Abstract
Few herbivores are well adapted to feeding on all foliage age classes available and most have evolved traits that are attuned to the characteristics of either developing or mature foliage; however, recent evidence has shown a number of insect herbivores that may mix different-aged foliage as a means of enhancing fitness. We carried out a series of laboratory and field experiments to investigate whether larvae of Asian gypsy moth [L. umbrosa (Butler) = L. dispar hokkaidoensis Goldschmidt] (Lepidoptera: Lymantriidae) engage in and benefit from foliage-age dietary mixing in common conifer species that naturally occur in its native range of Hokkaido, Japan. In a laboratory experiment, early instar larvae were observed on both developing and mature foliage when both age classes were available; however, larval survival and weight were highest on hosts with developing foliage available (larch, fir, and pine), whereas all larvae died on spruce where only mature foliage was available. In contrast, laboratory and field experiments indicated that late-instar larvae often consumed both developing and mature foliage on all conifer species studied, although there was general preference bias towards mature foliage. Field bioassays indicated that late-instar larvae provided both foliage age classes (a ‘mixed’ diet) had similar performance to those provided only developing or mature foliage. Results of this study indicate that larvae obtain limited performance benefits from mixing different foliage age-classes into their diet, other than perhaps the benefits accrued from having a broader resource pool available on a single host tree.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Variation in nutritional quality of different-aged foliage within plants can significantly influence the performance and associated distribution and abundance of herbivores. In general, developing foliage tends to be soft and nutritious but is often heavily defended by harmful allelochemicals—including terpenoids, alkaloids, and pyrethrins—that disrupt metabolic processes in herbivores (Cates 1980). In contrast, mature foliage tends to be tough and possesses fewer essential nutrients (nitrogen and water) but is also less toxic and less likely to produce defensive secondary-plant metabolites in response to herbivory (Cates 1980; Géri et al. 1993; Karban and Baldwin 1997; Moreau et al. 2003). Few herbivorous insects, in particular, are well adapted to feeding on all foliage age classes available, and the majority have evolved life history, physiological, and behavioral traits that are attuned to the characteristics of either developing or mature foliage (e.g., Carroll 1999; Moreau et al. 2003; Johns et al. 2009; Pinault et al. 2009; Johns and Quiring 2010).
Even insects that specialize on a particular foliage age class may sometimes consume small quantities of other age classes while foraging. In some instances, this may occur as the insect explores and ‘tastes’ the food available on its host; however, there are also examples where mixing small amounts of typically non-preferred foods in a diet may enhance fitness. Such adaptive dietary mixing of different-aged foliage has been reported in several larval moths (Carroll 1999; Johns et al. 2009; Pinault et al. 2009) and sawflies (Moreau et al. 2003; Johns and Quiring 2010; Johns et al. 2012) that feed on conifers, although the mechanisms underlying mixing vary somewhat from system to system. In some instances dietary mixing reflects changing nutritional needs or tolerances in developing larvae vs. mature foliage during development (Johns et al. 2009). Dietary mixing may also enhance insect performance through providing a more favorable nutrient balance or toxin dilution (Carroll 1999; Moreau et al. 2003; Johns et al. 2009; Pinault et al. 2009). There could also be benefits of dietary mixing associated with natural enemy avoidance and or deterrence, although this hypothesis remains largely unexplored (but see Stamp and Bowers 1990). Most past studies of foliage-age mixing in conifers have focused on monophagous insects (i.e., insects that feed on only a few closely related host species) (but see Johns et al. 2009). Whether more polyphagous (or generalist) insect species (i.e., those that may feed on a variety of host species) also engage in or benefit from foliage-age mixing remains unknown; however, numerous studies have demonstrated adaptive dietary mixing in both specialists and generalists involving either multiple different host-plant species or nutritionally manipulated artificial diets (Hägele and Rowell-Rahier 1999; Singer et al. 2002; Mody et al. 2007; reviewed in Behmer 2009).
Here, we discuss results from laboratory and field experiments carried out to investigate the foliage-age feeding preference and associated performance of larval gypsy moth (Lymantria dispar L.) (Lepidoptera: Lymantriidae) on four common conifer species in its native range of northern Japan. There are generally two main strains of gypsy moth, distinguished as European and Asian strains (Hajek and Tobin 2009). Asian gypsy moths differ in several ways from the European strain, including having larger late-instar larvae, winged adult females that can fly, and a wider feeding breadth that includes a greater number of conifer species (Baranchikov 1989; Baranchikov and Montgomery 1994). Within the Asian strain there is often further splitting into sub-strains, such as in China and Korea (L. dispar asiatica L.) and Japan (L. dispar japonica L.). Our study focuses on a strain in Hokkaido, Japan [L. umbrosa (Butler) = L. dispar hokkaidoensis Goldschmidt] (Pogue and Schaefer 2007; Kishida 2011), which will hereafter be referred to as HGM, with all other strains referred to for simplicity as ‘gypsy moth’. Although several studies have described the impressive host range available to gypsy moth larvae—perhaps as many as 500 plant species—(Liebhold et al. 1995; Matsuki et al. 2001; Onodera and Hara 2010), we know very little of how the insect interacts with the majority of these hosts. Several laboratory studies have showed that gypsy moth may enhance larval performance through switching during development from a deciduous host (e.g., oak) to a conifer host (e.g., pine) (Barbosa et al. 1986; Rossiter 1987; Sheppard and Friedman 1990; Joseph and Kelsey 1994; but see Stoyenoff et al. 1994). Whether larvae might further benefit from switching among different foliage age classes within single host trees remains unstudied.
