Abstract
The studies, which were conducted in southern Poland, focused on the recovery of the herb layer in 17-year-old post-fire silver birch and black alder forests. Although both types of stands, which are of the same age, developed spontaneously, the alder stands occupied damper sites (with thicker A horizons that survived the fire) than those in the birch forests. We surveyed the migration rates of 44 woodland species, primarily ancient woodland indicators, into both forests and the potential differences in these rates depending on their moisture regime and the community type represented by unburned forests, which were treated as the source of the woodland species pool. Additionally, the role of local depressions with high humidity that were covered by post-fire alder woods in the colonization process, as well as species survivorship and recolonisation, were estimated. Woodland species showed diverse migration paces among the sites; most of them migrated faster on more fertile sites with a higher humidity. Small patches of post-fire alder woods contributed to the recolonisation process since many woodland species in the herb layer survived the fire due to its high humidity, which inhibited the intensity of the forest fire. The recovery of woodland species in post-fire woods is the combined effect of regeneration, which relies on autochthonic propagules, and secondary succession, which is based on allochthonic propagules. Local depressions, which provide refuges for fire-sensitive, dispersal-limited species, contribute to their survivorship and thus to the successive recovery of herbaceous layers after a fire.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unlike in boreal ecosystems and other fire-prone communities, the fires in broad-leaved forests in temperate climates are not natural drivers that shape the vegetation physiognomy, structure, species composition and turnover, but are regarded as severe disturbances that are detrimental to their biodiversity. This is because the majority of plant species associated with canopy-closed deciduous forests do not display any adaptations to fire (Whelan 1995; Matlack 2013).
The influence of fire, both wildfires and anthropogenic fires, on plant communities is not uniform and depends on many factors. These include the characteristics of a fire (its frequency, periodicity, intensity, size, season of the burn and depth of the burn); the vegetation that existed prior to the ignition (in other words the amount and type of biomass that was available); habitat conditions and topography; and the weather pattern before the fire, during the fire and in the post-fire period (Kilgore 1987; Brown and Smith 2000; Flannigan et al. 2000; Keeley 2009). The alteration of the physical environment, which is one of the first order effects of fire, has great implications on the successive regeneration and development of the vegetation cover. The most important effects of fire, which shape the structure and functioning of the vegetation and are important drivers of the successive community recovery, are plant mortality and the loss of organic matter that is consumed by fire and lost as a result of wind erosion (Whelan 1995; Certini 2005). The mortality risk to plants depends on the amount of heat that they receive (high temperature plus exposure time) and on their susceptibility to fire, which is related to the amount of sensitive, meristematic tissue and the level of their exposure to heat (Brown and Smith 2000). Those plants that are able to survive a fire on a site are called sprouters (sensu Biswell 1974 and Keeley 1981), whereas seeders are the species that are killed by the flames and high temperature and therefore have to colonise the post-fire site from neighbouring communities (Whelan 1995). Species from the first group recover via vegetative propagation, whereas the latter rely on sexual reproduction (Tsuyuzaki et al. 2013). The survival and sprouting of herbaceous species (forbs and grasses) are strictly related to the depth of burn, which in turn is a function of the length of a fire, which is dependent on the amount of fuel that is available on a site. The survivorship and regeneration of plants depends on their life history traits. The way that herbs protect their buds and therefore the origin of their shoots (seeds, rhizomes, stolons, bulbs, etc.), the depth at which they are stored (in organic, combustible material or in the inflammable mineral layers of soil) and the initial soil moisture content are important factors that are responsible for the community restoration process following a forest fire (Faliński 1998a; Brown and Smith 2000; Kwiatkowska-Falińska 2008; Budzáková et al. 2013). The survivorship of some species in an area where a forest has burned allows for their regeneration. However, most woodland herbs are not capable of such survivorship and for this reason are poorly represented in early successional, post-fire forests (Faliński 1998a; Orczewska et al. 2010). For these reasons, forest recovery is usually the combined effect of regeneration (the process of rebuilding the destroyed or distorted parts of the community by propagules that are derived from the same community) and succession (a directional process of community recovery by propagules that originate from the exterior) (sensu Faliński 1998a), because most plant species have to recolonise the site from outside.
