Abstract
Tropical deforestation is well known to have serious negative consequences for biodiversity, terrestrial carbon sinks and the balance of atmospheric greenhouse gases. By contrast, selective logging of tropical forests is often regarded as having a lesser impact on the ecosystem particularly in long terms, even though there have been few critical evaluations of the practice, particularly in Africa. We compared field data from 511 plots in the tropical forest of Sierra Leone, Ghana, Cameroon and Gabon. These plots were subject to different forest management practices: no recent logging (primary forests), selective logging (up to 30 years old) and re-grown secondary forests post clear-cutting (at least 20 years ago). Our findings suggest that the vertical structure and plant richness of the selectively logged and secondary forests change in different amplitude from those of primary forests, but stem density and the prevalence of vine and weed species differ greatly. We show that the effects of selective logging are greater than those expected simply from the removal of commercial species, and can persist for decades. Selective logging, unless it is practiced at very low harvest intensities, can significantly reduce the biomass of a tropical forest for many decades, seriously diminishing aboveground carbon storage capacity, and create opportunities for weeds and vines to spread and slow down the ecological succession.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical forests attain the highest level of biodiversity of any terrestrial ecosystem (Kalipeni 2007), provide critical ecosystem services, such as protecting hill slopes and moderating local climate, and represent one of the most productive ecosystems on Earth, accounting for 59 % of the global carbon stock in forests (Dixon et al. 1994; Malhi and Grace 2000). However, tropical forest areas are subject to many anthropogenic impacts and are characterized by very high rates of deforestation, conversion to agriculture, logging and degradation by repeated harvesting of wood for human subsistence (Johns 1997).
Selective logging—the harvest of valuable timber trees above a threshold stem diameter with prescriptions designed to maintain the forest cover—is considered to be a better practice compared with clearcutting and it is increasingly embraced as an approach to protect forest integrity while allowing an appropriate use of resources (Rametsteiner and Simula 2003; Deckker and de Graaf 2003). In recent decades, many tropical forest nations have adopted the mechanized selective logging regimes for timber production (Gascon et al. 1998; De Wasseige and Defourny 2004; Drigo et al. 2009). This has been done for different reason: because of a change in the local economics and politics of timber extraction; partially because of the evidence that deforestation can increase atmospheric CO2 concentration (Houghton et al. 2000; DeFries et al. 2002); and in recognition that maintaining forest integrity is beneficial for carbon sequestration and biodiversity protection as well as productivity (Thompson et al. 2009; FAO 2010; Gibson et al. 2011; Pan et al. 2011). Information available on the impact of selective logging on carbon sequestration and biodiversity and the trade-off between these two ecosystem variables in function of intensity of timber harvesting are, despite its increasing geographical dimension, scarce in some continents (particularly in Africa) and where more abundant (particularly in South America) they are contrasting. This kind of forest practice is designed to maintain the forest cover including a significant understock of living biomass. Despite the weak scientific evidences, selective logging is considered as a “sustainable forest management” (SFM) practice and it is increasingly embraced as an approach to protect forest integrity while allowing an appropriate use of resources. In the past, the intensity of the disturbance caused by selective logging was solely quantified as the number of fallen trees, damage of other trees caused by logging operations and the construction of access roads and exploitation trails, while techniques used for decades to identify occurrences of forest loss by deforestation were not suitable to analyse variations of biodiversity and forest C-stocks in selectively logged areas.