We were interested in whether HGM could accrue benefits by mixing different-aged foliage while feeding on conifer hosts, as has been reported for other insect herbivores (Carroll 1999; Moreau et al. 2003; Johns et al. 2009, 2012; Pinault et al. 2009). In our first experiment, we examined the synchrony of HGM egg hatch with budburst of four native conifer species, and how differences in the initial availability of developing foliage influenced host suitability for newly hatched larvae. A second laboratory ‘choice’ experiment was carried out using mid- and late-instar larvae to determine the foliage-age feeding preference on each host during later instars. A final field experiment was carried out to compare the performance of larvae transferred from a common deciduous host (i.e., Betula sp.) to a conifer host, where they were split into treatments providing either a mix of developing and mature foliage, or only one or the other foliage type. We also performed foliage chemical analyses for each species and age class to determine whether patterns of foliage nutritional quality could explain observed feeding preference and performance of HGM within and among conifer hosts.
Methods
Study insect and site description
The life history of gypsy moth is similar for all strains and has been described previously in detail by Leonard (1981). Briefly, larvae hatch from egg masses in the spring and disperse to host foliage by walking or on silken threads carried by wind currents. Larvae feed through the spring and early summer on the foliage of their hosts and pupate by mid-summer. Adults emerge in late summer to mate and lay eggs in masses, often at the base of host tree trunks. Outbreaks in northern Japan are common in mixed deciduous forests (Betula, Alnus, Populus, Quercus, etc.) and in intensively managed stands of Japanese larch [Larix kaempferi (Lamb.) Carrière].
Studies were carried out in mature stands of intensively managed Japanese larch, Sakhalin fir [Abies sachalinensis (F. Schmidt) Mast.], Yezo spruce [Picea jezoensis (Siebold et Zucc.) Carrière], and Japanese white pine [Pinus parviflora Siebold et Zucc. var. pentaphylla (Mayr) A. Henry] (hereafter larch, fir, spruce, and pine) located in the experimental forest of Hokkaido Research Center, Forestry and Forest Products Research Institute in Hokkaido, Japan (N42°59′37.4″, E141°23′27.6″). These conifer species are all native to Japan and are common in the regions of Hokkaido where HGM outbreaks occur. On the three evergreen conifer species (spruce, fir, and pine), our experiments involved comparisons of HGM preference and performance on the developing buds/shoots vs. mature age-classes of foliage; we did not distinguish between the different years of mature foliage (e.g., one year old, two year old, etc.). Although there is some evidence that different age classes of mature foliage vary nutritionally, this variation is relatively low compared with that between developing and mature foliage (Moreau et al. 2003). For larch, all foliage is produced and shed in the same year, so we used analogues for ‘developing’ and ‘mature’ foliage to nutritionally and morphologically differentiate foliage types that are produced at different times of the same season. The fascicle foliage in larch is produced first in the spring along the surface of branches and is comparable in position and chemistry to the mature foliage in evergreens, whereas the long shoots of larch begin developing at the branch tips approximately 2–3 weeks after the fascicles and are analogous morphologically and nutritionally to the developing shoots of evergreens (Ohigashi et al. 1981; Johns et al. 2012). Stands were monocultures of similar ages and were located within an approximately 3 × 3 km area to minimize differences in soil and site characteristics. In all experiments, we attempted to select trees and branches located on the western side of stands and only used branches that were growing in open, sunlit conditions. The evergreen conifer stands (spruce, fir, and pine) showed no obvious signs of previous herbivory. In the larch stand, we observed very low densities of HGM and larch sawfly (Pristophora erichsonii Htg.), which when found on study branches were removed and placed on non-study trees. The HGM larvae used in our experiments were collected as egg masses (approximately 20 in total) in the fall of 2008 from a mature Japanese larch stand at the Hokkaido Forestry Research Institute in Bibai, Japan (43°17′12.2″N 141°51′18.2″E). The larvae that hatched from these egg masses in the spring of 2009 were used in all subsequent experiments.