The colonisation of a site by plant species via their seeds largely depends on seed availability, which is a function of seed production, the distance to the seed pool (adult, parent plants that produce seeds) and seed mobility (high for anemo-, epi- and endozoochores but low for auto-, baro- and myrmecochores) (Peterken and Game 1984; Ehrlén and Eriksson 2000; Honnay et al. 2002; Flinn and Vellend 2005). In the case of post-fire forests, the efficiency of this process is expected to be very low since most woodland herbaceous species predominantly rely on vegetative propagation and only produce a small number of seeds. Furthermore, many typical woodland species do not form a persistent seed bank (no seed bank, a transient or a short-time persistent seed bank type according to the Thompson et al. 1997 classification) (Bierzychudek 1982; Bekker et al. 1998; Whigham 2004). The lack of a seed bank combined with a poor dispersal potential of many woodland herbs (almost 1/3 of woodland species are either baro-, auto- or myrmecochores) (Hermy et al. 1999) are responsible for their limited distribution outside the forests that have had a long and continuous existence in the landscape (the so-called ancient forests and ancient forest species sensu Peterken 1974, 1977). Consequently, species with such characteristics are slow in colonising new sites because of a dispersal limitation mechanism that was reported by Peterken and Game (1984); Brunet and von Oheimb (1998), Bossuyt et al. (1999); Dzwonko (2001), Orczewska (2009a, 2011) and others. In other words, the ability of woodland species to migrate outside a forest habitat is very limited and highly dependent on the distance from a mature forest with abundant populations of woodland species to an available new site that is to be colonised. For these reasons the successful recovery of the herbaceous layer in woods that appear in a landscape recently (for example, in woods of a post-agricultural origin) is highly dependent on the area and spatial distribution of mature forests with a high species richness and diversity of their forest floor.
Another mechanism that is responsible for the expected slow recolonisation of the herb layer by woodland flora in recently developed stands is a recruitment limitation (Jacquemyn and Brys 2008; Baeten et al. 2009; Orczewska 2009b). This is defined as the difficulties that species have in establishing their populations on new sites because of biotic factors (mainly expressed as the vigorous growth of competitive species that inhibit the migration of woodland species and suppress their growth) and abiotic ones (such as altered physico-chemical soil conditions, light and water regime changes; for review of the literature concerning these topics see, for example, Flinn and Vellend 2005).
Mechanisms similar to those described above, related to the recovery of forests on abandoned agricultural land, are involved in the process of community restoration on post-fire sites. In places with such an origin, some species could survive a forest fire on the site. However, one may expect that woodland species were either not present or in a minority among them when one takes into account their ecological characteristics as described above. Furthermore, they are poorly represented in the post-fire woods because the viable, short-lived seeds of woodland species are burned during a fire. The survivorship of their propagules could be limited only to the dampest microhabitats, where the intensity of a fire is suppressed by a naturally high groundwater level that is persistent even during the hot, summer period. In most cases, however, woodland species have to migrate into post-fire forests from outside. Therefore, the existence of small patches of unburned forests that are rich in woodland herbs in their neighbourhood is of great importance in the herb layer recovery process. Therefore, we compared the herb layer recovery process in young, 17-year-old forests dominated by silver birch (Betula pendula Roth) that occupied fresh habitats and in black alder (Alnus glutinosa (L.) Gaertn.) stands of the same age, but which occurred on damp forest sites with thicker A horizons than those in the birch forests. Both types of stands had developed spontaneously on post-fire sites. In particular, the studies aimed to compare the migration rates of woodland species into post-fire birch and alder stands and the potential differences in these rates depending on their moisture regime and the community type that was represented by an unburned forest, treated as the source of the woodland species pool. Additionally, we tested whether the damp sites that are currently occupied by young black alder woods of a post-fire origin have richer woodland species pools in their herb layer than the post-fire birch forests that occur on drier habitats. If this was the case, we aimed to answer the question of whether post-fire alder woods contribute to hastening the herb layer recovery process in birch stands.
Study area
The studies were done in Kotlina Raciborska, the Rudziniec Forest District, Łącza Subdistrict in Upper Silesia in southern Poland (N50°18′4.61″; E18°25′48.29″). In this area one of the largest, man-induced forest fires ever to take place in central Europe occurred during the extremely hot and dry summer weather in late August 1992 (Hawryś 1998). The ignition originated from a railway track as a train was travelling through the forest complex. As a result of the fire, which lasted 26 days and spread through three administrative forest districts, over 9062 hectares (ha) of forests were completely burned. Of these 9062 ha, more than 5000 ha had originally been covered by mature stands, mainly pine–oak mixed moderately moist and humid forests (Hanak 1994; Szabla 1994).
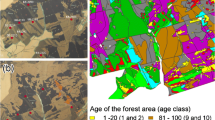
In the Rudziniec Forest District more than 2154 ha of forest were burned. The Łącza Subdistrict, which covers an area of 683.16 ha, was among those areas where almost 100 % of the forests were burned (94 %; 644.71 ha). Among the stands that survived, very young forests were predominant (15.67 ha), whereas there were only three small and isolated patches of mature woods (Fig. 1a). Almost half of the area of the young forests that replaced the burned ones originated from natural succession and they are primarily composed of either silver birch or black alder stands.