Studies of the ecological consequences of selective logging, in South or Central America (Verissimo et al. 1992; Magnusson et al. 1999; Villela et al. 2006; Lobo et al. 2007), South-East Asia (Cannon et al. 1994; Shearman et al. 2012) and Africa (Plumptre and Reynolds 1994; Plumptre 1996; Dranzoa 1998; Klop et al. 2008; Blanc et al. 2009; Lewis et al. 2009) have generally shown that this practice has low impact on diversity (Johns 1988; Cannon et al. 1998; Imai et al. 2012) but a significant impact on species composition (Ganzhorn et al. 1990; Silva et al. 1995; Luna et al. 1999; Mangusson et al. 1999), genetic diversity (Jennings et al. 2001), forest structure (Cannon et al. 1994; Hall et al. 2003; Okuda et al. 2003) and nutrient cycling (Herbohn and Congdon 1993). Remote sensing analyses have demonstrated non-negligible impacts of large-scale physical forest damage from selective logging on the extent of canopy openings, arising from operations and changes in the carbon stock (Asner et al. 2005). Tropical silvicultural systems are very well known by the 1930s and practiced for hundreds years (Dawkins 1998). Nevertheless, there appears to be a consensus that selective logging causes relatively minor disturbance (Deckker and de Graaf 2003; Medjibe et al. 2011; Schleuning et al. 2011; Picard et al. 2012) and is thus acceptable within the requirements of some certification schemes such as the Forest Stewardship Council (FSC), a highly regarded Sustainable Forest Management (SFM) standard established in 1993 and now linked to REDD programs (Rametsteiner and Simula 2003). However, evidence of long-term effects of selective logging on carbon sequestration and biodiversity is poor and sometimes overlooked, particularly in Africa, despite the increasingly wide adoption of this practice.
Although generalizations about the impact of selective logging on biodiversity has so far remained elusive in Africa and it depends on many different factors, such as intensity, round cycle and gaps dimension, the studies on net effect of logging varies with a prevalence of researches highlighted moderate or negative impacts on biomass and richness, both of fauna (Farwig et al. 2008; Ofori-Boateng et al. 2013) and flora (Chapman and Chapman 1997; Hall et al. 2003; Ouédraogo et al. 2011)
The present work aims at contributing to fill knowledge gap on this commercial practice by investigating the impact of selective logging in some African tropical forests (West and Central Africa), its effects on biomass and biodiversity and the similarities and differences of this practice against clearcutting.
We focused on selective logging in Africa because of the scarcity of studies in most areas (particularly in the Congo river basin) and the consideration that this is the most widespread area-extensive extractive industry, with logging concessions occupying 30–45 % of forests (Nasi et al. 2006). It has been already documented in Africa that the presence of heavy machinery and logging companies has effects on forest wildlife (White 1994; Johns 1997; White and Tutin 2001), but how it affects forest ecosystem with direct disturbance and modifications of the structure, species composition and ecosystem services is still under discussion. Simulation studies show that the rate of forest regeneration strongly depends on the size, number and spatial arrangement of canopy gaps following harvest (Pinard and Cropper 2000). The creation of canopy gaps by selective logging is a critical, and often unconsidered, mechanism by which this practice affects the forest. Canopy gaps have an immediate impact on light interception, heat fluxes, water stress and plant productivity (Healey et al. 2000). Recent studies also suggest that canopy openings decrease in size with distance from each felled tree crown, but in recently logged forest the area initially affected by harvesting of each tree is at least 50–100 m in radius (Asner et al. 2004). Creation of large canopy gaps promotes invasion by different species of weeds and vines which can suppress regeneration of late-successional trees (Schnitzer et al. 2000; Duclos et al. 2013). Natural gaps created by branch-fall or natural tree death tend to be smaller than logging gaps and within them shade-tolerant tree saplings seems to be prepared to grow fast enough to fill the canopy opening (Bais et al. 2003; Perry et al. 2005; Callaway and Mahall 2007; Dudley and File 2007; Thorpe et al. 2009) and reducing the likelihood of invasion by light-demanding trees, vines and weeds (Asner et al. 2004). We argue that changes in native tree communities caused by the selective logging result in local variation of forest structure with consequences for biodiversity and carbon stocks and to analyse these effects we also consider in our research the role of vines and weeds in areas subject to selective logging.
In order to test the effect of selective logging and weeds and vines invasion described above we compared three forest types from 511 plots in West and Central Africa (33 in Ghana, Cameroon and Gabon and 478 in Sierra Leone) as representative of different management regimes: unlogged old-growth, selectively logged and regenerated following clearcutting. Data were collected during the last three years researches for the “Africa GHG” project supported by the European Research Council (ERC) Grant. From these data we derived 12 distinct indices encompassing forest density, structure and biomass as well as diversity which we tested for the impact of forest management type.
