Abstract
Pastoralism is pervasive and has a long history across the rangelands of Trans-Himalaya. Disturbance associated with pastoralism can influence the behaviour of wild animals; hence, it is important to better understand its effects on wild animal behaviour. We compared the activity budget of the Himalayan marmot (Marmota himalayana) between areas experiencing both high and low levels of pastoralism, in the Upper Mustang region in Nepal. Scan sampling was used to collect diurnal activity budget data on adult marmots, whereas 2 min focal observations were made on foraging marmots to assess vigilance during foraging. Contrary to our prediction, there was no significant difference between areas of high and low pastoralism in terms of foraging behaviour. However, the vigilance activity of marmots was significantly influenced by the extent of disturbances associated with pastoralism. Marmots scanned the surroundings more often while foraging and spent more time scanning in high pastoralism sites as compared to marmots in low pastoralism sites. Although we found no direct negative effects of pastoralism on foraging time, marmots shifted the time of day when they foraged. This study suggests that marmots adjust their vigilance behaviour according to the environmental conditions in which they occur. These findings have important implications for the conservation of marmots in the wake of increasing pastoral activities and consequent increases in human-wildlife conflict in Nepal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Himalayan marmot (Marmota himalayana) lives in colonies in the high altitude mountains and rangelands of Nepal where nomadic pastoralism occurs (Nikol’skii and Ulak 2007). Himalayan marmots are considered one of the highest elevation living mammals in the world (Nikol’skii and Ulak 2006). The Trans-Himalayan landscape is undergoing considerable changes with expanding developmental projects and changing socio-economic conditions (NTNC 2008). This, coupled with predicted rapid climatic and phenological changes in the Himalaya (Shrestha et al. 2012; Aryal et al. 2014a), is likely to provide suitable conditions to expand and intensify pastoralist activities in Nepal. These predicted changes in the nature of Nepalese pastoralism may increase human-wildlife conflict in the region (Aryal et al. 2014b), and have potential detrimental effects on marmot foraging activities due to competition with grazing animals (Shrestha and Wegge 2008).
Foraging theory suggests that animals maximize their rate of energy intake whilst minimizing the risk of predation (Verdolin 2006). There is a trade-off for animals in terms of the benefits associated with obtaining food and risks of predation (Lima and Dill 1990). Animals often make two types of behavioural adjustment in response to the perceived risk of predation: vigilance and group formation (Caro 2005). Vigilance has long been recognized as an anti-predator behaviour, where animals scan the surroundings for predators (Lima and Bednekoff 1999; Treves 2000). Vigilance entails costs because it requires time and attention otherwise spent on activities such as foraging and resting (Fortin et al. 2004a). Vigilance not only influences the time spent foraging, but it also can affect their feeding efficiency through food intake rate (Fritz et al. 2002). If foraging is limited, this could affect energy intake, which may have significant consequences on an animal’s body mass and population viability (Ozgul et al. 2010).
Wild animals such as marmots normally perceive humans and their livestock as potential predators. Therefore the effects of human disturbance are analogous to natural predation risks (Frid and Dill 2002; Beale and Monaghan 2004). Previous studies have described how anthropogenic disturbances are associated with behavioural changes in marmot species (Neuhaus and Mainini 1998; Semenov et al. 2002). Human disturbance strongly influences the behaviour in marmots populations, and under perceived risk of disturbance, marmots become more vigilant (Griffin et al. 2007; Li et al. 2011). The cost of disturbance is a waste of time and energy (Houston et al. 2012) and in a human-dominated landscape such as the Trans-Himalayan rangelands, animals are much more likely to be disturbed by humans than by natural predators (Ciuti et al. 2012). Hence, quantifying activity budget to estimate the consequences of anthropogenic disturbance is an essential first step towards understanding the potential long-term energetic consequences for animals (Christiansen et al. 2013).
Foraging is particularly important for hibernating mammals such as marmots, which exhibit a circannual rhythm of energy intake and body mass (Dark 2005). This is because increased energy intake during summer active months is strongly correlated with winter survival and reproduction (Kuhn and Vander Wall 2008). Recent studies of other marmot species have found a relationship between increased body mass gained over the active season and survival during hibernation, reproduction rates, and subsequent litter and population size (Ozgul et al. 2010; Tafani et al. 2012). However the efficacy of successful foraging is strongly associated with time invested otherwise in vigilance and other behaviours.
Vigilance is influenced by several factors (Armitage 2014), including distance to burrow (Blumstein et al. 2001), visibility (Bednekoff and Blumstein 2009; Ferrari et al. 2009), distance to other neighbours (Fernández-Juricic et al. 2007), age and sex (Neuhaus and Mainini 1998; Lea and Blumstein 2011), reproductive status (Childress and Lung 2003), type of stimuli (Blumstein et al. 2009; Li et al. 2011), parental status (Lenti Boreo 2003), and human activities (Griffin et al. 2007). In general, animals invest more time in vigilance behaviour in areas experiencing high risks (Unck et al. 2009). Moreover, the response of animals often varies in terms of their familiarity to the risk (Crawford et al. 2012). Studies have shown that animals can learn about threat levels and adjust their behaviour accordingly (Ikuta and Blumstein 2003).
The aim of this study was to investigate the influence of anthropogenic disturbance associated with pastoralism on the activity budget and vigilance strategy of Himalayan marmots. Recent work on the Himalayan marmot (Poudel et al. 2015) found a temporal shift in activity patterns in relation to pastoralism. In Nepal, marmot colonies often occur in rangeland environments also used by domestic livestock (Aryal et al. 2013, 2015). Himalayan marmots are subject to livestock disturbances which have the potential to affect the time spent on different behavioural activities (e.g. foraging). We predict that marmots would spend more time being vigilant, and correspondingly less time foraging in areas experiencing high levels of disturbance associated with pastoralism. We also tested the prediction that marmots foraging in areas experiencing high levels of disturbance from pastoralism would scan the surroundings more often, and spend more time on scanning, than foraging marmots in low pastoralism sites.
Materials and methods
Study species and area
The Himalayan marmot (Sciuridae: Marmota himalayana Hodgson, 1841) is a social, burrowing, herbivorous mammal that lives in alpine and sub-alpine meadows in the mountains of central Asia (Armitage 2000; Nikol’skii and Ulak 2006). The species is categorized globally as ‘Least Concern’ on the IUCN Red List; it has a wide geographical distribution, but its population trend is largely unknown (Molur and Shrestha 2008). They occur in the areas between timber line and snow line, at elevations of 3000–5500 m above sea level (Nikol’skii and Ulak 2007; Aryal et al. 2015). They live in colonies, the size of a colony reaches up to 50 ha and consists of 5–30 family groups (Nikol’skii and Ulak 2007). The species remains active from April to September, and hibernates for the remainder of the year. The young are born toward the end of hibernation or after the animals have emerged from hibernation, after a 1-month gestation period (Smith et al. 2010). The litter size usually ranges 2–11 young (Smith et al. 2010). They dig numerous deep burrows (Nikol’skii and Ulak 2007), and are shared by colony members during winter hibernation (Molur and Shrestha 2008). They depend on burrows as a refuge (Berryman and Hawkins 2006).