Study area: a the Łącza Forest Subdistrict with the proportion of burned (white) and unburned forests (grey); b location of the transects: 1 patches of unburned mature forests; 2 post-fire silver birch forest; 3 post-fire black alder forests; T1–T6 transects; 225a, 225b, 225c forest sections that represent post-fire forest, unburned, alder–ash carr and oak–hornbeam communities, respectively; c transect scheme
Detailed studies on the process of the recovery of the herb layer were carried out in April and June/July 2010, in the post-fire silver birch and black alder stands that had originated from spontaneous succession. The stands (within forest section No. 225a cover 15.55 ha) are located in close proximity to two small patches of mature, unburned woodlands that remained, which cover an area of 4.27 and 0.96 ha and represent alder–ash carr Fraxino–Alnetum W. Mat. 1952 and oak–hornbeam Tilio cordatae–Carpinetum betuli Tracz. 1962 communities (according to the phytosociological nomenclature of plant communities by Matuszkiewicz 2001) (forest sections 225b and 225c, respectively; Fig. 1b). According to the foresters’ reports (Szabla 1994; Hawryś 1998), as a consequence of the fire the soil physico-chemical properties, and microbial and hydrological habitat conditions throughout the entire affected area were severely altered and degraded. The litter and humus layers were completely burned to a depth of 7–10 cm or deeper. Subsequently, the burned material was lost because of the very strong wind erosion that occurred for several months after the fire. In contrast, prior to the fire the soils of the sites studied were described as Mollic Gleysols that had a 30–40 cm deep humus layer (O + A horizons). A year after the fire, when the soils within all of the post-fires woods were studied for management guidance issues, the soils in this part of the forest complex were classified as Stagnic Phaeozem with a 3-cm O horizon and a 35-cm-deep Ah layer, and as Haplic Stagnosol that lacked a litter layer (O horizon) and with a 20-cm A horizon (Operat glebowosiedliskowy 1995). Thus, compared with the original soil conditions (rich and fertile soils), it can clearly be seen that the post-fire area, which is currently occupied by young, early successional forests that are composed of pioneer tree species, is a mosaic of edaphic conditions with different microsites reflecting the intensity of burn. In some parts, the original humus layer was twice as thick as the one that remained after the fire. In such cases not only was the litter burned but also approximately 20 cm of the humus layer was destroyed, which was enough for most of the woodland species to be lost during the fire. However, there were also some areas in which thicker layers of the A horizons survived.
Unfortunately, although 17 years have passed after the fire, there are no comparative data on the secondary succession in these forests because no research was conducted here after the fire (except for studies on the stand structure composition and changes). One may only assume the possible direction based on the forest site types that are occupied by the current post-fire woods, i.e. their fertility and moisture. This, combined with the fact that in the direct neighbourhood of the post-fire forests, rich, mesotrophic forests untouched by the fire still exist, is an indication that mixed–deciduous and deciduous forests can develop here in the future.
Data collection
Studies were conducted within six transects located perpendicularly either across the border between the young post-fire stands and the unburned mature forests or between a post-fire birch stand and a post-fire alder wood isolated from any mature, unburned forest, but that still had a reasonably rich pool of woodland species in its herb layer (Fig. 1b). Transects were 4 m wide and consisted of quadrat sample plots of 16 m2, which were laid out at intervals of 4 m (Fig. 1c). To assess the pool of herbaceous woodland species present in the mature forests that had been unaffected by the fire, four quadrats of each transect were situated within them. The number of sample quadrats in the young forests of a post-fire origin differed among the transects and ranged between six and ten, depending on the species composition of the herb layer (a transect ended once the latter was homogenous and did not contain any woodland species). Transects 1 and 2 crossed the boundary between the alder–ash carr community of the unburned wood and the post-fire birch stand, and ended in the post-fire alder stand that is isolated from any mature, unburned forest. Although an alder stand appeared spontaneously after the fire, the assumption was made that some woodland species could have survived the fire there because of the dampness of its habitat. The indirect evidence supporting this assumption is that there are places within the post-fire forests where most of the humus layer was not burned. One may expect that the moist microhabitats suppressed the intensity of the fire and contributed to the partial survivorship of the A horizons. Moreover, even if the water conditions were altered as a consequence of fire, these transformations must have only been temporary since local subsidence and subsequent waterlogging was recorded there in the spring period during the studies. Transect 3 was laid out across the alder–ash carr community and a young alder stand of a post-fire origin which was located in its direct neighbourhood. Transects 4 and 6 crossed the border between an unburned oak–hornbeam community and a post-fire birch stand, while transect 5 was located entirely within young stands of a post-fire origin of the same age, namely an alder forest that was isolated from any mature unburned forests (the same one as at the ends of transects 1 and 2) and the silver birch stands (Fig. 1b). Again, because of its dampness, which did not permit the total burning of the humus layer, the alder wood was treated as a potential source of forest species.
In each quadrat of the transects, the individual percentage cover of each herbaceous species was estimated according to the following scale: 1, 5, 10 % and then at 10 % intervals.