Early instar larvae
Budburst phenology, preference, and performance
To determine the effects of tree species and foliage age on the preference and performance of early instar gypsy moth larvae, we carried out a laboratory experiment in 2009 using foliage collected from each of our four conifer host species. Egg masses began hatching on 13 May 2009, which was approximately at the same time as populations became active at the collection site. Once larvae began hatching, foliage from each tree species (larch, spruce, fir, and pine) was collected from our study stands and transported to the laboratory, where the branch tips were then cut into smaller sections and inserted separately into wet floral foam. Branches were cut to provide similar numbers of both developing buds and mature shoots, although at the initiation of this study, the developing buds on some species had not yet burst and were thus unavailable to the young larvae. Overall, we used ten replicates of each of the five tree species for a total of 50 replicates. We placed on each of these branch replicates 10 larvae that had hatched within the previous 24 h and then placed the branches into a clay pot (20 cm diameter × 20 cm tall). To prevent larvae from escaping, the clay pots were covered with a fine-mesh cloth, which was secured over the top of the pot with an elastic band. All pots were then placed in the outdoor insectary. To determine larval feeding preference, we checked branches every 3 days and determined the location of larvae on the branch (i.e., on developing shoots/foliage, on old foliage, or off the branch). When checking larvae we added fresh foliage to the pot and assessed whether the buds on collected foliage had yet burst. Budburst was defined as when the bud cap had been shed such that the developing needles were exposed and available for feeding. After 2 weeks, we counted and weighed surviving larvae.
To assess differences in the percentage of larvae on developing vs. mature foliage, we used mixed generalized linear models with the random effect of sample date removed as a random factor (proc GLIMMIX, dist = bin link = logit, SAS Institute Inc 1999). To determine the effects of host species on final larval survival, we used a generalized linear model (proc GENMOD, dist = bin, link = logit, SAS Institute Inc 1999). Differences in larval weight among tree species were assessed using a one-way analysis of variance (ANOVA) (proc GLM, SAS Institute Inc 1999).
Late-instar larvae
Preference
To assess the foliage age-feeding preference of mid- to late-instar larvae, we conducted a ‘choice’ experiment in a laboratory in 2009. In two iterations initiated on 2 and 8 July, we provided individual HGM larvae access to developing and mature foliage of each conifer host in clay-pot arenas (as described above) for 24 h. Up until each experiment, larvae were provided in the laboratory with a diet of cut birch leaves, which were replaced every 2–3 days. Pairs of developing and mature foliage from each host were placed in separate arenas for each tree species. The shoots were cut to similar length and placed at opposite ends of the arena. We could not observe directly the preference of the larvae during the daytime as they generally prefer to feed at night. Thus, preference was assessed by weighing the amount of frass deposited at the base of each foliage type over the 24 h period. It should be noted that the use of frass production as a proxy for feeding preference may not be ideal as some caterpillars may compensate for poor quality food by increasing feeding rates (and thus frass production) (Albert and Bauce 1994); however, we noted in our experiments that frass production generally corresponded well to visual estimates of shoot defoliation on either side of the arena (unpubl. data). In each of 10 replicates, one mid- to late-instar larvae that had been feeding on cut foliage of the same host (all age classes of foliage available) for 1 week, was placed into each of 5 arenas and left for 24 h to feed. Enough foliage of each age class was provided so as to never be limiting, and the arena was wide enough that frass falling to the base of each foliage type never overlapped with that on the other side of the arena. After the 24 h, frass from each pot was collected and placed in a paper bag and dried at 80 °C in a convection oven for 24 h. Dried frass was subsequently weighed. To assess differences in the total weight of frass at the base of each host and foliage type, we carried out a generalized linear model with pairwise comparisons of treatments (proc GENMOD, dist = bin link = logit, SAS Institute Inc 1999).
Performance
To determine the effects of host species and foliage age on late-instar larval performance, we carried out a field experiment in 2009 in the same forests used in the above experiments. Along the western perimeter of each stand we selected 15 trees and marked three unshaded, west-facing branches in the mid crown of each tree. These branches were randomly assigned a treatment of: (1) Both age classes (‘mixed’); (2) only developing foliage (‘developing’); or (3) only mature foliage (‘mature’). To prevent caterpillars from feeding on foliage age classes other than those specified in the treatment, we used small mesh-cloth sleeve cages and strips of the same material to cover unwanted age classes, as in Johns et al. (2009). Prior to the experiment, caterpillars had been reared in the laboratory on cut birch foliage, which was replaced with fresh leaves every 3 days. Using this deciduous host rather than larch ensured that the larvae were not acclimatized to feeding on any of our conifer hosts prior to experiments. At approximately mid-instar these larvae were placed in groups of 3 on each treatment branch and enclosed in a fine mesh-cloth sleeve cage (30 × 45 cm). Sleeve cages were checked every 2–3 days for pupae, which were removed immediately when found and transported to the laboratory. Collected pupae were weighed and placed in a petri dish and were monitored daily in an outdoor insectary for eclosion and to identify adult sex.
