups and populations: SB-CK control group/dry-climate population, SB-WS drought treatment/dry-climate, YL-CK control/wet-climate, YL-WS drought treatment/wet-climate. Different small letters indicate significant differences in two populations at all points in time of control and drought periods (P < 0.05)
Antioxidative enzymes
SB and YL showed similar SOD activities under control conditions (Fig. 8a). When exposed to drought stress, SOD activity in YL increased significantly at the beginning of the T1 period (P < 0.05), and subsequently decreased to control levels during the T2 and T3 periods. SOD activity in SB remained at higher levels than the control during the entire drought period (Table S13; P < 0.05). CAT activity was significantly increased by drought on days 6, 9 and 12 (Fig. 8b; Table S14; P < 0.01). However, CAT activity in stressed-SB was significantly higher than that in stressed-YL at each point during the T1 stage (P < 0.05). SB showed significantly higher POX activity than YL under both control (Table S15; P < 0.01) and drought conditions (Fig. 8c; P < 0.01). Compared with the control, POX activity in stressed-YL decreased on day 12, and increased when re-watered. POX activity in stressed-SB increased to and remained at a significant higher level than that in the control after 9 days of drought stress (P < 0.01). APX activity in SB was higher than that in YL at many measurement days under both control and drought conditions (Fig. 8d; Table S16). When exposed to drought stress, APX activity in SB and YL increased significantly on days 3 and 6 (P < 0.01), and decreased after 12 days of drought stress (P < 0.05). The GR activity in both populations was declined markedly during the drought treatment on day 3 (Fig. 8e; P < 0.01), but significantly improved with during stress (P < 0.01), and recovered after the re-watering period. However, GR activity in SB showed significantly higher levels than that in YL under both drought conditions and during the re-watering treatment (Table S17; P < 0.05). Except for the difference in enzyme activities, SB showed a longer active period of SOD, CAT, and POD than YL.
Effects of progressive soil drying (T1, on days 0, 3, 6, 9 and 12), sustained water supply (T2, on day 25) and re-watering treatment (T3, on day 30) on superoxide dismutase (SOD) (a), catalase (CAT) (b), peroxidase (POX) (c), ascorbate peroxidase (APX) (d), and glutathione reductase (GR) (e) production in two Sour jujube populations. Values are mean ± SE, N = 3. Acronyms for treatment groups and populations: SB-CK control group/dry-climate population, SB-WS drought treatment/dry-climate, YL-CK control/wet-climate, YL-WS drought treatment/wet-climate. Different small letters indicate significant differences in two populations at all points in time of control and drought periods (P < 0.05)
Non-enzymatic antioxidants
Drought treatment significantly increased GSH content in both populations on day 12 (Table S18; P < 0.05), and enhanced ASC content in YL on day 9 and in SB on day 12 (Fig. 9a, b; Table S19; P < 0.05). Drought treatment significantly enhanced the reduction state of GSH in SB on days 3, 6, 9 and 25, but only on day 9 in YL (Fig. 9c; Table S20; P < 0.05). Drought treatment also increased the reduction state of ASC in SB on days 3 and 30, and that in YL on days 3, 25 and 30 (Fig. 9d; Table S21; P < 0.05). However, the reduction states of GSH in YL and ASC in SB decreased significantly on days 12 and 25, respectively (Fig. 9c, d; P < 0.05). Under control conditions, the reduction state of ASC in YL was lower than that in SB (Fig. 9d; P < 0.05). Drought treatment markedly increased Car content in SB plants on days 3, 6, 9, and 25 and in YL plants on days 6, 9, and 12 (Fig. 9e; Table S22; P < 0.05). Compared with the control, flavonoids accumulated (Fig. 9f) gradually both in SB (increased by 42 %) and YL (increased by 35 %) during the T1 stage, but significantly decreased on day 25 (Table S23; P < 0.01). Re-watering enhanced flavonoid contents in both populations. We also found that the free proline content gradually increased (SB: 8.8 times; YL: 5.2 times, on day 12) in both populations during T1 period (Table S24). However, proline content declined to the control level during the T2 period (Fig. 9g).