The daily activity pattern of Himalayan marmot, like all other species of marmots, is bimodal, mostly active during morning and late afternoon (Poudel et al. 2015). Livestock grazing pastures provide an ideal habitat for the marmots (Nikol’skii and Ulak 2006). Himalayan marmots serve as important prey for many predators, for example snow leopards (Panthera uncia), and brown bears (Ursus arctos) (Aryal et al. 2012, 2014c; Devkota et al. 2013). They are ecologically important and sometimes described as an ‘ecosystem engineer’ because of the role they perform in vegetation dynamics (Bagchi et al. 2006; Davidson et al. 2012). Marmots are especially vulnerable to environmental changes associated with climate change (Inouye et al. 2000; Ozgul et al. 2010; Armitage 2013). The Himalayan marmot is one of the least-understood marmot species (Le Berre and Ramousse 2007), whose ecology is poorly known (Nikol’skii and Ulak 2007).
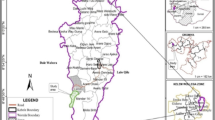
This study was conducted in the Upper Mustang region (N29°10′, E83°54′) of the Annapurna Conservation Area in northern Nepal (Fig. 1). The Upper Mustang region is located in the Himalayan rain shadow area; it is a desert-like landscape, and characterized by an arid climate (average annual rainfall <200 mm) and strong desiccating winds (Ohba et al. 2008). Most precipitation occurs in the form of snow during winter (from November until February), whilst some rainfall occurs during the monsoon (June–August). The daily temperature falls below freezing for 191 days, and has a maximum temperature of 18 °C in July and a minimum of −12 °C in January (Pokharel and Chetri 2006). The vegetation has been characterized as a Trans-Himalayan steppe dominated by a scarce and scattered patches of thorny cushion plants (e.g., Caragana spp., Astragalus spp., and Lonicera spp.), while grasses, sedges and forbs are also common on some meadows and grasslands (Ohba et al. 2008).
Location of the Upper Mustang region of Annapurna Conservation Area (shaded) in Nepal (a), and location of the study area (within the box) in the Upper Mustang region (b). Upper Mustang region includes seven Village Development Committee areas (VDCs)—Lhomanthang, Chhonhup, Chhoser, Surkhang, Chhusang, Ghami and Charang. VDC is the smallest political unit in Nepal and commonly in use
We conducted the study in the Lhomanthang and Chhonhup Village Development Committee (VDC) areas (Fig. 1) where marmots are widely distributed, located in a typical high alpine meadow, ranging above 4000 m asl. There is a long history of animal husbandry and pastoralism in these high altitude rangelands (Schaller 1977). The livestock assemblages of the region typically include: goat (Capra hircus), sheep (Ovis aries), cow (Bos indicus), yak (Bos grunniens) and horse (Equus caballus) (Aryal et al. 2014b). We conducted a reconnaissance survey before the beginning of this research and characterized the present grazing regime. We estimated approximately 8500 livestock (5600 goats/sheep, 800 cows, 600 horses and 1500 yaks) grazed in the study area during the study season. Pastoralists corral the livestock every evening inside their villages, or near to a pastoral camp, and then herded out to surrounding grazing areas each morning accompanied by herders and their herding dogs. Yaks were semi free-ranging, i.e., herders release the yaks for grazing in the morning and are herded back to campsites in the evening, but did not follow the yak herds throughout the day, whilst goats, sheep and cows were herded at farther distances from the campsites (or villages) by herders and brought to the campsites (or villages) in the evening. Goat and sheep were herded in mixed herds, the size of which generally ranged 100–300 individuals. Detailed descriptions of the grazing system in the region can be found in Pokharel and Chetri (2006) and Paudel and Andersen (2010).
The Tibetan woolly hare (Lepus oiostolus) is the only wild herbivore that shares the study area with marmots during summer. Snow leopards and brown bears are known to use this area (Aryal et al. 2014b), especially during winter, but no terrestrial predators were observed during the fieldwork period. The Golden eagle (Aquila chrysaetos) is the only avian predator known to feed on marmots. Hence we assumed that the risk from natural predators was similar across sites.
Selection of study sites
Behavioural data was collected from thirty different marmot sites selected across the study area. Because the average area of a family group of Himalayan marmot generally ranges 0.5–1.7 ha (Nikol’skii and Ulak 2007), and maximum distance travelled from their burrows is 48 m (Poudel et al. 2015), we defined a marmot ‘site’ as an area encompassing the burrows occupied by a family group or interacting family group within a radius of approximately 100 m, that was separated from other marmot sites by a minimum of 270 m. This process of site selection was developed to avoid resampling the same individual in more than one site, and ensure sites were independent of each other in terms of behavioural interactions.
At each site, the extent of disturbances associated with pastoralism was assessed using the following attributes: presence of livestock, humans, and dogs (Griffin et al. 2007; Namgail et al. 2007); distance to camps (Sasaki et al. 2009; Dorji et al. 2013); and the density of major human/tractor trails (Cingolani et al. 2008; Paudel and Andersen 2010). We continuously recorded the number of livestock herds, humans and guard dogs that passed through each site during daylight hours, from 0700 to 1900 h over two consecutive days. The distance of each site to the nearest pastoral camp was measured. The total length of major human/tractor trails was estimated in each site. Each attribute was first scaled and assigned an ordinal value between 0 and 5 based on their intensity of effects. We then calculated a combined index of disturbance for each site. Owing to a marked bimodal distribution of this index, we divided the sites into the two pastoralism disturbance categories: ‘low’ and ‘high’ pastoral activity sites.
This process resulted in the selection of 20 ‘low’ and 10 ‘high’ pastoral intensity sites for subsequent marmot observations. In general, ‘high’ sites were closer to pastoralist camps, and frequently exposed to disturbances from domestic livestock, human and dogs; whereas ‘low’ sites were farther away from camps and were infrequently disturbed by pastoralist activities (see supplementary material, Table S1). Through the use of multiple sites in each level of pastoralism (high and low) and use of mixed modelling approach (see below), we minimized and segregated the effects of site-specific conditions on marmot behaviour. Using this approach, we also ensured that the average group size of adult marmots in high and low sites was similar (‘high’: mean = 3.6, range = 2–7 individuals; ‘low’: mean = 3.0, range = 2–5 individuals; Mann–Whitney test: U = 89, P = 0.63, n = 30).