To confirm that late-instar caterpillar feeding preference in the field was consistent with observations in our above laboratory experiment, we also assessed relative defoliation on the developing vs. mature on the ‘mixed’ treatment branches. Defoliation was assessed visually before and after larval feeding for all shoots enclosed in sleeve cages by assigning each shoot a defoliation class of 0, 5, 20, 40, 60, 80, 95, or 100 %, similar to what has been done previously in fir (Piene 1991), spruce (Johns et al. 2006), and larch (Johns et al. 2012). Sleeve cages enclosed up to 4 years of foliage on the evergreens and a similar number of shoots on larch and food was never observed to be limiting.
We assessed effects of tree species and foliage age treatment on overall larval survival, the percentage of survivors that were female (i.e., percent female), male and female pupal weight, and eclosion time using generalized linear models with treatment nested in tree species (proc GENMOD, for percent survival and percent female dist = bin link = logit; for pupal weight and eclosion time dist = Poisson link = log, SAS Institute Inc 1999). To test overall effects of treatments on HGM performance with all host species pooled, a second analysis was carried out using generalized linear models with the same model parameters described above and with pairwise comparisons of treatments.
Foliage chemistry
For each study tree used in the late-instar caterpillar field experiment, we collected west-facing mid-crown branches and placed them immediately in a freezer at −60 °C until foliage chemical analyses could be carried out. Details of similar foliage chemical analyses are described in detail in Johns et al. (2012). Briefly, in 2009 we analysed foliage chemistry for one branch collected from each of the trees used in the field bioassays. Foliage was collected on 22 June 2009 after most HGM were mid- to late-instar. For all branches, we separated shoots into developing and mature foliage (for larch long and short shoots) and placed them in separate Ziplock® bags for immediate processing. Rubber gloves were used throughout collection and chemical analyses to limit contamination of foliage by contact with skin. Estimates of fresh and dry mass, and water content (%) were assessed for 20 needles on both developing and mature foliage for each collected branch. These needles were weighed and reweighed after being dried in a paper bag in a convection oven at 80 °C for 48 h. The remaining foliage from branches was removed using tweezers and placed in paper bags, then dried and dehydrated in an Eyela Freeze Dryer (FD-5 N) for 48 h. Freeze-dried needles were separated from bark and petioles, then ground into powder using a grinding mill. To reduce contamination between samples, the grinding mill was cleaned between samples with a 99 % ethanol solution. Ground foliage was stored in sealed glass vials at room temperature until chemical analysis. Prior to chemical analysis, we measured 20 mg of powder from each sample and placed it into a 2 ml Eppendorf tube and performed extractions in a 2 ml solution of 50 % methanol. As controls, we set up two test tubes containing the 50 % methanol solution.We next placed all Eppendorf tubes in a sonic cleanser at 40 °C for 1 h, then transferred them to a centrifuge for 2 min at 15,000 revolutions/min. The resulting filtrate was analyzed for condensed tannins using the hydrochloric acid–butanol method (Bate-Smith 1977), and for total phenols using the Folin–Ciocalteu method (Julkanen-Tiitto 1985). We also analyzed foliage nitrogen content using a CN analyzer (Vario Max CN, Elementar Analysensysteme GmbH, Hanau, Germany) for foliage that had been dried at 80 °C for 48 h.
To assess differences in foliage nutrients and allelochemicals between foliage age classes and among tree species, we carried out using mixed-model analysis of variance with foliage age nested in tree species and with the random effect of tree removed from the model (proc MIXED, SAS Institute Inc 1999).
Results
Early instar larvae
Budburst phenology, preference, and performance
The availability of developing foliage at the time of natural larval hatch varied among conifer species (Fig. 1). The ‘mature’ foliage on larch had burst several weeks before the experiment, however, the ‘developing’ foliage was not available until weeks later (Fig. 1a). Whereas developing foliage was available on fir and pine almost as soon as the larvae hatched (Fig. 1c, d), the developing foliage on spruce was not available until at least 2 weeks into larval development (Fig. 1b). Larvae on host species with both developing and mature foliage available (i.e., fir and pine) were found on developing and mature foliage but differed significantly in number between the two foliage types (F 1,94 ≥ 6.90, P ≤ 0.01) (Fig. 1c, d). However, the distribution of larvae appeared to shift somewhat from mature towards developing foliage from the beginning to the end of the 2-week experiment (Fig. 1c, d). At the end of 2 weeks, larval survival differed significantly among tree species (χ 2 = 168.36, df = 3, P < 0.001), with the highest survival on larch, moderate survival on fir and pine, and no survival on spruce (Fig. 2a). Similarly, larval weight varied among tree species (with spruce removed due to a lack of survivors) (F 2,23 = 21.44, P < 0.001) with trends similar to survival (Fig. 2b).