Data analyses
All of the studies that have dealt with the recovery of the herb layer species in forests of a post-agricultural origin (Brunet and von Oheimb 1998; Bossuyt et al. 1999; Dzwonko 2001; Orczewska 2009a; 2011) followed the procedure proposed by Matlack (1994). This was also implemented in our studies. Therefore, the migration rates were counted based on the occurrence of the furthest individual (FI) and the most distant occurrence of the maximum cover of species (MC) in the plots that were located in the post-fire forests. Similar to Matlack (1994), the assumption was made that the boundary of the unburned forest that adjoined the young post-fire stands was the starting point of the migration process of herbaceous species into the post-fire woods and that the entire woodland species’ pool was present there once the recovery began. Once such a design is accepted, one should be aware that the migration rates are underestimated because it is assumed that all of the species considered were present on the edge of the unburned forest once their migration into the post-fire woods began. In addition, not all species from the unburned species pool began their colonisation at the same time. The drawbacks of such a design were pointed out by Bossuyt et al. (1999) and other researchers who calculated species migration rates. Nevertheless, these simplifications have been widely accepted as the procedure to use to obtain estimations about species colonisation capacities as expressed by their migration rates; and a similar procedure allows comparisons with the results of earlier studies. The border between the unburned and post-fire forests was easily detectable and was indicated by a line of old trees, which had been untouched by fire. Furthermore, the management activities on the burned sites, which involved the mechanical disturbances connected with the removal of dead trees followed by the preparation of the sites for new tree generation, ‘sharpened’ the boundary with the unburned woodland patches. To estimate the migration rates per year, the distance was divided by the forest’s age. The statistical significance in the mean migration rates of species among the sites was checked using the Kruskal–Wallis test (Statistica Copyright© StatSoft, Inc. (2014). STATISTICA (data analysis software system), Tulsa, OK, 74104, USA, version 12. www.statsoft.com.).
In the case of transects 1 and 2, which began in the mature alder–ash carr community, crossed a post-fire birch wood and ended in a post-fire alder wood isolated from any unburned woodland (for a detailed description of their locations see the “Data collection” section and Fig. 1b), only the migration rates for the species that were colonising the birch wood from the alder–ash carr community were calculated. For these reasons, once we had studied the distribution of species along all of the transects carefully, we decided to take into account only those plots that we had located at the maximum distances of 98 m (transect 1) and 102 m (transect 2) from the border of the mature forest. This was decided because at these distances none of the woodland species were present any longer. However, on the plots that were located further from the alder–ash community but closer to the boundary with the post-fire alder wood, the gradual appearance and successive increase in the percentage share of woodland species were recorded. Such a distribution pattern of woodland herbs indicates that the colonisation of a birch wood along the entire length of transects 1 and 2 took place from two directions, namely from the alder–ash carr community (taking into account in our calculations of species’ migration rates) and from the post-fire alder woodland.
As the next step, detrended correspondence analysis, DCA, was used to look for the main patterns in plot variation and for the possible environmental variables that were responsible for the distribution of the plots that were observed (Canoco for Windows 4.5, Copyright© Biometris—Plant Research International, Wageningen, Netherlands). This allowed any floristic similarities among the sites to be determined, especially between the unburned forest patches and the post-fire alder woods. Finally, to look for any species that showed an affinity to either the post-fire alder or birch wood, the frequencies of the species that were recorded in their herb layers were compared using the Fisher exact probability test (Statistica Copyright© StatSoft, Inc. (2014). STATISTICA (data analysis software system), Tulsa, OK, 74104, USA, version 12. www.statsoft.com.). For these calculations, the plots that had originated from the alder woods isolated from and adjacent to the mature woodland patches were merged into one group because of the low frequencies of individual species within them.
Results
The mean migration rates of herb layer species in the post-fire silver birch and black alder stands, which were based on the FI and furthest occurrence of species MC, differed among the sites (Table 1). They reached higher values in the alder and birch woods that were adjacent to the alder–ash carr community (exceeding 2 m year−1 for the FI measure and 1.7 m year−1 for MC) compared with the birch woods that had developed in the proximity of the oak–hornbeam community (1.26 and 1.08 m year−1 for FI and MC, respectively). The lowest mean migration values for both FI and MC were noted in the case of the birch wood adjacent to the post-fire alder stand that was isolated from any mature forest patches (0.77 and 0.71 m year−1, respectively). Statistically significant differences in the case of the FI measure were recorded between the birch and alder woods adjacent to the alder–ash carr communities and the other two types of sites. However, in the case of the MC values, only the differences between the post-fire birch and alder woods adjacent to the alder–ash carr community and the birch woods that had developed in the proximity of the post-fire alder stand were significant (Table 1).
The migration pace of most individual species differed among the sites. Most species migrated into the birch or alder stands from the adjacent alder–ash community at a quicker pace than to the birch woods located in direct proximity to the oak–hornbeam community or post-fire alder stands (Table S1). Of the 44 species for which the migration rates were calculated, 11 species were present on all four types of sites. Most of them showed the same pattern of recolonisation pace to the one described above with the exception of Brachypodium sylvaticum, which showed a reverse tendency (lower migration rates in the stands that were adjacent to the alder–ash carr community) and with Maianthemum bifolium, which was indifferent in its behaviour among the sites. Moreover, Ranunculus auricomus, which was present in the alder–ash carr community, did not migrate to any post-fire woods that had developed in its neighbourhood, similar to Mycelis muralis, which did not migrate to the birch stand from oak–hornbeam. Another ten species migrated only to some sites whereas, in other situations, they persisted exclusively in the unburned woods (Table S1).