Effects of progressive soil drying (T1, on days 0, 3, 6, 9 and 12), sustained water supply (T2, on day 25) and re-watering treatment (T3, on day 30) on the content of glutathione (GSH) (a), ascorbate acid (ASC) (b), carotenoids (Car) (e), total flavonoids (f) and proline (g), and the reduction states of GSH (GSH %) (c) and ASC (ASC %) (d) in two Sour jujube populations. Values are mean ± SE, N = 3. Acronyms for treatment groups and populations: SB-CK control group/dry-climate population, SB-WS drought treatment/dry-climate, YL-CK control/wet-climate, YL-WS drought treatment/wet-climate. Different small letters indicate significant differences in two populations at all points in time of control and drought periods (P < 0.05)
Principal component analysis (PCA) of ROS and antioxidant indices
PCA was carried out to obtain a broad view on the changes of ROS and antioxidant indices. Four principal components (PC1, PC2, PC3 and PC4) were extracted, and these dimensions together explained over 80 % of the total variability (Fig. 10). Data dimensions were therefore reduced from 15 to 4 for further data processing.
Principal component analysis of reactive oxygen species and antioxidants in two populations under control and drought treatments. a, b Loading plots of PC1–PC2 and PC3–PC4 variables. c, d Sample score plots of PC1–PC2 and PC3–PC4. PC1–PC4, the first, second, third and fourth principal components, respectively. S, dry climate Sour jujube; (Y) wet climate Sour jujube; (0, 3, 6, 9, 12, 25, 30) onset day after treatment; (C) control group; (W) water stress group. PC1 mainly includes catalase (CAT), glutathione (GSH), proline, and flavonoids (a). PC2 mainly includes peroxidase (POX), glutathione reductase (GR), carotenoids (Car), the reduction state of GSH (GSH%) and hydroxyl radical (·OH) (a). PC3 mainly includes hydrogen peroxide (H2O2), superoxide dismutase (SOD), and superoxide radical (·O2 −) (b). PC4 mainly includes ascorbate peroxidase (APX) and the state of ASC (ASC%) (b)
PC1 was heavily and positively associated with CAT, GSH, proline, and flavonoids (Fig. 10a). PC2 gave a high weighting to POX, GR, Car, the reduction state of GSH and ·OH (Fig. 10a). PC1 appeared to separate samples of the T1 stage (mainly on days 6, 9 and 12) from their corresponding stressed-samples (Fig. 10c). PC2 appeared to separate SB stressed-samples from YL stressed-samples on days 6, 9 and 12 (Fig. 10c).
Stressed-samples of YL on day 3 were separated mainly by PC3, which included H2O2, SOD, and ·O2 − (Fig. 10b, d). However, stressed-samples of SB on day 3 and 30 (completely re-watering) were mainly separated by PC4, including APX and the reduction state of ASC (Fig. 10b, d).
Discussion
Growth strategies of Sour jujube in response to drought stress
As a result of long-term natural selection and co-evolution, plants in drought-prone regions have evolved multiple mechanisms to counteract water deficit stress (Mullet and Whitsitt 1996). A wide range of characteristics and processes, from morphological traits to physiological and metabolic characteristics are involved in plant adaptation to drought stress (Reddy et al. 2004; Lei et al. 2006; Bai et al. 2009; An et al. 2013). Drought stress can cause an alteration in water status such as shoot water potential or RWC of the plant (Chaves et al. 2009; An et al. 2013). Under drought stress, RWC in SB was higher than that in YL on day 10, indicating that the dry-climate population exhibits a better ability to retain water than the wet-climate population (Colom and Vazzana 2003; Xu et al. 2008; Wang et al. 2012). To save water and enhance WUE, dry-climate populations of plants always presents lower height, less total biomass and leaf area, and a higher root/shoot ratio than wet-climate populations (Li 2000; Yin et al. 2004; Xu et al. 2008). In this study, the dry-climate populations (SB) of Sour jujube showed significantly smaller leaves and biomass, lower height and water consumption, but higher WUE than the wet-climate population (YL) under control conditions (Fig. 4; Table 2). This finding indicates that SB Sour jujube have evolved to a small size to reduce water transpiration and increase WUE under drought environments. The small size of the plants also contributes to avoiding potential damage under intense radiation which always accompanies drought stress (Yin et al. 2004; Ren et al. 2007). The different morphological traits of SB and YL indicate that internal constitutive differences exist between them, and they may be considered as two ecotypes.