Mean number (±1 SE) of early instar gypsy moth larvae feeding on either developing or mature foliage on each of four conifer species (a larch, b spruce, c fir, and d pine) over 2 weeks following egg hatch (i.e., approximately first to third instar). The number and asterisk represent the approximate day on which ≥50 % of buds on branches had burst (D developing, M mature), making the developing foliage available to larvae
Mean (±1 SE) a survival and b larval weight of gypsy moth larvae that had been provided developing buds and mature foliage of four conifers (larch, spruce, fir, and pine) for 2 weeks following egg hatch (i.e., approximately first to third instar). Letters above means represent the results of pairwise contrasts between treatments
Late-instar larvae
Preference
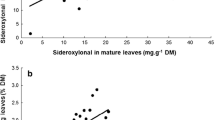
From laboratory bioassays, the mean dry weight of frass beneath shoots (i.e., our proxy for feeding preference) varied significantly among host species (F 3,312 = 6.45, P < 0.001) and between developing and mature foliage (F 2,312 = 11.45, P < 0.001), however, there was no species x foliage age interaction (F 3,312 = 1.59, P = 0.19). Although there was frass found beneath both shoot types in all tree species, there was generally more beneath mature shoots (Fig. 3a). Similarly, defoliation on the ‘mixed’ treatment branches in our field bioassays (discussed below) differed significantly among host species (F 3,112 = 37.01, P < 0.001) and between foliage age classes (F 2,112 = 33.67, P < 0.001), with generally more defoliation occurring on mature compared with developing foliage (Fig. 3b). There were no significant species x foliage age interactions (F 3,112 = 1.58, P = 0.20).
Foliage-age feeding preference of mid- to late-instar gypsy moth larvae based on a a laboratory choice experiment measuring larval frass dry weight near shoots of developing vs. mature foliage, and b percent defoliation on live branches in the field where larvae had access to both foliage types on each of four conifer host species. Error bars represent ±1SE
Performance
Foliage age treatment had no significant effect on any of the parameters measured to assess late-instar larval performance (Table 1). Survival of late-instar larvae, female pupal weight, and male and female eclosion time varied significantly among host species (Table 1), although results were somewhat variable (Fig. 4a). Although survival was generally higher on fir (Fig. 4a), female pupal weight was higher on larch, and adult eclosion time was earlier on larch, spruce, and pine (Fig. 4b–d). When all trees were pooled together to test for an overall foliage age treatment effect the only differences in performance detected were in survival, which was overall slightly lower for larvae fed only developing foliage (χ 2 = 4.04, df = 1, P = 0.04, Fig. 5).
Overall mean (±1SE) percent survival of late-instar gypsy moth larvae on both developing and mature foliage (‘mixed’), only developing foliage, or only mature foliage for all conifer species pooled (larch, spruce, fir, and pine). Letters above means represent the results of pairwise contrasts between treatments
Foliage chemistry
Water and N were generally higher in the developing compared with mature foliage of all conifers, with the exception of larch, which had higher N in mature shoots (Table 2). Dry needle mass was also significantly higher in developing compared with mature foliage of all of the evergreens, but not larch (Table 2). Total phenolics between developing and mature foliage were only significantly different for spruce and fir (Table 2); however, whereas in spruce phenolics were higher in the developing foliage, in fir phenolics were higher in mature foliage (Table 2). There were no significant differences in tannins between developing and mature foliage in any host (Table 2).
Discussion
Numerous studies have been carried out to examine the feeding habits of gypsy moth larvae, reporting a myriad of behavioral and physiological adaptations that allow it to thrive on a variety of hosts, despite enormous variations in host allelochemical and nutrient content (Lindroth et al. 1990; Lazarević et al. 2002). Comparatively few studies on conifers have been carried out under field conditions or within the gypsy moth’s native range. Our study indicates that when different-aged foliage is available, HGM feeds on both foliage types in all of the conifer species we studied, although the relative preference for either developing or mature foliage may change as larvae age. During early instars, larvae were observed on both foliage types (if available), although from our general observations it appeared that most actual feeding occurred on the developing needles. In terms of performance, larvae on hosts that had developing foliage available at time of egg hatch (larch, fir, pine) had higher larval survival and larval weight compared with those on hosts with later budburst (spruce) (Fig. 2). Past laboratory studies of gypsy moth on North American conifers have reported reduced early instar larval growth rates on some species (e.g., white pine, Sheppard and Friedman 1990), whereas others have shown higher pupal growth on other species (e.g., Douglas fir, Joseph and Kelsey 1994); however, even on evergreens with early bursting shoots, larval performance of young larvae was still relatively low compared with that on the deciduous conifer (i.e., larch) (Fig. 2).