Like the observations on the herb layer recovery in recent woods of a post-agricultural origin, in the case of the post-fire stands, there were groups of fast and slow colonisers. The following species reached the highest values of their migration rates, which ranged from 3 to 5 m year−1 for the FI measure (listed in a decreasing order of values): Moehringia trinervia, Luzula luzuloides, Brachypodium sylvaticum, Vaccinium myrtillus, Trientalis europaea, Lysimachia nemorum, Dryopteris carthusiana, Anemone nemorosa, Luzula pilosa, Athyriuum filix-femina and Scrophularia nodosa in the birch stands adjacent to the alder–ash carr community and Festuca gigantea and Moehringia trinervia in the birch woods adjacent to the oak–hornbeam community. On the other hand, there was also a large group of slow colonisers of the herb layer (for which the migration rates did not exceed 1 m year−1) that were present on all the four types of sites (see Table S1 for details). It is worth emphasising that among that group the instances of very slow colonisers, for which the migration rates reached 0.11–0.33 m year−1, were much more frequent in the birch woods adjacent to either oak–hornbeam community or the post-fire black alder stands than on the other two types of sites, namely, the birch or alder woods adjacent to the alder–ash carr community.
As was already mentioned, in the case of transects 1 and 2, the recovery of the herb layer in the birch wood took place from two directions, from the alder–ash carr community and from the post-fire alder wood that was isolated from any mature unburned forest patch. This is well confirmed when the distribution pattern of woodland herbs along these transects is observed. A decrease in their number and abundance until their total disappearance in the plots located further away from the unburned forest was assisted by their successive increase towards the border with the post-fire alder wood (see Tables S2, S3 for the species composition and abundance in transects 1 and 2).
The distribution of plots in the ordination diagram according to the first two DCA axes showed some interesting tendencies. The first axis significantly explains the plot distribution pattern (eigenvalue = 0.5636). It can be interpreted as the one that represents the forest origin gradient (with plots predominantly originating from the mature unburned forests on the left side of the graph vs plots from the post-fire birch woods, which are located on the right side) or as the gradient of decreasing moisture and fertility (with alder–ash carr, oak–hornbeam communities and post-fire alder woods on the left and the post-fire birch stands on the right side of the scatter plot) (Fig. 2). The interpretation of the second axis is not so clear (eigenvalue = 0.2923). The first DCA axis explains 13.4 % and the second 7 % of the variance in the species data.
There were only six species that were recorded significantly more frequently in the post-fire birch wood (seedlings of three tree species and three very common species of grasses and sedges, which are typical for disturbed forest sites with an open canopy—Agrostis capillaris, Calamagrostis epigejos and Carex brizoides). In contrast, 39 species showed an affinity to the black alder woods, of which 22 were ancient woodland indicator species (after Dzwonko and Loster 2001) (Table S4).
Discussion
The pace of forest recovery, especially the migration rates of individual woodland species on post-fire sites, has not been studied in detail. For these reasons, comparisons can only be made with the results of similar surveys in post-agricultural forests. The mean migration rates (based on the maximum cover and the furthest individual measures) calculated for the post-fire birch and alder stands adjacent to alder–ash carr (Fraxino–Alnetum) varied from 1.7 (1.72) to 2.16 (2.21) m year−1. In contrast, these values, when estimated for the post-agricultural alder woods of southern Poland that are adjacent to alder–ash carr forests were 0.79 and 1.26 m year−1 (Orczewska 2009a). The respective rates for the post-fire birch stand that was adjacent to the oak–hornbeam community (Tilio–Carpinetum), which ranged from 1.08 to 1.26 m year−1, were only slightly lower than those for the post-agricultural alder woods in this habitat (1.17–1.63 m year−1) (Orczewska 2009a). Nevertheless, they were much higher than in the case of the deciduous forests in southern Sweden (0.3–0.5 m year−1) (Brunet and von Oheimb 1998) or central Belgium (0.5–1.0 m year−1) (Bossuyt et al. 1999) and in the pine plantations growing in the habitat of deciduous forests in southern Poland (0.18–0.38 m year−1) (Dzwonko 2001). Therefore, the herb layer restoration in the post-fire woods that we studied proceeded faster than in similar habitats in the post-agricultural forests in Sweden, Belgium and Poland. In addition, the migration rates for many individual species in the post-fire woods that we surveyed exceeded those that were calculated by Brunet and von Oheimb (1998) and Bossuyt et al. (1999) in post-agricultural woods where species migrated at a pace that reached 0–1.25 m year−1, whereas in the post-fire woods, the rates were approximately three times higher. Slightly smaller differences were recorded in this respect compared with the post-agricultural alder woods in southern Poland and with deciduous forests of northeastern America, where the values for individual species reached 0–2.5 m year−1 (Matlack 1994). In contrast, both the mean migration rates (based on the maximum cover and the furthest individual measures) that were estimated for the post-fire birch wood adjacent to the alder wood of the same origin were significantly lower (0.71–0.77 m year−1) than those mentioned above. This indicates that the smaller number of forest species and the lower abundance of their populations in the isolated alder wood, compared with the unburned forest patches, contributes to a slower colonisation of the birch wood by forest herbs located in its direct proximity. As was reported from the studies in post-agricultural forests, forest recovery proceeds faster on more humid and fertile sites than in places with poor and dry soils (Dzwonko 2001; Verheyen et al. 2003; Orczewska 2009a, Orczewska and Fernes 2011). Such observations were confirmed in our studies because species migrated at a higher pace in the case of the alder woods than in the birch stands. Furthermore, the pace of herb layer restoration in the stands that were adjacent to the alder–ash carr was faster than in the case of those layers that had developed in the neighbourhood of the oak–hornbeam community.