Methods
Study sites
We analysed data from five sites in Sierra Leone and Ghana, representing the West African Upper Guinea forests, and Cameroon and Gabon representing the Central African Congolian forests (Supplementary Figure 1). Bia National Park in Ghana (6°30’11.38”N, 3° 4’42.82”O, 230 m s.l.m.) is part of a protected area of 306 km2, which includes 78 km2 of National Park in the north, and 228 km2 of Resource Reserve (where logging is permitted) in the south (Short 1983; Lieberman et al. 1987). Annual precipitation is 1,500–1,800 mm falling mostly in May, June, September and October, and mean daily temperatures are 24–28 °C. The site is in the transition zone between mixed evergreen forest in the south and mixed semi-deciduous forest in the north. More than 300 plant species per hectare have been recorded with widespread trees represented by Tieghemella, Ceiba, and Khaya.
The Ankasa Conservation Area in western Ghana (5°15’47.87”N, 2°34’42.61”O, 103 m s.l.m.) is a c. 500 km2 Wildlife Protected Area comprising Nini-Suhien National Park and the Ankasa Resource Reserve, separated by the Nini-Sushien river (Davis and Philips 2005). The southern parts of Ankasa were logged from the early 1960s up to c. 1974. Logging intensity was relatively low due to the low volumes of commercially valuable species available. Nini Sushien contains one of the few remaining blocks of relatively untouched forest in the country (IUCN 2003). Annual precipitation is 1,700–2,000 mm. Plant diversity is similar to Bia and Nini-Suhien is classified as wet evergreen and Ankasa as evergreen forest.
Sangha Tri-National Forest in eastern Cameroon, which include the Lobeké National Park (2°17’6.58”N, 15°41’14.72”E, 512 m s.l.m.), is c. 1,840 km2 (Laporte and Lin 2003). It is bordered to the east by the Sangha River, a tributary of the Congo River, and the mean annual precipitation is 1,400 mm, with the dry season occurring from December to February (Asner et al. 2004). The vegetation is semi-evergreen forest, with over 300 species of trees, the largest including Ceiba pentandra, Terminalia superba and members of the family Sterculiaceae (e.g. Triplochiton, Pterygota).
The forests of Woleu-Ntem and Moyen-Ogoouè in north and central Gabon (1°22’39.81”N, 12° 6’58.35”E, 522 m s.l.m.) cover c. 1,800 km2 (White 1994; Voysey et al. 1999). Annual precipitation is 1,750–3,050 mm with almost all rain falling between October and April. There is little, if any, rainfall May–September but humidity remains high. Mean daily temperature is c 27 °C and varies little throughout the year. The vegetation is moist and evergreen forest.
In these four sites the main commercial timber species are Entandrophragma cylindricum, Terminalia superba, Triplochiton scleroxylon and Heritiera utilis. Selective logging in this region typically has a minimum diameter for felling of 30–100 cm depending on species, and logging cycles are 15–30 years. For example, in Cameroon the minimum felling diameter is 100 cm for Entandrophragma cylindricum, 60 cm for Terminalia superba and 80 cm for Triplochiton scleroxylon and logging cycle is 15–30 years. Logging operations in the Cameroonian tropical forest managed by selective harvesting, is described in Maesano et al. (2013).
Gola Rainforest National Park in Sierra Leone (7°39’53.69”N, 10°54’41.05”O, 300 m s.l.m.) is c. 710 km2 and is the largest area of lowland evergreen moist forest remaining in Sierra Leone (Klop et al. 2008). Before the forest was gazetted as a National Park around 30 % of it was exploited for timber. Logging practice in the northern block was good in the 1960s and 1970s, but thought to have been poor in the 1980s. Most areas were only logged once but a few had two harvests. Offtake was concentrated in accessible areas so impact was patchy. Logging in the southern blocks was much more intensive. It occurred 1963–1965 then 1975–1989 and in the latter period was patchy, intensive and damaging (Lindsell and Klop 2013). For further details see Lindsell and Klop (2013).
Sampling methods
Due to difficulties to monitor big dimension sampling areas in tropical forests and the evidence that is better to have many little plots than few large ones to estimate biomass and biodiversity (Magurran Magurran 2004), in Ghana, Cameroon and Gabon we selected 33 rectangular plots of 500 m2 (20 m × 25 m or 10 m × 50 m) (Avery and Burkhart 1983; Condit 1998) with an high detail of information (sampling all trees with d.b.h. > 5 cm), adopting a random sampling scheme (adapted Whittaker sampling method, Barbour et al. 1980; Shmida 1984) from a grid overlain on a satellite image (or land-cover map derived from satellite imagery) of the study area surfaces. The plots where spatially random distributed within each sampling area. Locations closer than 50 m to access roads and extant logging trails were excluded.