Plants suffer from drought-stress either when the roots have difficulty obtaining water or when the transpiration rate becomes very high (Reddy et al. 2004). These two conditions often coincide under arid and semi-arid climates. Under drought conditions, a decrease in RWC, stomatal closure and damage to the photosynthetic apparatus may result in a decrease in photosynthetic rates (Reddy et al. 2004), finally resulting in the inhibition of plant growth. However, one early morphological response of plants to drought stress is believed to be a stress avoidance mechanism through the adjustment of the plant growth rate such as a reduction in shoot height (Chaves et al. 2009; Skirycz and Inzé 2010). In the present study, the shoot length and biomass accumulation in SB exhibited lower inhibition than those in YL (Fig. 5a; Table 2), which is consistent with studies of other plants (Li 2000; Yin et al. 2004; Lei et al. 2006). This indicated that growth of YL is more sensitive to drought stress than SB. The lower inhibition in SB growth is probably associated with its lower water consumption and higher RWC which resulted to a smaller degree of drought stress (Hsiao 1973).
The optimal/maximal photochemical efficiency of PSII in the dark (F v/F m) indicates intrinsic PSII efficiency (Flexas et al. 1998). F v/F m ranges from 0.80 to 0.83 in all higher plants under favorable conditions, and the ratio decreases in photo-inhibited plants (Maxwell and Johnson 2000). In this study, F v/F m in Sour jujube seedlings remained higher than 0.81 during the entire experiment (Fig. 6a), indicating that plants have evolved resistant mechanisms to protect PSII from drought stress-induced injury (Maxwell and Johnson 2000; Lu et al. 2003; Xu et al. 2008, 2014). ΔF/\(F_{\rm m}^\prime\) indicates the actual photochemical efficiency of PSII in the light-adapted leaves (Maxwell and Johnson 2000). qP reflects the proportion of the opened PSII reaction centers or the proportion of the oxidized state of QA (Genty et al. 1989; Lu et al. 2003). In the present study, ΔF/\(F_{\rm m}^\prime\) and qP in both SB and YL significantly declined on day 9, which is perhaps associated with the accumulation of ROS. However, ΔF/\(F_{\rm m}^\prime\) and qP in SB recovered to the control level on day 25 and increased after the re-watering period, while those in YL did not recover to the control level even during the re-watering period (Fig. 6b, c). These results indicate that the dry-climate population is superior in maintaining PSII efficiency to overcome adverse conditions. NPQ is conventionally used to estimate the harmless thermal dissipation of excess excitation energy through the xanthophyll cycle function (Demmig-Adams et al. 1996). Compared with the control, the NPQ of SB population increased by 100 % under drought on day 9, which again indicates that the dry-climate population possesses better protective mechanism by non-photochemical quenching to maintain PSII efficiency (Colom and Vazzana 2003; Xu et al. 2008).