Similar to the studies on the restoration of the herb layer in post-agricultural woodlands, species differed in their migration capacity in the post-fire forests. On the one hand there was a group of species, predominantly dispersed by ants (dispersal modes after Van der Pijl 1982), that did not colonise the post-fire forest, which indicates the presence of a dispersal limitation mechanism (for example, Carex sylvatica, Galeobdolon luteum, Mercurialis perennis and Ranunculus auricomus). On the other hand, there were both slow and fast colonisers of the post-fire forests present in their herb layer. Although the mechanisms of recruitment limitation have not been investigated, no doubt such factors, namely increased light availability and competitive exclusion of woodland species by vigorous and expansive grasses, played a significant role in the vegetation recovery in the post-fire birch and alder woods. The massive development of grasses and sedges that sprout after a fire is well known and has been reported by many authors (Daubenmire 1968, Singh 1993; cited by Whelan 1995) and was also observed during our studies. Such species either sprout from their meristems, which are hidden at the leaf base and surrounded by thick, compact tussocks (Whelan 1995), or from their underground parts (for example, long stolons, dense roots and horizontal rhizomes) which are well protected in the deeper layers of soil. The sprouting mechanisms described above refer to Carex brizoides and Calamagrostis epigejos, and allowed them to regenerate quickly and to conquer the vast areas that had been denuded by the fire. Both species are regarded as inhibitors of succession because they suppress the establishment and growth of other plants on the forest floor (Faliński 1998a, b). Faliński (1998b) observed that the dominance of the herb layer by Carex brizoides in an oak–hornbeam forest limits the abundance of populations of many woodland species including Hepatica nobilis, Sanicula europaea, Viola reichenbachiana, Galeobdolon luteum, Galium odoratum and Stellaria holostea. Similar cases were also reported by Kwiatkowska-Falińska (2008), who observed the presence of Holcus mollis and Corynephorus canescens in a post-fire site on poor soils that had originally been covered by Pinus sylvestris stands; and by Budzáková et al. (2013) on sites of spruce forests in the Tatra Mountains, burned and successively dominated by nitrophilous, competitive species such as Rubus idaeus. As was mentioned by Budzáková et al. (2013), any disturbance, including fires, creates open sites that have perfect conditions for the germination of seeds and for the vegetative spread of plants that are normally suppressed on the forest floors of undisturbed forests.
In the post-fire birch wood that we studied, where much more of the humus layer was lost in the fire than in alder woods, except for the Calamagrostis epigejos and Carex brizoides, only a few species were potentially able to survive a fire on a site (either their seeds, stolons or rhizomes), especially those that were deeply rooted. Seeds, and other diaspores of woodland species that were stored in the litter or buried in the upper parts of the A horizons, were consumed by the fire in the birch wood. According to Parusel (2006), who studied post-fire forests in the neighbouring Rudy Raciborskie where all the humus layer was burned, the presence of Calamagrostis epigejos, C. villosa, Carex nigra, Deschampsia caespitosa, Juncus conglomeratus, J. effusus, Molinia caerulea, Pteridium aquilinum and Rubus plicatus were of such an origin (typical sprouters sensu Biswell 1974 and Keeley 1981), whereas most species must have naturally colonised the herb layer of these forests afterwards.
In contrast, in one of the alder woods that we studied, where the losses of humus were distinctively smaller because of the natural dampness of the site (Operat glebowosiedliskowy 1995), the woodland species pools were noticeably larger. The rhizomes and viable seeds of some woodland species that are intolerant to fire managed to endure it there because the intensity of the fire in these places was suppressed when compared with the neighbouring sites currently occupied by birch stands. Otherwise, if the entire seed bank in the alder wood had been lost in the fire, the woodland species that were noted there would have migrated there from the unburned alder–ash carr forest, which was more than 200 m from the post-fire alder wood. As most woodland species are dispersal-limited, such a situation is unlikely.
Conclusions
The recovery of the post-fire forest communities is not a uniform process. On the one hand, it depends heavily on the presence of patches of mature forests. Such woods maintain the rich populations of woodland species that successively colonise post-fire woods. Their direct proximity to post-fire stands allows the recovery process to be hastened. This is similar to the restoration that takes place in forests of post-agricultural origin that are adjacent to ancient woodlands, and which has been reported by many authors (Peterken and Game 1984; Matlack 1994; Brunet and von Oheimb 1998; Dzwonko 2001). On the other hand, even a small mosaic of moist microsites such as deep forest ditches, local depressions and other places where water can stagnate in the summer, even when they are isolated from mature, unburned forests, can contribute to the survivorship of populations of forest herbaceous species and can subsequently speed up the forest recovery process.