Plot locations were established on the ground using a GPS and compass and the positions of all trees in the plot with d.b.h. > 5 cm were mapped. For each tree we measured d.b.h. (at 1.37 m or 50 cm above buttress) and tree height using a laser hypsometer–dendrometer and identified to genus or species where possible. Weed, shrub and vine cover on the understory was recorded on an imaginary grid (10 m × 50 m or 20 m × 25 m, with cells of 5 m × 5 m and sub-cells of 1 m × 1 m) overlaid on each plot and measuring the surface occupied in terms of square meters per plot.
We used every available information on logging records, either derived from assessment plans of each national park where available or personal communications by forest dwellers and managers, to determine felling densities for selectively logged plots. These ranged from 4 (low harvest intensity, 2–4 commercial species) to 38 (high harvest intensity, 7–9 commercial species) trees/ha, with a mean of 1 ± 0.6 commercial trees logged per plot (500 m2).
Plots were classified as one of six management categories: primary forest (PF) was old-growth forest for which we have documented evidence that they were untouched by mechanized logging for some centuries; secondary forest (SF) was subject to clear cutting in the 1990s and left to recover for at least 20 years; selectively logged in the 1990s (SL20) or in the 1980s (SL30); twice logged in 1960s and 1990s (SLD) for one plot in Cameroon only; and cleared then selectively logged after c. 50 years (SFD) for two plots in Ankasa only. All plots had negligible a slope and lay on plains with the exception of two primary forest plots (PF) lying on hillsides (Table 1).
In Sierra Leone we used a subset of 609 circular plots of 0.125 ha reported by Lindsell and Klop (2013), excluding those disturbed by rivers, swamps, trails, farming activities, and those where the slope was not measured. We also only considered trees larger than 30 cm dbh. Using field observations, available logging records and records of concessionaire performance (IUCN 2003) we classified the plots as primary forest which had been untouched by commercial operations and showed no sign of recent illegal activity (PF, 223 plots), well-managed selectively logged forest which retained a largely intact canopy (SL-WM, 119 plots) and heavily logged with extensive canopy openings (SL, 136 plots). This classification is based on historical records (Iles et al. 1993) and field observations. “Unlogged” areas were untouched by commercial operations and showed no indication of recent illegal activity. “Well-managed” and “logged” areas were distinguished according to the recorded performance of the relevant concessionaires (Dudley and File 2007) with “well-managed” areas having a largely intact canopy and “over-logged” areas having extensive canopy openings. Because of the different sampling methodologies, we analyse the data from Sierra Leone reported by Lindsell and Klop (2013) and (478 circular plots—Dataset2) separately from the others 33 plots in Ghana, Cameroon and Gabon (Dataset1).
Data analysis
D.b.h., height and published wood density values (Chave et al. 2009; Zanne et al. 2009) were used to calculate aboveground biomass adopting allometric equations from Chave et al. (2005), of which carbon content was taken to be 47 % (Solomon et al. 2007). We derived 12 indices for each plot to describe forest structure, tree density and diversity (Table 2), except Sierra Leone for which only stem density and normalized diameter were derived using slope-corrected plot areas. We do not combine or compare Sierra Leone results directly with the other sites due to differences in sampling and size thresholds. For each index, we compared the values scored by primary forest plots with those scored by secondary forest and selectively logged forest plots of the Dataset1. Our null hypothesis (namely that the index shows no difference between PF and either SF or SL) was tested with a Mann–Whitney U test, being our data distribution non-normal or unknown and the samples size small. From the value of the statistics U we compute, using Harding’s algorithm (Press et al. 2007), the probability P of the null hypothesis, namely that the two samples are drawn from the same distribution (we report P value only when lower than 0.05).
Beta-diversity of logged and secondary plots was compared against that of primary forest (considered to be the reference level, 1 or 100 %) using the Sørensen index (65). The Sørensen index used in this analysis scored value from 0 % (completely dissimilarity) to 100 % (complete similarity).