Better antioxidant ability in SB Sour jujube than YL Sour jujube
The homeostasis of ROS in plants is easily disrupted. The ROS levels will increase when plants have suffered from adverse conditions, especially after the inhibition of photosynthetic electron transfer (Foyer and Noctor 2012). In this study, YL showed larger increases in the production rate of ·O2 −, H2O2 and ·OH than SB during the T1 stage of drought stress (Fig. 7), indicating that SB suffers from less oxidative damage than YL. Our results are consistent with other reports that indicate more ROS accumulate in drought-sensitive plants and wet-origin plants under drought (Cia et al. 2012; Wang et al. 2012).
To counteract the damaging effects of ROS accumulation, plants have evolved antioxidants such as ASC, GSH, carotenoids, flavonoids, proline, and antioxidative enzymes such as SOD, POX, CAT, APX, and GR (Mittler 2002). Responses of antioxidants and antioxidative enzymes to drought depend on the plant species and genotypes involved, the length and severity of water deficit, as well as the age and stage of development of the plant (Reddy et al. 2004). An et al. (2013) revealed that the antioxidative system and proline play an important role in drought adaptation of Periploca sepium, a native woody vine on the Loess Plateau. Shan and Liang (2010) demonstrated that ASC and GSH are important for Agropyron cristatum to adapt to the dry climate of the loess hills. Here, SB showed higher POX and APX activities than YL at many measurement days under control conditions and higher CAT, POX, APX and GR activities at many measurement days under drought stress (Fig. 8; Tables S14, S15, S16 and S17). These results indicate that SB has evolved better antioxidant enzyme systems than YL. Compared with the control, SOD and CAT activities in SB under drought stress significantly increased at many measurement days (Tables S13, S14); however, APX activity increased notably in the first 6 days of drought stress, and POX and GR increased largely after 6 days of drought stress. These results indicate that all of these five antioxidant enzymes, especially in combination, contribute to the antioxidant capacity of SB, playing important roles in the adaptation of SB plants to drought-prone environments. Our results were consistent with many earlier research studies (Türkan et al. 2005; Xu et al. 2008; Chugh et al. 2011; Cia et al. 2012; Wang et al. 2012), which reported that when under drought stress dry-origin or drought-tolerant plants had higher antioxidant enzyme activities than the wet-origin or drought-sensitive plants. The longer active periods of SOD, CAT, and POD in SB than those in YL indicate that the long active period of antioxidant enzymes and early activation of this period may also contribute to the antioxidant capacity of SB.
ASC and GSH are considered to be important players in determining cellular redox status (Queval and Noctor 2007; Foyer and Noctor 2011). Our results showed that both populations increased GSH on day 12, and the ASC content was enhanced in YL on day 9, and in SB on day 12 (Fig. 9a, b), which confirmed this fact. In addition, SB showed higher GSH and ASC content than YL on day 12 under drought stress (Fig. 9a, b), which suggests that SB might have higher accumulation capability than the YL under more serious drought conditions. GSH as an important cellular thiol–disulfide buffer, plays an important role in protecting protein thiol from oxidation by ROS (Foyer and Noctor 2011). In our study, SB showed a continously higher reduced state of GSH (except on day 12) in the stressed-group, compared with the control (Fig. 9c). However, the reduced state of GSH in stressed YL showed strong fluctuation. It was significantly lower than that in the control group on days 12 and 25 (Fig. 9c). These results indicate that SB possesses a better antioxidant system in the ASC-GSH cycle than YL.
Carotenoids perform a major function in controlling ROS accumulation in plastids, especially in singlet oxygen levels within thylakoid membranes (Mittler 2002). In addition, carotenoids are very important in protection against photo-oxidation (Lu et al. 2003; Xu et al. 2008). As a large family of secondary plant metabolites, flavonoids play a role in plant cell detoxification by the flavonoid–POX reaction (Yamasaki et al. 1997; Winkel 2002). An enhanced rate of proline biosynthesis in chloroplasts during stress can maintain the low NADPH:NADP+ ratio, contribute to sustaining electron flow between photosynthetic excitation centers, stabilize the redox balance, and reduce photoinhibition and damage of the photosynthetic apparatus (Szabados and Savoure 2010). In this study, stressed SB plants showed better Car accumulation ability than YL on days 3 and 6, as well as after a long-term drought treatment (day 25). This agrees with reports that plants in drought-prone regions possess a better Car accumulation ability that allows them to adapt to drought-prone environments (Xu et al. 2008). Flavonoids and free proline accumulated gradually in both SB and YL in the T1 stage, but SB showed higher proline accumulation than YL at the late stage of T1. These results indicate that carotenoids, flavonoids and free proline may also contribute to the antioxidant capacity of Sour jujube, especially in SB.