Although there was no direct experimental test of feeding preference in early instar larvae vs. late-instar larvae, our results suggest that there may be an ontogenetic shift in overall larval feeding preference on evergreen hosts. Whereas early instar larvae appear to require soft, developing foliage as they initiate development (Fig. 1), later instars had a general preference for mature conifer foliage (Fig. 3). In bioassays using individual conifer host species, we were unable to detect any large differences in most performance parameters between the mixed vs. single age-class diets (Table 1; Fig. 4); however, overall survival pooled across all hosts to test for an overall effect of foliage age across species indicated that mature conifer foliage tends to yield higher larval survival than developing foliage (Fig. 5). This suggested ontogenetic shift most likely reflects changes in the tolerance and foliage consumption that occurs with larval development (Hochuli 2001). Young larvae often struggle to penetrate the hardened cuticle of mature foliage and may thus be constrained to feeding on soft, developing leaves (Raupp et al. 1988; Hunter and Lechowicz 1992). This may at least partially explain why gypsy moth outbreaks rarely occur in pure conifer stands. Older larvae, in contrast, may have less trouble with hardened leaves, but may risk accumulating excess toxins when consuming large quantities of developing foliage (e.g., Johns et al. 2009). Similar explanations have been provided to explain host-switching behaviors in gypsy moth from broadleaf to evergreen coniferous hosts (Barbosa et al. 1986; Rossiter 1987; Sheppard and Friedman 1990), although these benefits depend largely on the deciduous-conifer combination examined and may not always manifest (Joseph and Kelsey 1994; Stoyenoff et al. 1994).
Although dietary mixing by late-instar larvae of different-aged foliage was observed on all hosts studied (Fig. 3), we found no evidence that this mixing was associated with nutrient balancing or toxin dilution (Figs. 4, 5). In particular, there was no difference between groups of larvae feeding only on mature foliage vs. those feeding on a mix of foliar age classes. This contrasts another generalist herbivore, the white marked tussock moth, which can enhance performance on balsam fir through mixing small amounts of mature foliage in its main diet of developing foliage (Johns et al. 2009). HGM differs in that it seems quite capable, at least during mid to late instars, of feeding on both developing and mature foliage with little consequence on performance.
Outbreaks of HGM occur primarily in deciduous forests, although evergreen conifers may also be exploited if available (Onodera and Hara 2010). The apparent capacity of HGM to consume multiple age classes of foliage could be a concern to foresters managing outbreaks in or near evergreen stands. Most herbivores of evergreen conifers tend to prefer only developing or mature foliage (Cates 1980), and defoliation of either foliage age class can dramatically impact conifer growth (Piene 1980; Parsons et al. 2003). Complete defoliation of both developing and old foliage, however, can even more significantly impact the capacity of trees to recover, and may lead to rapid tree mortality (Kulman 1971). Intense feeding by gypsy moth on conifers during outbreaks may, therefore, be expected to have a higher impact on tree growth and mortality than that occurring during outbreaks by other insect herbivores.
Foliage chemical analyses yielded no clear explanation for the apparent preference and performance of HGM on different foliage age classes or among hosts (Table 2). Larvae tended to prefer and perform better (overall) on mature foliage despite relatively low N levels compared with that found in developing foliage. Phenolics, including tannins, did not explain HGM preference or performance. Although not analyzed here, it is likely that further analyses of other allelochemicals, such as terpenoids or alkaloids, could better explain some of the trends in HGM preference and performance within and among conifer hosts. Alkaloids in particular appear to have a strong effect foliage suitability for gypsy moth in both deciduous and coniferous hosts (Barbosa and Krischik 1987; Shields et al. 2006) and at least one previous study has shown that alkaloid concentrations in two species of pine (Pinus sp.) are higher in developing than in mature foliage (Gerson and Kelsey 1998).
Numerous laboratory and field studies have reported adaptive benefits from dietary mixing of different foods by both generalist and specialist herbivores, which are often related to the animal’s need to balance nutrient intake and/or dilute ingested toxins (Hägele and Rowell-Rahier 1999; Singer et al. 2002; Mody et al. 2007; reviewed in Behmer 2009). Our study provides evidence that HGM in conifers may mix multiple age classes of foliage within their diet, with few clear benefits or costs to performance. It is possible that the benefits of dietary mixing for HGM might only be seen in more subtle parameters such as relative growth rate and feeding efficiency (e.g., Barbosa et al. 1986; Rossiter 1987; Sheppard and Friedman 1990). It is possible also, however, that the foliage-age mixing observed is simply a reflection of adaptations in HGM allowing a more expansive diet breadth: having an expansive diet breadth allows for a broader resource pool, which is considered to be one of the major benefits of being a generalist herbivore (Bernays and Minkenberg 1997). Past studies of foliage-age dietary mixing by larval insects have drawn attention to potential benefits of obtaining a complementary diet from within a single plant, rather than risking dispersal to other hosts (Johns et al. 2009; Johns and Quiring 2010). Even though the mixing itself may not be important for HGM larvae, being able to consume multiple foliage age classes within a single host tree with limited fitness costs may still be beneficial when compared with possible risks of dispersing to other hosts, including predation and starvation.