In the post-fire forests the recovery of woodland species populations is the combined effect of both regeneration (based on autochthonic diaspores) and secondary succession (relying on allochthonic diaspores), and depends heavily on the intensity of the fire. Secondary succession plays a much bigger role than regeneration on sites whose humus horizons, where most woodland species store their diaspores, have been consumed by fire.
References
Baeten L, Hermy M, Verheyen K (2009) Environmental limitation contributes to the differential colonization capacity of two forest herbs. J Veg Sci 20:209–223. doi:10.1111/j.1654-1103.2009.05595.x
Bekker RM, Bekker JP, Grandin U, Kalamees R, Milberg P, Poschlod P, Thompson K, Willems JH (1998) Seed size, shape and vertical distribution in the soil: indicators of seed longevity. Funct Ecol 12:834–842. doi:10.1046/j.1365-2435.1998.00252.x
Bierzychudek P (1982) Life histories and demography of shade-tolerant temperate forest herbs: a review. New Phytol 90:757–776. doi:10.1111/j.1469-8137.1982.tb03285.x
Biswell HH (1974) Effects of fire on chaparral. In: Kozlowski TT, Ahlgren CE (eds) Fire and ecosystems. Academic Press, New York, pp 321–364
Bossuyt B, Hermy M, Deckers J (1999) Migration of herbaceous plant species across ancient-recent forest ecotones in central Belgium. J Ecol 87:628–638. doi:10.1046/j.1365-2745.1999.00379.x
Brown JK, Smith JK (eds) (2000) Wildland fire in ecosystems: effects of fire on Flora. General Technical Report RMRS-GTR-42, vol 2. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Ogden
Brunet J, von Oheimb G (1998) Migration of vascular plants to secondary woodlands in southern Sweden. J Ecol 86:429–438. doi:10.1046/j.1365-2745.1998.00269.x
Budzáková M, Galvánek D, Littera P, Šibík J (2013) The wind and fire disturbance in Central European mountain spruce forests: the regeneration after four years. Acta Soc Bot Pol 82:13–24. doi:10.5586/asbp.2013.002
Certini G (2005) Effects of fire on properties of forest soils: a review. Oecologia 143:1–10. doi:10.1007/s00442-004-1788-8
Dzwonko Z (2001) Migration of vascular plant species to a recent wood adjoining ancient woodland. Acta Soc Bot Pol 70:71–77. doi:10.5586/asbp.2001.010
Dzwonko Z, Loster S (2001) Wskaźnikowe gatunki roślin starych lasów i ich znaczenie dla ochrony przyrody i kartografii roślinności. Prace Geogr 178:119–132
Ehrlén J, Eriksson O (2000) Dispersal limitation and patch occupancy in forest herbs. Ecology 81:1667–1674
Faliński JB (1998a) Deciduous woody pioneer species (Juniperus communis, Populus tremula, Salix sp. div.) in the secondary succession and regeneration. Phytocoenosis N.S. 10:1–156
Faliński JB (1998b) Invasive alien plants, vegetation dynamics and neophytism. Phytocoenosis 10 (Supplementum Cartographiae Geobotanicae 9):163–187
Flannigan MD, Stocks BJ, Wotton BM (2000) Forest fires and climate change. Sci Total Environ 262:221–229. doi:10.1016/S0048-9697(00)00524-6
Flinn KM, Vellend M (2005) Recovery of forest plant communities in post-agricultural landscapes. Front Ecol Environ 3:243–250. doi:10.1890/1540-9295(2005)003[0243:ROFPCI]2.0.CO;2
Hanak B (1994) Koncepcja zagospodarowania wielkich pożarzysk leśnych na terenie Regionalnej Dyrekcji Lasów Państwowych Katowice. Sylwan 138:85–93
Hawryś Z (1998) Odnowienie pożarzyska w lasach rudzko-rudziniecko-kędzierzyńskich. Przyroda Górnego Śląska 14:7
Hermy M, Honnay O, Firbank L, Grashof-Bogdam C, Lawesson JE (1999) An ecological comparison between ancient and other forest plant species of Europe, and the implications for forest conservation. Biol Conserv 9:9–22. doi:10.1016/S0006-3207(99)00045-2
Honnay O, Bossuyt B, Verheyen K, Butaye J, Jacquemyn H (2002) Ecological perspectives for the restoration of plant communities in European temperate forests. Biodivers Conserv 11:213–242. doi:10.1023/A:1014531011060
Jacquemyn H, Brys R (2008) Effects of stand age on the demography of a temperate forest herb in post-agricultural forests. Ecology 89:3480–3489. doi:10.1890/07-1908.1
Keeley JE (1981) Reproductive cycles and fire regimes. In: Fire regimes and ecosystem properties. USDA Forest Service General Technical Report WO-26
Keeley JE (2009) Fire intensity, fire severity and burn severity: a brief review and suggested usage. Int J Wildland Fire 18:116–126. doi:10.1071/WF07049