Taken together, SB showed higher activities of several antioxidant enzymes and accumulation of many antioxidants than YL, explaining well why SB displays less ROS level than YL under drought stress.
Conclusions
In summary, under drought conditions the dry-climate populations exhibited lower growth and biomass inhibition, and more efficient water use strategy than the wet-climate populations, indicating the higher drought tolerance of the dry-climate population. Compared with the wet-climate population, the dry-climate population showed lower levels of ROS, better performance of the photo-protective system, higher levels of antioxidative enzymes and antioxidants under drought conditions; this suggested the dry-climate population has a better capacity to produce antioxidants. Our data provide useful information related to the use of native species in drought-prone regions.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
An Y, Zhang M, Liu G, Liang Z (2013) Proline accumulation in leaves of Periploca sepium via both biosynthesis up-regulation and transport during recovery from severe drought. PLoS One 8:e69942. doi:10.1371/journal.pone.0069942
Bai J, Gong CM, Chen K, Kang HM, Wang G (2009) Examination of antioxidative system’s responses in the different phases of drought stress and during recovery in desert plant Reaumuria soongorica (Pall.) Maxim. J Plant Biol 52:417–425. doi:10.1007/s12374-009-9053-7
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. doi:10.1007/BF00018060
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot Lond 103:551–560. doi:10.1093/aob/mcn125
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Chen L, Wei W, Fu B, Lü Y (2007) Soil and water conservation on the Loess Plateau in China: review and perspective. Prog Phys Geog 31:389–403. doi:10.1177/0309133307081290
Chugh V, Kaur N, Gupta AK (2011) Evaluation of oxidative stress tolerance in maize (Zea mays L.) seedlings in response to drought. Indian J Biochem Bio 48:47–53
Cia MC, Guimaraes ACR, Medici LO, Chabregas SM, Azevedo RA (2012) Antioxidant responses to water deficit by drought-tolerant and -sensitive sugarcane varieties. Ann Appl Biol 161:313–324. doi:10.1111/j.1744-7348.2012.00575.x-
Colom MR, Vazzana C (2003) Photosynthesis and PSII functionality of drought-resistant and drought-sensitive weeping lovegrass plants. Environ Exp Bot 49:135–144. doi:10.1016/S0098-8472(02)00065-5
Demmig-Adams B, Adams WW III, Barker DH, Logan BA, Bowling DR, Verhoeven AS (1996) Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol Plant 98:253–264. doi:10.1034/j.1399-3054.1996.980206.x
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620. doi:10.1016/0003-2697(76)90488-7
Flexas J, Escalona JM, Medrano H (1998) Down-regulation of photosynthesis by water deficit under field conditions in grapevine leaves. Aust J Plant Physiol 25:893–900. doi:10.1071/PP98054
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25. doi:10.1007/BF00386001
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18. doi:10.1104/pp.110.167569
Foyer CH, Noctor G (2012) Managing the cellular redox hub in photosynthetic organisms. Plant Cell Environ 35:199–201. doi:10.1111/j.1365-3040.2011.02453.x
Gay C, Gebicki JM (2000) A critical evaluation of the effect of sorbitol on the ferric–xylenol orange hydroperoxide assay. Anal Biochem 284:217–220. doi:10.1006/abio.2000.4696
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA-Gen Subj 990:87–92. doi:10.1016/S0304-4165(89)80016-9
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314. doi:10.1104/pp.59.2.309