Marmot activity budget
We conducted behavioural observations using an instantaneous scan sampling method (Altmann 1974; Martin and Bateson 2007). For simplicity, marmots were classified into two age-classes based on body size: juvenile (less than one year or in their first summer) and adult (≥2 years old). Young were markedly smaller relative to the adult individuals. In the current study, only adult marmots were considered to control for age effects. At each site, the activities of all the visible adult marmots were recorded at 15-min intervals during daylight hours, between 0700 and 1900 h, over 2 consecutive days from mid-June to early July. The observation period coincided with the period when the young had emerged above-ground. Observations were made from an unobtrusive vantage point, usually behind the rock or shrub (at distances 60–150 m from the site, where a ‘site’ encompassed a buffer area of at least 100 m radius—see site selection criteria), at which they showed no evidence of reactions to observers. For each scan, we recorded the above-ground activity of each marmot, using binoculars (8 × 42) and a telescope (20–60×). We accumulated 735 h (high sites: 245, low: 490 h) of direct observations of marmots at 30 sites.
Activities were classified as: (1) foraging; (2) vigilance; (3) travelling; (4) resting; and (5) other activities. Foraging was defined as the act of ingesting forage with the animal’s head down. We defined vigilance behaviour as the scanning behaviour. Scanning is the best estimate of vigilance (Armitage and Chiesura Corona 1994; Childress and Lung 2003), where an animal pauses feeding or other behaviour, and raise their head up to scan the surroundings. Travelling included walking and running. Resting was defined as normal lying at rest and sunning-out activity. ‘Other’ activities (playing, fighting, burrow digging, and grooming activities) were also recorded.
Vigilance behaviour during foraging
Whilst marmots were foraging, we used a focal animal sampling approach (Altmann 1974; Martin and Bateson 2007) to record marmot scanning behaviour to estimate the extent of their vigilance. Each site was visited 2 times, in the morning (0800–1100 h) and in the evening (1500–1800 h) periods, when Himalayan marmots were most active (Poudel et al. 2015). During each time, focal observations were made on 2–3 individuals, so the number of observations in a site varied from 4 to 6. For each focal sample, a foraging marmot was randomly selected and observed for approximately 2.5 min, following the methods of Blumstein et al. (2001). The 2.5 min animal observation period was selected, because longer time periods would include activities other than foraging and vigilance (Blumstein 1996). Successive observations were separated by 1 h intervals. Because marmots were not marked, and individual identification was not always possible, it was probable that one marmot contributed to more than one point in our dataset (though changes in foraging group size were noted). To minimize potential pseudoreplication (Hurlbert 1984), we observed marmots in randomized feeding locations for each site in any time period (morning or evening). To avoid resampling of the same individual, we systematically shifted our focus to different individuals in a group. In total, 52 observations were recorded from 10 high pastoralism sites, and 101 observations from 20 low pastoralism sites.
Only adult marmots were considered to control age effects. Observations were made on foraging marmots (Blumstein et al. 2004) when no young were present. We conducted this study from mid-June to early July, 2014. The days of observation were randomized among 30 sites to prevent potential bias that would arise if we first observed one set of sites (‘low’ or ‘high’). It was not possible to discriminate sex of focal marmots from distance. We assumed that the bias associated with sex was minimal because we randomly selected focal animal and observed 152 marmots, which is large relative to the categories of sex. These observations were dictated into SONY IC Recorder (ICD-UX512F/UX513F). Marmots were observed from an unobtrusive vantage point, usually behind a rock or shrub, located at distances of 60–150 m from the focal animal. Care was taken not to disturb the focal animal prior to, or during the observation period. When the focal individual moved out of sight (e.g., behind a rock or a shrub) or fled to a burrow, the sampling attempt was aborted. Samples were also discarded when marmots were disturbed by the following: (1) calls from another individual, (2) the presence of a herder or the observer, or (3) a natural predator (e.g., a golden eagle). Samples were also discarded when an animal moved more than 10 m during an observation period.
For each observation, we recorded the time of day, foraging group size (number of marmots within 15 m from the focal marmot) and distance from nearest burrow, which were hypothesized to influence vigilance (Blumstein 1996; Blumstein et al. 2004). Distances were measured with a Laser Rangefinder (MDL LaserAce 1000). For each observation, SONY recorder data were downloaded, and the first 0.5 min of observation was discarded and not analysed to remove any biases associated with the initiation of the observation period (Griffin et al. 2007). Animal recordings were transcribed and scored using JWatcher 1.0 (Blumstein and Daniel 2007).
Statistical analysis
Activity budget
We calculated the proportion of time marmots allocated to each of the five activities per scan for each site. We used the maximum number of marmots observed in any one scan on any day as the total population. Temporal patterns of marmot activities were analysed during 3 time periods: morning (0700–1000 h), midday (1001–1600 h), and evening (1601–1900 h). For each period, we averaged the proportion of activity time spent above-ground from all scans to get a single value per site (N = 90, 30 sites × 3 periods). These periods followed the local time of livestock herders, and represented different periods of risk to marmots. In high pastoralism sites, mornings and evenings were considered high risk, when herders and their dogs were near to the marmots, and the midday period was considered low risk as the herders were far from the marmots, and vice versa in low pastoralism sites.
General linear mixed models (LMM) were used to investigate the effect of pastoralism and time of day on marmots’ activity budget. As the proportion of time spent in each of the five activities in an activity budget totaled 1, and hence were not independent, principal component analysis (PCA) was conducted before the LMM was run to extract the major axis of variation of marmot activity budget. PCA was performed based on the correlation matrix produced using PAST 3.0 (Hammer et al. 2001). Using this approach, components with eigenvalues greater than 1 (Kaiser’s criterion) were used as response variables in the LMM. The function ‘lmer’ of the library ‘lme4’ (Bates et al. 2014) in R 3.1.2 (R Core Team 2014) was used for fitting the LMM. We entered pastoralism (factor with 2 levels), time of day (factor with 3 levels) and their interaction (pastoralism × time) as fixed effects into the model. To account for potential pseudoreplication and an unbalanced design with unequal sample sizes between levels of pastoralism, we used site as a random variable for all models (Pinheiro and Bates 2000; Bolker et al. 2009). P-values were obtained by likelihood ratio tests of the model with the effect in question, against the model without the effect in question, to test for significance.