References
Albert PJ, Bauce E (1994) Feeding preference of fourth- and sixth-instar spruce budworm (Lepidoptera: Tortricidae) larvae for foliage extracts from young and old balsam fir hosts. Env Entomol 23:645–653
Baranchikov YN (1989) Ecological basis of the evolution of host relationships in Eurasian gypsy moth populations. In: Wallner WE, McManus KA (eds) Proceedings Lymantriidae. A comparison of features of new and old world tussock moths. USDA Forest Service. Gen Tech Rpt NE-123, Broomall, pp 319–338
Baranchikov YN, Montgomery ME (1994) Tree suitability for Asian, European and American populations of gypsy moth. USDA Forest Service, Gen Tech Rpt NE-188, p 4
Barbosa P, Krischik VA (1987) Influence of alkaloids on feeding preference of eastern deciduous forest trees by the gypsy moth Lymantria dispar. Am Nat 130:53–69
Barbosa P, Martinat P, Waldvogel M (1986) Development, fecundity, and survival of the herbivore Lymantria dispar and the number of plant species in its diet. Ecol Entomol 11:1–6
Bate-Smith EC (1977) Astringent tannins of Acer species. Phytochemistry 16:1421–1426
Behmer ST (2009) Insect herbivore nutrient regulation. Annu Rev Entomol 54:165–187
Bernays EA, Minkenberg PJM (1997) Insect herbivores: different reasons for being a generalist. Ecology 78:1157–1169
Carroll AL (1999) Phyiological adaptation to temporal variation in conifer foliage by a caterpillar. Can Entomol 131:659–669
Cates RG (1980) Feeding patterns of monophagous, oligophagous, and polyphagous insect herbivores: the effect of resource abundance and plant chemistry. Oecologia 46:22–31
Géri C, Allais JP, Auger MA (1993) Effects of plant chemistry and phenology on sawfly behavior and development. In: Wagner MR, Raffa KF (eds) Sawfly life history adaptations to woody plants. Academic Press, New York, pp 173–210
Gerson EA, Kelsey RG (1998) Variation in Ponderosa (Pinus ponderosa) and lodgepole pine (P. contorta) foliage from central Oregon. J Chem Ecol 24:815–827
Hägele BF, Rowell-Rahier M (1999) Dietary mixing in three generalist herbivores: nutrient complementation or toxin dilution? Oecologia 119:521–533
Hajek AE, Tobin PC (2009) North American eradications of Asian and European gypsy moth. In: Hajek AE, Glare TR, O’Callaghan M (eds) Use of microbes for control and eradication of invasive arthropods. Springer, Berlin, pp 71–89
Hochuli DF (2001) Insect herbivory and ontogeny: How do growth and development influence feeding behaviour, morphology and host use? Austral Ecol 26:563–570
Hunter AF, Lechowicz MJ (1992) Host foliage acceptability during the dispersal period of gypsy moth larvae. Oecologia 89:316–323
Johns RC, Quiring DT (2010) Spatial heterogeneity within an evergreen conifer promotes foliage-age dietary mixing by a specialist herbivore. Anim Behav 80:659–666
Johns RC, Ostaff DP, Quiring DT (2006) Sampling methods for evaluating yellowheaded spruce sawfly density and defoliation in juvenile black spruce. J Acad Entomol Soc 2:1–13
Johns RC, Quiring DT, Lapointe R, Lucarotti CJ (2009) Foliage-age mixing within balsam fir increases the fitness of a generalist caterpillar. Ecol Entomol 34:624–631
Johns RC, Ozaki K, Tobita H (2012) Dietary mixing within the crown of a deciduous conifer enhances the fitness of a specialist sawfly. Anim Behav 84:1393–1400
Joseph G, Kelsey RG (1994) Acceptibility and suitability of Douglas-fir as a secondary host for gypsy moth (Lepidoptera: Lymantriidae). Pop Ecol 23:396–405
Julkanen-Tiitto R (1985) Phenoloic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agr Food Chem 33:213–217
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Kishida Y (2011) Lymantriidae. In: Kishida Y (ed) The standards of moths in Japan, vol 2. Gakken Education Publishing, Tokyo, pp 139–147
Kulman HM (1971) Effects of insect defoliation on growth and mortality of trees. Annu Rev Entomol 16:289–324
Lazarević J, Perić-Mataruga V, Stojković B, Tucić N (2002) Adaptation of the gypsy moth to an unsuitable host plant. Entomol Exp Appl 102:75–86
Leonard DE (1981) Bioecology of gypsy moth. The gypsy moth: Research toward Integrated Pest Management. In: Doane CC, McManus ML (eds) USDA Technical Bulletin 1584. US Department of Agriculture, Washington, pp 9–30