Hendriks T, van den Berg BM, Schram AW (1985) Cellular location of peroxidase isoenzymes in leaf tissue of Petunia and their affinity for Concanavalin A-Sepharose. Planta 164:89–95. doi:10.1007/BF00391030
Hsiao TC (1973) Plant responses to water stress. Ann Rev Plant Physiol 24:519–570. doi:10.1146/annurev.pp.24.060173.002511
Kozlowski TT, Pallardy SG (2002) Acclimation and adaptive responses of woody plants to environmental stresses. Bot Rev 68:270–334. doi:10.1663/0006-8101(2002)068[0270:AAAROW]2.0.CO;2
Lei Y, Yin C, Li C (2006) Differences in some morphological, physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiol Plant 127:182–191. doi:10.1111/j.1399-3054.2006.00638.x
Li C (2000) Population differences in water-use efficiency of Eucalyptus microtheca seedlings under different watering regimes. Physiol Plant 108:134–139. doi:10.1034/j.1399-3054.2000.108002134.x
Li ZJ, Li PR, Shi YG, Zhang SL (2010) Effects of long-term fertilizer management regimes on availability of soil micronutrient elements. Plant Nutr Fert Sci 6:23 (in Chinese)
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Liu Y, Fu B, Lü Y, Wang Z, Gao G (2012) Hydrological responses and soil erosion potential of abandoned cropland in the Loess Plateau, China. Geomorphology 138:404–414. doi:10.1016/j.geomorph.2011.10.009
Lu C, Qiu N, Lu Q, Wang B, Kuang T (2003) PSII photochemistry, thermal energy dissipation, and the xanthophyll cycle in Kalanchoe daigremontiana exposed to a combination of water stress and high light. Physiol Plant 118:173–182. doi:10.1034/j.1399-3054.2003.00061.x
Maatallah S, Ghanem ME, Albouchi A, Bizid E, Lutts S (2010) A greenhouse investigation of responses to different water stress regimes of Laurus nobilis trees from two climatic regions. J Arid Environ 74:327–337. doi:10.1016/j.jaridenv.2009.09.008
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668. doi:10.1093/jexbot/51.345.659
Medaa A, Lamien CE, Romito M, Millogo J, Nacoulma OG (2005) Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 91:571–577. doi:10.1016/j.foodchem.2004.10.006
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. doi:10.1016/S1360-1385(02)02312-9
Mullet JE, Whitsitt MS (1996) Plant cellular responses to water deficit. Plant Growth Regul 20:119–124. doi:10.1007/978-94-017-1299-6_6
Pnueli L, Hallak-Herr E, Rozenberg M, Cohen M, Goloubinoff P, Kaplan A, Mittler R (2002) Molecular and biochemical mechanisms associated with dormancy and drought tolerance in the desert legume Retama raetam. Plant J 31:319–330. doi:10.1046/j.1365-313X.2002.01364.x
Queval G, Noctor G (2007) A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: application to redox profiling during Arabidopsis rosette development. Anal Biochem 363:58–69. doi:10.1016/j.ab.2007.01.005
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202. doi:10.1016/j.jplph.2004.01.013
Ren J, Dai W, Xuan Z, Yao Y, Korpelainen H, Li C (2007) The effect of drought and enhanced UV-B radiation on the growth and physiological traits of two contrasting poplar species. For Ecol Manag 239:112–119. doi:10.1016/j.foreco.2006.11.014
Schopfer P, Plachy C, Frahry G (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol 125:1591–1602. doi:10.1104/pp.125.4.1591
Shan C, Liang Z (2010) Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Plant Sci 178:130–139. doi:10.1016/j.plantsci.2009.11.002
Skirycz A, Inzé D (2010) More from less: plant growth under limited water. Curr Opin Biotech 21:197–203. doi:10.1016/j.copbio.2010.03.002