Vigilance during foraging
Three variables were used to assess vigilance behaviour: (1) the number of times marmots scanned in a two minute period (“Number of scan”), (2) the proportion of time spent on scanning (“Percent time scanned”), and (3) the average time spent on scanning (“Average scan time”). Data were checked to meet assumptions for outliers, normality, and homogeneity according to protocols described by Zuur et al. (2010). To reduce the heterogeneity of the variances and increase the residual normality, the factor “Number of scan” was square-root transformed, and “Average scan time” and “Percent time scanned” were log-transformed. A Shapiro–Wilk’s test (P > 0.05), and visual inspection of histograms and box plots, showed that the transformed data were normally distributed for both high- and low-pastoralism sites.
To investigate which vigilance parameters differed between the high- and low pastoralism sites, we repeated the LMM approach (Zuur et al. 2009). We used the extent of pastoralism (factor with 2 levels) as a fixed factor. Site was used as a random factor to account for potential correlations amongst observations within sites, because of repeated observations from the same site, and due to uneven sample sizes amongst sites. Prior to running the LMM, we used correlations and the general linear model to determine whether distance to burrow and foraging group size (both continuous) and time of the day (factor with 2 levels: morning and evening) influenced any of the three scanning behaviours. We found a significant relationship on scan frequency, so included them as covariates for all models. We analysed the three vigilance variables in separate models, with pastoralism, group size, distance and time as predictor variables.
We defined a set of a priori models (supplementary material, Table S2) and used the Akaike Information Criterion (AIC) to select the most parsimonious ones (Burnham and Anderson 2002). For each analysis, we present the best models (ΔAIC ≤ 2; Burnham and Anderson 2002), as well as the null model for comparison. The relative importance of factors was determined by summing the Akaike weight of the models containing these factors (ΔAIC ≤ 2). Model averaged coefficients and relative importance of factors were calculated using the ‘model.avg’ function of the ‘MuMIn’ package (Barton 2014). A factor was considered significant if P ≤ 0.05, and model R2 was used to evaluate the ability of each model. Marginal R2 (proportion of variance explained by the fixed factors) and conditional R2 (proportion of variance explained by both the fixed and random factors) were calculated according to Nakagawa and Schielzeth (2013). All analyses were carried out in R 3.1.2 (R Core Team 2014).
To assess whether the marmots could be correctly classified into pastoral categories (high and low pastoralism) based on vigilance behaviour, we conducted a nested permuted discriminant function analysis (pDFA) (Mundry and Sommer 2007). The pDFA was conducted in R 3.1.2 (R Core Team 2014) using a function (provided by R. Mundry) with the ‘MASS’ package (Venables and Ripley 2002) loaded. Before conducting the pDFA, we performed PCA on the correlation matrix to reduce the number of vigilance variables because of high inter-correlations. PCA analysis generated 2 components (PCA 1 and PCA 2) with eigenvalues greater than 1. These two components explained 97.5 % (PCA 1: 58.28 %, PCA 2: 39.22 %) of the total variation in the original vigilance behaviour. These components scores were used as variables for pDFA. The pDFA used 20 sites and four samples per site (number of cases selected = 80) to derive the discriminant functions. Remaining cases (153 − 80 = 73) were used for cross validation. The random selection of observations to be cross-validated was repeated 100 times and the results were averaged. A total of 1000 permutations were conducted for the analysis. Results were considered significant at P < 0.05. All data are reported as means ± 95 % confidence intervals unless otherwise stated.
Results
Activity budget of Himalayan marmots
Marmots spent more time in their burrows than in any other activity for both low and high pastoralism sites (low = 65.7 % of daylight hours; high = 67.5 %). When above-ground, marmots spent more time foraging (approximately 54 % for all sites) than in any other activity. Resting was the other major above-ground activity (low = 29.7 %; high = 23.6 %), followed by vigilance and travelling behaviours (Fig. 2).
The PCA produced two components that together accounted for 62.7 % (PCA 1 = 39.1, PCA 2 = 23.6) of the variation in activity budget of Himalayan marmots (Fig. 3). The first component (PCA 1) had a high positive value for resting (0.61) and high negative value for foraging (−0.68). Traveling and vigilance (0.55 and 0.42 respectively) were highly positively associated with PCA 2, while ‘other’ activity was negatively correlated (−0.69) with PCA 2 (Fig. 3). This analysis showed few patterns in above-ground behaviours in low pastoralism sites. However in high pastoralism sites, marmots tended to show greater vigilant behaviour (Fig. 3).
LMM analyses showed that pastoralism had a significant effect on the vigilance/travelling behaviour of marmots (PCA 2: P = 0.01; Table 1), and explained 36 % of the variation in our model. Marmots were more vigilant in high (mean = 15.0 % of activity) as compared to low (mean = 7.8 % of total time) pastoralism sites. However pastoralism had no significant influence on foraging and resting behaviours (PCA 1: P > 0.05; Table 1). Time of the day had no significant influence on foraging or vigilance behaviour (P > 0.05), however there was a significant interaction between pastoralism and time of day on foraging/resting behaviour (PCA 1: P = 0.01; Table 1). Marmots increased their foraging activities at midday and rested more frequently in the evening in high pastoralism sites, and vice versa in low pastoralism sites. Compared with morning and evening, marmots spent more time resting during midday in low pastoralism sites, whereas this pattern was opposite in high pastoralism sites (Table 2).
Vigilance behaviour during foraging
“Number of scans”
Marmots in high pastoralism sites scanned more frequently, and spent more time on scanning, than in low pastoralism sites (Fig. 4). Using an Akaike Information Criterion (AIC) approach, model results showed that the individual scanning behaviour of marmots was strongly influenced by the extent of pastoralism (Table 3). “Number of scan” was best modelled by including the factors pastoralism, group size, time, and distance as predictor variables (model weight = 0.55; marginal R2 = 0.39, conditional R2 = 0.53) (Table 3). In terms of their relative importance, most of the included predictor variables for the model “number of scan” were statistically significant (P < 0.05; Table 4). This analysis suggests that marmots were more vigilant during the evening, and scanned at high pastoralism sites more often than at low pastoralism sites (Table 4). By holding other predictors constant, further analysis showed that the mean “number of scan” at high pastoralism sites was 0.79 times greater than for low pastoralism sites.
“Average scan time” and “percent time scanned”
The most parsimonious model for “average scan time” contained the single variable group size, which only explained 22 % of variability (marginal R2 = 0.02, conditional R2 = 0.22). Pastoralism was not a factor in this top ranked model, but further models ΔAIC < 2 did contain pastoralism and were considered as competitive models, however model weights were relatively low (supplementary material, Table S2). Similarly, the best parsimonious model for “percent time scanned” included pastoralism and group size as factors, and explained 33 % of the total variation (marginal R2 = 0.06, conditional R2 = 0.33), however these models were also not strong (supplementary material, Table S2).