Kilgore BM (1987) The role of fire in wilderness: a stage-of-knowledge review. In: Lucas, Robert C., comp. Proceedings—National Wilderness Research Conference: issues, state-of-knowledge, future directions, Fort Collins, CO. General Technical Report INT-220. U. S. Department Agriculture, Forest Service, Intermountain Research Station, Ogden, 23–26 July 1985, pp 70–103
Kwiatkowska-Falińska A (2008) Post-fire succession on abandoned fields in coniferous habitat (north-east Poland). Acta Soc Bot Pol 77:235–254
Matlack GR (1994) Plant species migration in a mixed-history forest landscape in eastern North America. Ecology 75:1491–1502
Matlack GR (2013) Reassessment of the use of fire as a management tool in deciduous forests in Eastern North America. Conserv Biol 27:916–926. doi:10.1111/cobi.12121
Matuszkiewicz W (2001) Przewodnik do oznaczania zbiorowisk roślinnych Polski. Vademecum Geobotanicum. Państwowe Wydawnictwo Naukowe, Warszawa
Operat glebowosiedliskowy (1995) Materiały dokumentacyjne. RDLP w Katowicach. Nadleśnictwo Rudziniec, Cz. 2. Biuro Urządzania Lasu i Geodezji Leśnej. Oddział w Przemyślu, Pracownia Gleboznawczo-Siedliskowa w Rzeszowie, Rzeszów
Orczewska A (2009a) Migration of herbaceous woodland flora into post-agricultural black alder woods planted on wet and fertile habitats in south western Poland. Plant Ecol 204:83–96. doi:10.1007/s11258-008-9570-3
Orczewska A (2009b) The impact of former agriculture on habitat conditions and distribution patterns of ancient woodland plant species in recent black alder (Alnus glutinosa (L.) Gaertn.) woods in south-western Poland. Forest Ecol Manag 258:794–803. doi:10.1016/j.foreco.2009.05.021
Orczewska A (2011) Colonization of post-agricultural black alder (Alnus glutinosa (L.) Gaertn.) woods by woodland flora. In: Wallace EB (ed) Woodlands: ecology, management and conservation. Nova Science Publishers, Inc., New York, pp 13–48
Orczewska A, Fernes M (2011) Migration of herb layer species into the poorest post-agricultural pine woods adjacent to ancient pine forests. Pol J Ecol 59:113–123
Orczewska A, Obidziński A, Żołna K (2010) Wpływ czyszczeń na rozwój roślinności runa w spontanicznych odnowieniach brzozowych po pożarze. Studia i Materiały Centrum Edukacji Przyrodniczo-Leśnej 12:377–387
Parusel JB (2006) Sukcesja roślinności na wielkim pożarzysku w Lasach Rudzkich. In: Environmental Changes and Biological Assessment III: Scripta Facultatis Rerum Naturalium Universitatis Ostraviensis 163, pp 77–87
Peterken GF (1974) A method for assessing woodland flora for conservation using indicator species. Biol Conserv 6:239–245. doi:10.1016/0006-3207(74)90001-9
Peterken GF (1977) Habitat conservation priorities in British and European woodlands. Biol Conserv 11:223–245. doi:10.1016/0006-3207(77)90006-4
Peterken GF, Game M (1984) Historical factors affecting the number and distribution of vascular plant species in the woodlands of central Lincolnshire. J Ecol 72:155–182. doi:10.2307/2260011
Szabla K (1994) Warunki powstania i rozwoju pożarów, niektóre działania organizacyjne oraz aktualne zagadnienia hodowlane i ochronne na pożarzysku w Nadleśnictwie Rudy Raciborskie. Sylwan 138:75–83
Thompson K, Bakker JP, Bekker R (1997) The soil seed banks of north west Europe: methodology, density and longevity. Cambridge University Press, New York
Tsuyuzaki S, Narita K, Sawada Y, Harada K (2013) Recovery of forest-floor vegetation after a wildfire in a Picea mariana forest. Ecol Res 28:1061–1068. doi:10.1007/s11284-013-1087-0
Van der Pijl L (1982) Principles of dispersal in higher plants. Springer, Berlin
Verheyen K, Bossuyt B, Honnay O, Hermy M (2003) Herbaceous plant community structure of ancient and recent forests in two contrasting forest types. Basic Appl Ecol 4:537–546. doi:10.1078/1439-1791-00210
Whelan RJ (1995) The ecology of fire. Cambridge University Press, Cambridge
Whigham DF (2004) Ecology of woodland herbs in temperate deciduous forests. Annu Rev Ecol Evol S 35:583–621. doi:10.1146/annurev.ecolsys.35.021103.105708
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Orczewska, A., Prukop, M. & Strzelczyk, A. Recovery of the herbaceous layer in the young silver birch and black alder stands that developed spontaneously after a forest fire. Ecol Res 31, 125–133 (2016). https://doi.org/10.1007/s11284-015-1321-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-015-1321-z