Sun G, Zhou G, Zhang Z, Wei X, McNulty SG, Vose JM (2006) Potential water yield reduction due to forestation across China. J Hydrol 328:548–558. doi:10.1016/j.jhydrol.2005.12.013
Sun YF, Liang ZS, Shan CJ, Viernstein H, Unger F (2011) Comprehensive evaluation of natural antioxidants and antioxidant potentials in Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou fruits based on geographical origin by TOPSIS method. Food Chem 124:1612–1619. doi:10.1016/j.foodchem.2010.08.026
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97. doi:10.1016/j.tplants.2009.11.009
Treutter D (2006) Significance of flavonoids in plant resistance: a review. Environ Chem Lett 4:147–157. doi:10.1007/s10311-006-0068-8
Türkan I, Bor M, Ozdemir F, Koca H (2005) Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci 168:223–231. doi:10.1016/j.plantsci.2004.07.032
Wang GH (2006) Can the restoration of natural vegetation be accelerated on the Chinese Loess Plateau? A study of the response of the leaf carbon isotope ratio of dominant species to changing soil carbon and nitrogen levels. Ecol Res 21:188–196. doi:10.1007/s11284-005-0103-4
Wang CX, Yue XJ, Ge XZ, Wang XD (2010) Effect of different cultivation measures on activity and bound forms of organic carbon in Lou Soil. Agric Res Arid Areas 28:58–63 (in Chinese)
Wang S, Liang D, Li C, Hao Y, Ma F, Shu H (2012) Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant Physiol Biochem 51:81–89. doi:10.1016/j.plaphy.2011.10.014
Winkel SB (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5:218–223. doi:10.1016/S1369-5266(02)00256-X
Xu G, Duan B, Li C (2008) Different adaptive responses of leaf physiological and biochemical aspects to drought in two contrasting populations of seabuckthorn. Can J For Res 38:584–591. doi:10.1139/X07-164
Xu WZ, Deng XP, Xu BC, Gao ZJ, Ding WL (2014) Photosynthetic activity and efficiency of Bothriochloa ischaemum and Lespedeza davurica in mixtures across growth periods under water stress. Acta Physiol Plant 36:1033–1044. doi:10.1007/s11738-013-1481-9
Yamasaki H, Sakihama Y, Ikehara N (1997) Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol 115:1405–1412. doi:10.1104/pp.115.4.1405
Yang HB, An SQ, Sun OJ, Shi ZM, She XS, Sun QY, Liu SR (2008) Seasonal variation and correlation with environmental factors of photosynthesis and water use efficiency of Juglans regia and Ziziphus jujuba. J Integr Plant Biol 50:210–220. doi:10.1111/j.1744-7909.2007.00391.x
Yin C, Duan B, Wang X, Li C (2004) Morphological and physiological responses of two contrasting poplar species to drought stress and exogenous abscisic acid application. Plant Sci 167:1091–1097. doi:10.1016/j.plantsci.2004.06.005
Zheng S, Shangguan Z (2007) Spatial patterns of foliar stable carbon isotope compositions of C3 plant species in the Loess Plateau of China. Ecol Res 22:342–353. doi:10.1007/s11284-006-0024-x
Acknowledgments
We thank the Key Project of the National 12th-Five Year Research Program of China (2015BAC01B03), Knowledge Innovation Project of the Chinese Academy of Science (KZCX2-XB2-05-01) and the Key Project of the Chinese Academy of Science (KZCX2-YW-443) for funding this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Wang and Y. An contributed to the work equally and should be regarded as co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Wang, Y., An, Y., Yu, J. et al. Different responses of photosystem II and antioxidants to drought stress in two contrasting populations of Sour jujube from the Loess Plateau, China. Ecol Res 31, 761–775 (2016). https://doi.org/10.1007/s11284-016-1384-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-016-1384-5