Comparison of vigilance behaviour during foraging between high and low pastoralism sites
Nested permuted discriminant function analysis (pDFA) showed that the marmots could be discriminated between areas based on the three scanning activities (pDFA: percent correctly cross-classified = 77.2 % compared with a 51.1 % random expected cross-classification; N = 73 cross-classified observations, P = 0.007). The function correctly classified 72.7 % of the total observations selected to derive the discriminant function to type of site (high and low pastoralism), as compared to 57.8 % using a random expected classification (pDFA: P = 0.009, N = 80). Therefore, the vigilance behaviour of marmots was different in high versus low pastoralism sites.
Discussion
Activity budget of marmots and pastoralism effects
This study presents the first data on the activity budget and behaviour of Himalayan marmots. In the Nepalese Trans-Himalaya rangelands, this species spend approximately half (54 %) of their above-ground time foraging. The percentage of time spent foraging by Himalayan marmots was higher compared with other marmot species, such as yellow-bellied marmots (up to 23 %; Armitage et al. 1996; Armitage and Chiesura Corona 1994), golden marmots (about 30 %; Blumstein 1998), hoary marmots (up to 40 %; Barash 1980; Taulman 1990), Olympic marmots (about 33 %; Griffin et al. 2007).
Time spent foraging by marmots is influenced by several factors, including food availability, season, sex, reproductive status, and other environmental factors (Armitage et al. 1996; Armitage 2014). In comparison to other marmot studies, we recorded differences in the foraging time of Himalayan marmots, which may be explained by seaonsal differences in study timing, and availability of food resources for different marmot species. Food availability is regarded as the most important factor in determining an animals’ activity budget (e.g. Bertolino et al. 2004; Hanya 2004). For example, Armitage (2014) has suggested that food abundance has a key influence on the foraging time in marmots. Lower food availability can increase the searching time of an animal, which in turn could increase foraging time. Our results suggest that Himalayan marmots need to forage as much as possible because food resources are often scarce in high-altitude rangelands in Nepal. Compounding this issue, these rangelands are also grazed upon by other domestic livestock, placing further pressures on limited food resources.
In our study, we sampled marmots when the young had emerged above-ground and coincided with the lactation period. This may account for greater foraging times we recorded for Himalayan marmots, as compared to other species (e.g. Johns and Armitage 1979; Taulman 1990; Armitage et al. 1996). For example, Armitage et al. (1996) found that yellow-bellied marmots in Colorado spent more time foraging during lactation. Methodological differences in the definition of individual behaviours also makes comparison between other marmot studies problematic. However, given that Himalayan marmots have limited above-ground time (Poudel et al. 2015) would explain why much of this time is spent foraging as compared to other species.
We found that disturbances associated with pastoralism significantly influenced the above-ground activities of marmots, where PCA showed that marmots altered the relative time spent conducting foraging, resting and vigilance behaviours in relation to grazing intensity. Though marmots showed similar patterns of foraging in both sites, marmots in high pastoralism sites spent more time conducting vigilance behaviour (see main vigilance discussion below). Vigilance is considered energetically costly if the time spent vigilant reduces the time available for foraging (Frid and Dill 2002; Fortin et al. 2004a). We found that Himalayan marmots traded resting time for increased vigilance time in high pastoralism sites. Resting is a fundamentally important behaviour in terms of an animal’s physiology (Lusseau 2004). Other studies have found that reduced resting can cause an increase in the expenditure of energy of an animal because its metabolic rate is considerably lower in resting than in any other activity (Bishop 1999). Consequently, increased vigilance (rather than resting) may affect the ability of marmots to build up fat for greater body condition required for overwinter survival.
In terms of foraging time, the lack of response by marmots to pastoralism confirms that foraging is an important above-ground activity for marmots. We frequently observed marmots simultaneously foraging with domestic livestock (Poudel et al., pers. obs.). Other studies have documented how summer foraging is strongly correlated with over-winter survival in fossorial squirrels (Kuhn and Vander Wall 2008). Likewise, marmots must obtain sufficient food to replace reserves, breed and then build up reserves again for the next winter. The body mass gained during the growing season is not just critical for winter survival, but for subsequent reproduction in marmots (Ozgul et al. 2010).
We also found a significant interaction between pastoralism and time of day, with marmots foraging more during the evening and resting more during the daytime in low pastoralism sites, and vice versa in high pastoralism sites. Increased foraging time in high pastoralism sites at midday, as compared to morning and evening periods, can be attributed to reduced pastoralist activities during this time. Therefore, marmots in high pastoralism sites appear to have reduced the temporal overlap with pastoralist activities, by adjusting their diurnal patterns of activity and the distances moved from their burrows in relation to the timing of pastoralist activities (temporal niche shift) (Poudel et al. 2015).
Our findings suggest that marmots do not compromise their daily energetic needs, rather, they adjust their behaviour according to prevailing conditions. As such, Himalayan marmots exhibit behavioural plasticity in relation to their activity budgets (sensu Armitage 2014; Maldonado-Chaparro et al. 2015). Such behavioural flexibility is considered an important trait which can facilitate adaptations to human-induced environmental changes (Tuomainen and Candolin 2011; Sih 2013).
Vigilance behaviour during foraging
The vigilance time of Himalayan marmots varied from 7.8 % (low pastoralism sites) to 15.0 % (high pastoralism sites), which is consistent with previous data recorded for the yellow-bellied marmot (Armitage et al. 1996). Differences in vigilance time between low and high pastoralism sites can be explained by different risks associated with each site. Marmots at high pastoralism sites are exposed to high levels of anthropogenic disturbance than low pastoralism sites, where the presence of humans is known to change the vigilance behaviour of other marmots (Griffin et al. 2007).
Pastoralism was found to strongly influence the vigilance behaviour of Himalayan marmots. Our data suggests that Himalayan marmots are perhaps less vigilant (i.e. in terms of scan frequency, scan time) in comparison to other Marmota species (Blumstein et al. 2001), however their response to the presence of humans is consistent with other published work on yellow-bellied marmots (Armitage et al. 1996; Li et al. 2011), Olympic marmots (Griffin et al. 2007), and alpine marmots (Neuhaus and Mainini 1998). For example, Griffin et al. (2007) found marked differences in scan frequency and total time spent on scanning between sites experiencing low to high levels of recreational use. Likewise, Li et al. (2011) reported that yellow-bellied marmots significantly increased the proportion of time they were vigilant when exposed to high vehicular pressures associated with human activities.