The distribution of stem sizes in the Sierra Leone plots (Dataset2) was compared for the primary forest, well-managed and heavily logged plots using pairwise tests.
The large dataset of Sierra Leone allows us to estimate in a reliable way the distribution of the measured quantities. Therefore, we may test whether the logging activities change the vertical structure of the forest. This is accomplished by means of a pairwise Kolmogorov–Smirnov test between the ‘unlogged’ and ‘well-managed’ samples, and between the ‘unlogged’ and ‘logged’ samples (Press et al. 2007). The probability density functions (pdf) plotted in Fig. 5 are estimated with a kernel density method (based on the Epanechnikov kernel, using Silverman’s rule for bandwidth selection (Preston 1962). We estimate the pdf of the diameters of individual trees, and of the plot-based indices “tree density” and “normalized diameter”. With these two indices we also perform pairwise Mann–Whitney U tests. In order to properly weight the contribution of plots on sloping grounds, we use the vertical projection of the surface area in the computation of the values of the indices (namely, \(A_{l} = A_{0} { \cos }(\varphi_{l} )\), where \(A_{0}\) = 0.125 ha is the (constant) surface area of the plot, and φ is the slope of the ground as measured in situ with a clinometer).
Note that the actual values of these two indices are not directly comparable with those of the same indices of the 33 plots from the Dataset1, because in the former case the samples include all trees above 5 cm dbh, while in Sierra Leone only the trees above 30 cm dbh were measured and, overall, the sampling protocols are quite different (see “Sampling Methods”).
Results
Tree-level and plot-level indices provide contrasting perspectives on the state of the logged forests. When tree-level indices are compared (Fig. 1), secondary and selectively cut forest are found to be statistically indistinguishable from primary forest in terms of mean height (U = 25.0, P = 0.034 and U = 47.0, P not significant, respectively for secondary and selectively logged sites) but to have lower mean biomass (U = 23.0, P = 0.023 and U = 29.0, P = 0.006, respectively) and (in the case of selectively harvested forests) lower mean diameter (U = 34.0, P = 0.015).
Indices characterizing the distribution of tree height, biomass and diameter in each plot of Ghana, Cameroon and Gabon. a Mean tree height, b height of the 80th percentile, c mean biomass and d mean diameter. The abscissa shows a serial number attributed to each plot (which is held the same in all the figures). The marker shape identifies the geographical location of the plot (circles Cameroon; triangles Gabon; hexagons Ghana—Bia; diamonds: Ghana—Ankasa). Plots from Cameroon and Gabon are clumped together in Table 3 as “Central Africa”, Bia and Ankasa as “West Africa”. The colours identifies the type of forest management (black primary; dark grey selective; light gray secondary). We show the values of the Mann–Whitney U statistics, above the yellow symbols when comparing primary vs selectively cut forest plots. If the probability P of the null hypothesis is lower than 0.05 we report it under the value of U
One selectively logged plot in Gabon scores the highest value (16.6 m) in all four tree-level indices, reflecting that large trees may remain uncut following selective logging operations (Fig. 1). Plot-level comparisons (density with normalized indices in Fig. 2), show that secondary and selectively logged forests have lower stem density (U = 13.0, P = 0.02 and U = 20.5, P = 0.01, respectively), normalized height (U = 21.0, P = 0.016 and U = 22.0, P = 0.002, respectively) and normalized diameter (U = 27.0, P = 0.048 and U = 27, P = 0.004, respectively) than old growth forest, even though no logging had taken place in any of the sites for at least 30 years; biomass was also low in selectively logged forest (U = 42.0, P = 0.047), but secondary forests were not statistically distinguishable from old growth forests (U = 40.0, P not significant) because one plot in Gabon had exceptionally high biomass (Fig. 2). A general portrait in structure and gaps distribution differences are illustrated in Figs. 3 and 4, which compare the vertical profiles and canopy covers of four sampling plots of old growth and selectively logged forests in Cameroon and Ghana. When one compares a primary forest plot containing a few big, tall trees with a selectively logged forest deprived of tall trees, the averages are in both cases dominated by medium and low height trees, that by far outnumber tall trees even in primary forest plots (Asner et al. 2004, 2005). However, a sparseness of trees in selectively logged plots compared with the denser, thicker and more uniform spatial tree distribution in primary forest plots is evident.