Liebhold AM, Gottschalk KG, Muzika R, Montgomery ME, Young R, O’Day K, Kelly B (1995) Suitability of North american tree species to the gypsy moth: a summary of field and laboratory tests. USDA General Technical Report NE-211, USDA, Forest Service, Delaware
Lindroth RL, Anson BD, Weisbrod AV (1990) Effects of protein and juglone on gypsy moths: growth performance and detoxification enzyme activity. J Chem Ecol 16:2533–2547
Matsuki M, Kay M, Serin J, Floyd R, Scott JK (2001) Potential risk of accidental introduction of Asian gypsy moth (Lymantria dispar) to Australasia: effects of climatic conditions and suitability of native plants. Agr For Entomol 3:305–320
Mody K, Unsicker SB, Linsenmair KE (2007) Fitness related diet-mixing by intraspecific host-plant-switching of specialist insect herbivores. Ecology 88:1012–1020
Moreau G, Quiring DT, Eveleigh ES, Bauce E (2003) Advantages of a mixed diet: feeding on several foliar age classes increases the performance of a specialist insect herbivore. Oecologia 135:391–399
Ohigashi H, Wagner MR, Matsumura F, Benjamin DM (1981) Chemical basis of differential feeding behavior of the larch sawfly, Pristophora erichsonii (Hartig). J Chem Ecol 7:599–614
Onodera K, Hara H (2010) Defoliation of Sakhalin fir by gypsy moth that outbreaked in multi-layered plantation. Koshunai-kihou 158:6–9 (in Japanese)
Parsons K, Quiring DT, Piene H, Farrell J (2003) Temporal patterns of balsam fir sawfly defoliation and growth loss in young balsam fir. For Ecol Manage 184:33–46
Piene H (1980) Effects of insect defoliation on growth and foliar nutrients of young balsam fir. For Sci 26:665–673
Piene H (1991) The sensitivity of young white spruce to spruce budworm defoliation. North J Appl For 8:168–171
Pinault L, Thurston G, Quiring DT (2009) The interaction of foliage and larval age influence the preference and performance of a geometrid caterpillar. Can Entomol 141:136–144
Pogue MG, Schaefer PW (2007) A review of selected species of Lymantria Huber (1819) (Lepidoptera: Noctuidae: Lymantriinae) from subtropical and temperate regions of Asia, including the descriptions of three new species, some potentially invasive to North America. US Department of Agriculture Forest Health Technology Enterprise Team, Morgantown
Raupp MJ, Werren JH, Clifford S (1988) Effects of short-term phonological changes in leaf suitability on the survivorship, growth, and development of gypsy moth (Lepidoptera: Lymantriidae) larvae. Env Entomol 17:316–319
Rossiter MC (1987) Use of a secondary host by non-outbreak populations of the gypsy moth. Ecology 68:857–868
SAS Institute Inc (1999) The SAS system version 8 for Windows. SAS Institute, Cary
Sheppard CA, Friedman S (1990) Influence of host plant, foliar phenology and larval dietary history on Lymantria dispar larval nutritional indices. Entomol Exp Appl 55:247–255
Shields VD, Rodgers EJ, Arnold NS, Williams D (2006) Feeding responses to selected alkaloids by gypsy moth larvae, Lymantria dispar (L.). Naturwissenschaften 93:127–130
Singer MS, Bernays EA, Carriere Y (2002) The interplay between nutrient balancing and toxin dilution in foraging by a generalist insect herbivore. Anim Behav 64:629–643
Stamp NE, Bowers MD (1990) Variation in food quality and temperature constrains the foraging of gregarious caterpillars. Ecology 7:1031–1039
Stoyenoff JL, Witter JA, Montgomery ME, Chilcote CA (1994) Effects of host switching on gypsy moth (Lymantria dispar L.) under field conditions. Oecologia 97:143–157
Acknowledgments
We thank A. Komatsu for processing foliage for chemical analyses. We are also grateful to T. Yamaguchi for allowing us to use his Japanese larch stand for manipulative experiments, and to D. Carleton, E. Moise, D. Quiring, C. Simpson, J. Sweeney, Z. Sylvain, and several anonymous reviewers for comments on earlier versions of the manuscript. Financial support was provided through the Japanese Society for the Promotion of Science and from a JSPS Postdoctoral Fellowship for Foreign Researchers (25252030 and 19.07779).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Johns, R.C., Tobita, H., Hara, H. et al. Adaptive advantages of dietary mixing different-aged foliage within conifers for a generalist defoliator. Ecol Res 30, 793–802 (2015). https://doi.org/10.1007/s11284-015-1280-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-015-1280-4