The most striking difference between high and low pastoralism sites was in the number of scans marmots performed. We found that pastoralism increased both the frequency of scanning behaviours and the total time marmots spent on scanning. Higher scan frequency, and correspondingly higher time spent scanning, in marmots at high pastoralism sites can be explained by high chance of encountering livestock, herders and their dogs (supplementary material, Table S1). However, the duration of each scan was relatively (although not significant) greater in low pastoralism sites (Fig. 4b). Fritz et al. (2002) described how the cost of scan duration is greater than that of scan rate through instantaneous intake rate. This suggests that marmots may partly reduce the vigilance costs experienced with more frequent scans, by minimizing the scan duration in high pastoralism sites. We also observed marmots chewing while scanning (Poudel et al., pers. obs.), which suggests that multi-tasking strategies are used to reduce the cost of vigilance (Fortin et al. 2004b; Makowska and Kramer 2007). However, our analysis could not separate ‘foraging-alert’ or ‘foraging-vigilance’ from other vigilance behaviours, as recorded by Armitage et al. (1996).
Vigilance is considered a component of wariness (Armitage and Chiesura Corona 1994; Brilot et al. 2012). Increased vigilance recorded by marmots in high pastoralism sites may therefore indicate increased wariness. Besides behavioural changes, human disturbance can also elicit physiological stress responses in wildlife (Creel et al. 2002; Ellenberg et al. 2006; Walker et al. 2006) because animals perceive humans as a threat (Frid and Dill 2002; Beale and Monaghan 2004). Quantifying behavioural and physiological responses and their impacts on wildlife is important as these can negatively impact wildlife (French et al. 2011; Strasser and Heath 2013). For example, Pangle and Holekamp (2010) showed increased vigilance and higher mortality in spotted hyenas (Crocuta crocuta) in response to human disturbance associated with pastoralism. As Griffin et al. (2007) discussed, further detailed demographic data is required to draw inferences on the behaviour of marmots in relation to disturbances caused by human activities. Although we did not tag individual marmots, by using a mixed modeling approach, we statistically controlled these confounding effects on vigilance and identified the effects of pastoralism on marmot behaviour.
Conclusions
The Nepalese high altitude rangelands is a stressful environment where resources are often scarce. Therefore any potential conflict between human activities and wildlife may have a significant effect on the ecology and survival of species. For marmots, obtaining sufficient food while reducing the perceived risks associated with pastoralism is particularly important, as they have to feed efficiently to obtain a critical body mass during the short summer feeding period to survive winter hibernation. Our results suggest that although the total daily foraging time is not affected by pastoralism, marmots showed increased levels of vigilance during foraging in areas experiencing high levels of pastoralism.
However the conventional supposition that vigilance incurs direct foraging costs was not supported. Rather, increased time spent in vigilance was performed at the expense of time spent in resting and ‘other’ behavioural activities. Such increased vigilance activity suggests greater wariness of marmots in relation to pastoralism, which may have detrimental impacts on the fitness of marmots. Previous studies also suggest that marmots can adjust their behaviour to avoid potential negative demographic effects associated with human disturbance (Griffin et al. 2007; Li et al. 2011; Poudel et al. 2015). Further experimental studies are required to quantify the effects of changes in marmot vigilance behaviour associated with pastoralism in terms of their long-term survival and reproduction success.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267
Armitage KB (2000) The evolution, ecology, and systematics of marmots. Oecol Mont 9:1–18
Armitage KB (2013) Climate change and the conservation of marmots. Nat Sci 5:36–43
Armitage KB (2014) Marmot biology: sociality, individual fitness, and population dynamics. Cambridge University Press, Cambridge
Armitage KB, Chiesura Corona M (1994) Time and wariness in yellow-bellied marmots. J Mt Ecol 2:1–8
Armitage KB, Salsbury CM, Barthelmess EL, Gray RC, Kovach A (1996) Population time budget for the yellow-bellied marmot. Ethol Ecol Evol 8:67–95
Aryal A, Hopkins JB, Raubenheimer D, Ji W, Brunton D (2012) Distribution and diet of brown bears in the Upper Mustang region, Nepal. Ursus 23:231–236
Aryal A, Brunton D, Pandit R, Kumar Rai R, Shrestha UB, Lama N, Raubenheimer D (2013) Rangelands, conflicts, and society in the Upper Mustang region, Nepal. Mt Res Dev 33:11–18
Aryal A, Brunton D, Raubenheimer D (2014a) Impact of climate change on human-wildlife-ecosystem interactions in the Trans-Himalaya region of Nepal. Theor Appl Climatol 115:517–529
Aryal A, Brunton D, Ji W, Barraclough RK, Raubenheimer D (2014b) Human–carnivore conflict: ecological and economical sustainability of predation on livestock by snow leopard and other carnivores in the Himalaya. Sustain Sci 9:321–329
Aryal A, Brunton D, Ji W, Karmacharya D, McCarthy T, Bencini R, Raubenheimer D (2014c) Multipronged strategy including genetic analysis for assessing conservation options for the snow leopard in the central Himalaya. J Mammal 95:871–881
Aryal A, Brunton D, Ji W, Rothman J, Coogan SCP, Adhikari B, Su J, Raubenheimer D (2015) Habitat, diet, macronutrient, and fiber balance of Himalayan marmot (Marmota himalayana) in the Central Himalaya, Nepal. J Mammal 96:308–316
Bagchi S, Namgail T, Ritchie ME (2006) Small mammalian herbivores as mediators of plant community dynamics in the high-altitude arid rangelands of Trans-Himalaya. Biol Conserv 127:438–442
Barash DP (1980) The influence of reproductive status on foraging by Hoary Marmots (Marmota caligata). Behav Ecol Sociobiol 7:201–205
Barton K (2014) MuMIn: multi-model inference. R package version 1.10.5. http://CRAN.R-project.org/package=MuMIn. Accessed 18 Dec 2014
Bates D, Maechler M, Bolker B, Walker S (2014) lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7. http://CRAN.R-project.org/package=lme4. Accessed 18 Dec 2014
Beale CM, Monaghan P (2004) Human disturbance: people as predation-free predators? J Appl Ecol 41:335–343