Indices of forest density in each plot. a Normalized height, b tree density, c biomass, d normalized diameter. Symbols, colours and legends as in Fig. 1. Expected density indices of primary forest vs density indices of selective logged and secondary plots. The expected indices are obtaining omitting the two tallest trees of each plot for the normalized height (a); the two heaviest trees for the biomass density (b); any two trees for the tree density (c) and the two trees with the largest diameter for the normalized diameter (d). Symbols, colours and legends as in Fig. 1
Concerns that our inferences were influenced by a few large trees in some plots led us to explore the implications of removing outliers, but our findings keep robust. For all the old-growth forest plots in dataset 1, the value of the four density indices were recalculated after omitting the two tallest trees from each plot for the normalized height, the two heaviest trees for the biomass density, and the two trees with the largest diameter for the normalized diameter and any two trees for the tree density. This choice is justified by the fact that the estimated number of felled trees per plot in the selectively logged plots of Dataset 1 is 1 ± 0.6. In this way we have a rather pessimistic estimate of what those indices would be expected to score, if those plots were subject to a selective logging whose only effect was the disappearance of the two most valuable trees of each plot. We then compare the scores of the expected indices of primary forest plots (blue indicators in Fig. 2) with those of the other two types of forest in Fig. 2. In spite of the simulated logging, they remain significantly above the scores of selectively logged plots, except for biomass density, where the difference, albeit existing, is only marginally significant.
In order to establish whether test results were affected by geographic proximity, we applied the Mann–Whitney test grouping together only the plots from West Africa and, separately, only the plots from Central Africa (Table 3). Because of the reduced size of samples, it is impossible to reject the null hypothesis with very high confidence even when the value of U is near zero: with samples of this size the P-values can never be very small. However, the general pattern appears to be unaffected by the geographic location, namely that the first four indices, together with entropy and evenness are not particularly skilled in distinguishing plots of primary forest from the other two forest types, and that the density indices, together with species richness and vines cover fraction are generally able to perform the distinction. Notable exceptions are normalized height and tree density for West Africa plots of primary vs selectively logged forest. In these two cases, however, the value of the U statistics (U = 9.0, P not significant for normalized height and U = 11.5, P not significant for tree density) is almost completely determined by the low values recorded at a single plot of primary forest in Bia (plot number 7 in the figures), characterized by having relatively few, unusually low trees, probably because it lies on the side of a steep hill.
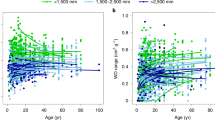
Probability density functions (pdfs) of the diameters of stems in the Sierra Leone forests (Fig. 5) are remarkably similar for old-growth vs well-managed and old-growth vs hystorically logged stands (K–S = 0.046, P = 0.060 and K–S = 0.049, P = 0.0381, respectively). The P-values of a Kolmogorov–Smirnov test confirm that differences were marginally significant and arose from slightly greater numbers of very large trees (> 75 cm dbh) in old growth forest. In contrast, the pdfs of tree density (U = 11,502, P = 6.18E−05) and normalized diameter (U = 10,576, P = 7.57E−07) reveal very clear differences between historically logged and old-growth forests, with much lower densities of stems > 30 cm dbh in the logged forests. Interestingly, these differences are not evident in comparisons of old-growth and well-managed forests (U = 13,187, P = 0.463 and U = 12,504, P = 0.190, respectively for tree density and normalized diameter). Tree species richness (U = 10.5, P = 0.001 and U = 17.0, P = 5E−04, respectively for primary vs secondary and primary vs selective), Shannon entropy (a measure of diversity, U = 21.0, P = 0.016 and U = 38.0, P = 0.027, respectively) and vine cover (U = 0.0, P = 6E−06 and U = 0.0, P = 4E−07, respectively) differ greatly among management types (Fig. 6).