Bednekoff PA, Blumstein DT (2009) Peripheral obstructions influence marmot vigilance: integrating observational and experimental results. Behav Ecol 20:1111–1117
Berryman AA, Hawkins BA (2006) The refuge as an integrating concept in ecology and evolution. Oikos 115:192–196
Bertolino S, Mazzoglio PJ, Vaiana M, Currado I (2004) Activity budget and foraging behavior of introduced Callosciurus finlaysonii (Rodentia, Sciuridae) in Italy. J Mammal 85:254–259
Bishop CM (1999) The maximum oxygen consumption and aerobic scope of birds and mammals: getting to the heart of the matter. Proc R Soc Lond B Biol Sci 266:2275–2281
Blumstein DT (1996) How much does social group size influence golden marmot vigilance? Behaviour 133:1133–1151
Blumstein DT (1998) Quantifying predation risk for refuging animals: a case study with golden marmots. Ethol 104:501–516
Blumstein DT, Daniel JC (2007) Quantifying behavior the JWatcher way. Sinaur, Sunderland
Blumstein DT, Daniel JC, Bryant AA (2001) Anti-predator behavior of Vancouver Island marmots: using congeners to evaluate abilities of a critically endangered mammal. Ethol 107:1–14
Blumstein DT, Runyan A, Seymour M, Nicodemus A, Ozgul A, Ransler F, Daniel JC (2004) Locomotor ability and wariness in yellow-bellied marmots. Ethol 110:615–634
Blumstein DT, Ferando E, Stankowich T (2009) A test of the multipredator hypothesis: yellow-bellied marmots respond fearfully to the sight of novel and extinct predators. Anim Behav 78:873–878
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Brilot BO, Bateson M, Nettle D, Whittingham MJ, Read JCA (2012) When is general wariness favored in avoiding multiple predator types? Am Nat 179:e180–e195
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Caro T (2005) Antipredator defenses in birds and mammals. University of Chicago Press, Chicago
Childress MJ, Lung MA (2003) Predation risk, gender and the group size effect: does elk vigilance depend upon the behaviour of conspecifics? Anim Behav 66:389–398
Christiansen F, Rasmussen MH, Lusseau D (2013) Inferring activity budgets in wild animals to estimate the consequences of disturbances. Behav Ecol 24:1415–1425
Cingolani AM, Renison D, Tecco P, Gurvich D, Cabido M (2008) Predicting cover types in a mountain range with long evolutionary grazing history: a GIS approach. J Biogeogr 35:538–551
Ciuti S, Northrup JM, Muhly TB, Simi S, Musiani M, Pitt JA, Boyce MS (2012) Effects of humans on behaviour of wildlife exceed those of natural predators in a landscape of fear. PLoS One 7:e50611
Crawford BA, Hickman CR, Luhring TM (2012) Testing the threat-sensitive hypothesis with predator familiarity and dietary specificity. Ethol 118:41–48
Creel S, Fox JE, Hardy A, Sands J, Garrott B, Peterson RO (2002) Snowmobile activity and glucocorticoid stress responses in wolves and elk. Conserv Biol 16:809–814
Dark J (2005) Annual lipid cycles in hibernators: integration of physiology and behavior. Annu Rev Nutr 25:469–497
Davidson AD, Detling JK, Brown JH (2012) Ecological roles and conservation challenges of social, burrowing, herbivorous mammals in the world’s grasslands. Front Ecol Environ 10:477–486
Devkota BP, Silwal T, Kolejka J (2013) Prey density and diet of snow leopard (Uncia Uncia) in Shey Phoksundo National Park,Nepal. Appl Ecol Environ Sci 1:55–60
Dorji T, Totland O, Moe SR (2013) Are droppings, distance from pastoralist camps, and pika burrows good proxies for local grazing pressure? Rangel Ecol Manag 66:26–33
Ellenberg U, Mattern T, Seddon PJ, Jorquera GL (2006) Physiological and reproductive consequences of human disturbance in Humboldt penguins: the need for species-specific visitor management. Biol Conserv 133:95–106
Fernández-Juricic E, Beauchamp G, Bastain B (2007) Group-size and distance-to-neighbour effects on feeding and vigilance in brown-headed cowbirds. Anim Behav 73:771–778
Ferrari C, Bogliani G, von Hardenberg A (2009) Alpine marmots (Marmota marmota) adjust vigilance behaviour according to environmental characteristics of their surrounding. Ethol Ecol Evol 21:355–364
Fortin D, Boyce MS, Merrill EH, Fryxell JM (2004a) Foraging costs of vigilance in large mammalian herbivores. Oikos 107:172–180
Fortin D, Boyce MS, Merrill EH (2004b) Multi-tasking by mammalian herbivores: overlapping processes during foraging. Ecology 85:2312–2322
French SS, Gonzalez-Suarez M, Young JK, Durham S, Gerber LR (2011) Human disturbance influences reproductive success and growth rate in California sea lions (Zalophus californianus). PLoS One 6:1–8
Frid A, Dill L (2002) Human-caused disturbance stimuli as a form of predation risk. Ecol Soc 6:11
Fritz H, Guillemain M, Durant D (2002) The cost of vigilance for intake rate in the mallard (Anas platyrhynchos): an approach through foraging experiments. Ethol Ecol Evol 14:91–97
Griffin SC, Valois T, Taper ML, Mills LS (2007) Effects of tourists on behavior and demography of olympic marmots. Conserv Biol 21:1070–1081
Hammer O, Harper D, Ryan P (2001) Past: paleontological statistics software package for education and data analysis. Paleontol Electron 4:1–9
Hanya G (2004) Seasonal variations in the activity budget of Japanese macaques in the coniferous forest of Yakushima: effects of food and temperature. Am J Primatol 63:165–177
Houston AI, Prosser E, Sans E (2012) The cost of disturbance: a waste of time and energy? Oikos 121:597–604
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Ikuta LA, Blumstein DT (2003) Do fences protect birds from human disturbance? Biol Conserv 112:447–452
Inouye DW, Barr B, Armitage KB, Inouye BD (2000) Climate change is affecting altitudinal migrants and hibernating species. PNAS USA 97:1630–1633
Johns DW, Armitage KB (1979) Behavioral ecology of alpine yellow-bellied marmots. Behav Ecol Sociobiol 5:133–157
Kuhn KM, Vander Wall SB (2008) Linking summer foraging to winter survival in yellow pine chipmunks (Tamias amoenus). Oecologia 157:349–360
Le Berre M, Ramousse R (2007) Bibliographia marmotarum: an update. In: Esipov AV, Bykova EA, Brandler OV, Ramousse R, Vashetko EV (eds) The marmots of Eurasia: origin and current status. International Marmot Network, Tashkent, pp 64–71
Lea AJ, Blumstein DT (2011) Age and sex influence marmot antipredator behavior during periods of heightened risk. Behav Ecol Sociobiol 65:1525–1533
Lenti Boreo D (2003) Spotting behaviour and daily activity cycle in the Alpine Marmots (Marmota marmota): a role for infant guarding. Oecol Mont 12:1–6
Li C, Monclúsb R, Terry R, Maulc L, Jianga Z, Blumstein DT (2011) Quantifying human disturbance on antipredator behavior and flush initiation distance in yellow-bellied marmots. Appl Anim Behav Sci 129:146–152
Lima SL, Bednekoff PA (1999) Back to the basics of antipredatory vigilance: can nonvigilant animals detect attack? Anim Behav 58:537–543
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lusseau D (2004) The hidden cost of tourism: detecting long-term effects of tourism using behavioral information. Ecol Soc 9:2
Makowska IJ, Kramer DL (2007) Vigilance during food handling in grey squirrels (Sciurus carolinensis). Anim Behav 74:153–158
Maldonado-Chaparro AA, Martin JGA, Armitage KB, Oli MK, Blumstein DT (2015) Environmentally induced phenotypic variation in wild yellow-bellied marmots. J Mammal 96:269–278
Martin P, Bateson PPG (2007) Measuring behaviour: an introductory guide. Cambridge University Press, Cambridge
Molur S, Shrestha TK (2008) Marmota himalayana. IUCN Red List of Threatened Species. Version 2014.3 http://www.iucnredlist.org. Accessed 11 Oct 2014
Mundry R, Sommer C (2007) Discriminant function analysis with nonindependent data: consequences and an alternative. Anim Behav 74:965–976
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Method Ecol Evol 4:133–142
Namgail T, Fox JL, Bhatnagar YV (2007) Habitat shift and time budget of the Tibetan argali: the influence of livestock grazing. Ecol Res 22:25–31
Neuhaus P, Mainini B (1998) Reactions and adjustment of adult and young alpine marmots Marmota marmota to intense hiking activities. Wildl Biol 4:119–123
Nikol’skii AA, Ulak A (2006) Key factors determining the ecological niche of the Himalayan marmot (Marmota himalayana Hodgson, 1841). Rus J Ecol 37:46–52
Nikol’skii AA, Ulak A (2007) The Ecology of the Himalayan marmot (Marmota himalayana Hodgson, 1841) in Nepal. In: Esipov AV, Bykova EA, Brandler OV, Ramousse R, Vashetko EV (eds) The marmots of Eurasia: origin and current status. International Marmot Network, Tashkent, pp 101–107
NTNC (2008) Sustainable development plan of Mustang (2008–2013). National Trust for Nature Conservation, Kathmandu
Ohba H, Iokowa Y, Sharma LR (2008) Flora of Mustang,Nepal. Tokyo, Kodansha Scientific Ltd
Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T (2010) Coupled dynamics of body mass and population growth in response to environmental change. Nature 466:482–485
Pangle WM, Holekamp KE (2010) Lethal and nonlethal anthropogenic effects on spotted hyenas in the Masai Mara National Reserve. J Mammal 91:154–164
Paudel KP, Andersen P (2010) Assessing rangeland degradation using multi temporal satellite images and grazing pressure surface model in Upper Mustang, Trans Himalaya, Nepal. Remote Sens Environ 114:1845–1855
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Pokharel A, Chetri M (2006) Traditional grazing system and seasonal pasture use in Upper Mustang,Nepal. Our Nat 4:29–41
Poudel BS, Spooner PG, Matthews A (2015) Temporal shift in activity patterns of Himalayan marmots in relation to pastoralism. Behav Ecol 26:1345–1351
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 2 Sept 2014
Sasaki T, Okubo S, Okayasu T, Jamsran U, Ohkuro T, Takeuchi K (2009) Management applicability of the intermediate disturbance hypothesis across Mongolian rangeland ecosystems. Ecol Appl 19:423–432
Schaller GB (1977) Mountain monarchs: wild sheep and goats of the Himalaya. University of Chicago Press, Chicago
Semenov Y, Louis S, Giboulet O, Ramousse R (2002) Accommodation behaviour of alpine marmot (Marmota marmota, Linne 1758) under direct anthropogenic influence. In: Rumiantsev VY (ed) KB Armitage. Holartic marmots as a factor of biodiversity ABF Publishing House, Moscow, pp 358–362
Shrestha R, Wegge P (2008) Wild sheep and livestock in Nepal Trans-Himalaya: coexistence or competition? Environ Conserv 35:125–136
Shrestha UB, Gautam S, Bawa KS (2012) Widespread climate change in the Himalayas and associated changes in local ecosystems. PLoS One 7:e36741
Sih A (2013) Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim Behav 85:1077–1088
Smith AT, Xie Y, Hoffmann RS, Lunde D, MacKinnon J, Wilson DE, Wozencraft WC, Gemma F (2010) A guide to the mammals of China. Princeton University Press, New Jersey
Strasser EH, Heath JA (2013) Reproductive failure of a human-tolerant species, the American kestrel, is associated with stress and human disturbance. J Appl Ecol 50:912–919
Tafani M, Cohas A, Bonenfant C, Gaillard J-M, Allainé D (2012) Decreasing litter size of marmots over time: a life history response to climate change? Ecology 94:580–586
Taulman JF (1990) Late summer activity patterns in hoary marmots. Northwest Nat 71:21–26
Treves A (2000) Theory and method in studies of vigilance and aggregation. Anim Behav 60:711–722
Tuomainen U, Candolin U (2011) Behavioural responses to human-induced environmental change. Biol Rev 86:640–657
Unck C, Waterman J, Verburgt L, Bateman P (2009) Quantity versus quality: how does level of predation threat affect cape ground squirrel vigilance? Anim Behav 78:625–632
Venables WN, Ripley BD (2002) Modern applied statistics with S. Springer, New York
Verdolin JL (2006) Meta-analysis of foraging and predation risk trade-offs in terrestrial systems. Behav Ecol Sociobiol 60:457–464
Walker BG, Dee Boersma P, Wingfield JC (2006) Habituation of adult Magellanic penguins to human visitation as expressed through behavior and corticosterone secretion. Conserv Biol 20:146–154
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Method Ecol Evol 1:3–14
Acknowledgments
We would like to thank Iain R Taylor and Hem S Baral for their helpful advice during the design of this study. We also thank Roger Mundry for his statistical advice and Gary Luck for his comments on an earlier draft of this manuscript. We thank research assistants Prabin Shrestha, Mahesh Neupane, Shankar Tripathi, and Thokme Lowa for their help with data collection. A special thanks to the community of the Upper Mustang and staffs at ACAP-Lhomanthang Sector Office who provided generous hospitality during field work. This study was supported by a post graduate scholarship from Charles Sturt University (to BSP) and grant from Holsworth Wildlife Research Endowment (CT#22178). This study was conducted under an approved protocol by the Animal Care and Ethics Committee of the Charles Sturt University, Australia (Protocol No. 13/013), and research permit from National Trust for Nature Conservation/Annapurna Conservation Area Project, Nepal.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Poudel, B.S., Spooner, P.G. & Matthews, A. Pastoralist disturbance effects on Himalayan marmot foraging and vigilance activity. Ecol Res 31, 93–104 (2016). https://doi.org/10.1007/s11284-015-1315-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-015-1315-x