Probability density functions of a diameters of individual stems; b tree densities; and c normalized diameters of Sierra Leone plots. Blue line unlogged plots; green line well-managed plots; red line logged plots. The very steep decline to zero on the left side of the curves in a is due to having sampled only trees larger than 30 cm d.b.h. K–S stands for the pairwise Kolmogorov–Smirnov test value
Indices of diversity and vine/weeds coverage. a Species richness, b Shannon’s entropy, c evenness, d cover fraction. Symbols, colours and legend as in Fig. 3
Evenness values (Table 3; Fig. 6) were generally very high (U = 49.0, P not significant and U = 61.0, P not significant, respectively), indicating that many trees species were represented by only one or two individuals. Historically logged forests were indistinguishable from old growth forests in terms of evenness of diversity, but had slightly lower entropy values, and much lower species richness: this is the expected consequence of the fact that the number of individuals per plot is less in selectively logged than in primary forests. Finally, vines contributed very little to the canopy cover of old-growth forests but were a significant component in historically logged forests.
Similarity indices (β-diversity based on genera) for species recorded in different management types of forests in each study area was calculated using the incidence-based Sørensen’s index. The results of pairwise comparisons are summarized in Table 4. These results cannot be considered as representative of the whole forest ecosystems because they are referring to small sampling plots (500 m2) which do not reach the Species–Areas Curve saturation (Press et al. 2007). The analysis suggests that selectively logged forests are more compositionally similar to old-growth forests than are secondary forests (Table 4). However, primary forests plots replicates share more common genera than if compared to selectively logged areas.
Discussion and conclusion
In recent decades, many tropical countries have adopted the mechanized selective logging as forest resource extraction practice (De Wasseige et al. 2004; Gascon et al. 1998; Drigo et al. 2009), regarding it as a management procedure that has few dangerous effects on the ecosystem, even though researches are scanty in critically evaluating its impact on forest structure, biodiversity and ecosystem services. To better understand the effect of selective logging on tropical forests, we compared three different types of forest management: primary forest, namely old-growth (not subject to mechanized logging) forest; secondary forest, namely forest subjected to clear-cutting management; selectively logged forests with different degrees of intensity. The data allowed us to form 12 distinct indices (summarized in Table 2) on forest biomass, tree diversity, tree density, etc. that we examined in order to determine which of them show statistically significant differences among the forest types, and which do not.
Our results indicate that the distribution of heights, diameters and biomasses, and the diversity of trees in a logged forest are not largely different from those of a primary forest (Fig. 1), while all indices of density (stem density U = 13.0, P = 0.02 and U = 20.5, P = 0.01; normalized height U = 21.0, P = 0.016 and U = 22.0, P = 0.002 and normalized diameter U = 27.0, P = 0.048 and U = 27, P = 0.004) show a marked decrease of the logged forest together with the concomitant invasion by vines and weeds (U = 0.0, P = 6E−06 and U = 0.0, P = 4E − 07 in Fig. 6). In other words, the forest subjected to selective logging does not seem to suffer evident effects on its vertical structure but rather shows a well-marked arboreal rarefaction.
The single exception to these findings are the “well-managed” plots of the Gola National Park in Sierra Leone (Dataset 2), where the density indices appear to have roughly the same values than in the “unlogged” plots (U = 13,187, P = 0.463 and U = 12,504, P = 0.190, respectively for tree density and normalized diameter). On the other hand, the hystorically “logged” plots of Gola show a drop in the density indices even though they were logged several decades ago (tree density U = 11,502, P = 6.18E − 05; normalized diameter U = 10,576, P = 7.57E − 07). This result suggests the key that allows for an understanding of the observed data. In the “well-managed” plots of Gola the harvesters took care to minimize as much as possible the amount of canopy damage exerted by the logging activities. No such care was taken in the “logged” plots. This, in turn, implies that the amount of extra light filtering to the lower forest layers, and to the ground, was limited for “well-managed” plots, and more abundant and spread out for “logged” plots.
It is reasonable to assume that the extra light caused by canopy openings larger than some threshold, triggers a complex chain of effects on the plant community (Chapman and Chapmap 1997; Blanc et al. 2009), of which we may guess a few links: (1) young trees of dominant species (such as Celtis mildbraedii) taking advantage of the free space and reduced shade due to the removal of parent plants or other logged, tall commercial species, replace some species of the secondary or tertiary layer (e.g. Baphia nitida); (2) many of the shade-intolerant trees, being suddenly exposed to direct sunlight, experience much increased mortality rates, and leave space for vines and weeds such as Aframomum alboviolaceus, Halopegia azurea, Manniophyton fulvum and Haumania danckelmania; (3) vines and shrubs, in the occasional spots where they manage to become dense enough, suppress the growth of seedlings of typical primary forest species (Santos et al. 2009).
We further investigate this issue by evaluating the expected normalized indices, for all the primary forest plots of Dataset 1, namely how would those indices be if plots were subject to a selective logging whose the only effect was the disappearance of the two most valuable trees of each plot.
We notice that, in spite of the simulated logging, they remain significantly above the scores of selectively logged plots (blue indicators in Fig. 2), except for biomass density, where the difference, albeit existing, is only marginally significant. This result suggests a scenario where the logging damage, unless the harvesting is extremely careful, non only persists for decades after the logging, but in some respects (e.g. tree density, normalized diameter, normalized height) becomes worse with time.
If, as a consequence of logging activities, a portion of forest remains locked for decades in a low-density, vines-rich state, undoubtedly there will be effects on its biodiversity (Schnitzer et al. 2000). However, those effects may be such to escape easy quantification. At the scale of the plots most species appear only once or twice, regardless of the forest type. Therefore entropy and evenness give very high scores (for instance in Gabon it is ≈ 1 in almost all plots, see Table 3). Richness of the plots appears to drop for logged forests (from a maximum value of 42 species in old-growth to 10 and 15 species in clearcutted and selectively logged forests, respectively), and this may easily be explained by the reduced density of the forest: a lower number of trees per plot inevitably leads to a reduced number of species (Cazzolla Gatti 2011), if each species is represented only once or twice (singletons or doubletons).
Changes in the density of suitable animal dispersers, directly or indirectly linked to forest alterations induced by logging activities (Brodie et al. 2009) may be among the possible causes of reduction of plant species at the forest-wide scale. In Central Africa, this phenomenon can be due to the reduction of herbivorous populations (elephants, antelopes, etc.) and other relevant animals such as monkeys, insects, etc. which are important for seed dispersal. Because many pioneer species are wind dispersed, clear-cutting encourages the establishment of plants which are wind dispersed. These include Funtumia elastica and Terminalia superba, which were responsible for 65 % of the total abundance of the SF in one study performed in Cameroon (Terborg and Wright 1994). Furthermore, the elimination of species such as those belonging to the genera Entandrophragma, Ceiba, Triplochiton and Erythrophleum, removes the main feeding sites of arboreal monkeys and nesting sites of hornbills, which are both important seed dispersers and reduce the possibility to grow for lianas and epiphytes (Gerwing and Vidal 2002).
Finally, we compared the practice of clearcutting to selective logging and we analysed the secondary forests plot indices. Looking just at the figures of normalized biometrics (dbh and heigh) and biomass (Fig. 1, 2, 5) selective logging and clearcutting appear to have very similar impacts. However, the high loss of species and the resetting of the long-term ecological successions of a tropical forest make clear-cutting obviously the worst in terms of conservation. This is clear analysing the Sørensen indexes (Table 4) which show genus similarity higher (sometimes close to 1) among secondary forest plots themselves than if compared to primary ones.
We can no longer assume that, after selective logging, the forest ecosystem recovers on a trajectory towards its primary (pre-disturbance) state or can keep biomass and biodiversity within their old-growth levels (Odum et al. 1971). This research suggests instead that, although in most of the plots analysed only a few commercial trees per hectare are removed, a large structural alteration of the whole ecosystem causes the decline of biodiversity and ecosystem services (in our case carbon storage in terms of biomass). Moreover, our findings suggest that the delay required for a near-complete recovery after a round of selective logging is heavily dependent on the amount of damage inflicted by the logging activities: in Sierra Leone a customary 30 years long pause between consecutive logging rounds could be sufficient for “well-logged” plots to recover the same arboreal density as the “unlogged” plots (even if it does not always ensure the same tree species richness), but would be largely inadequate for the more intensely harvested “logged” plots.
It is very likely that changes of the dynamics of niches consolidated over hundreds or thousands of years (Cazzolla Gatti 2011) in tropical forests due to the removal of certain key tree species lead to the development of dynamics (Herbohn and Congdon 1993; Thompson et al. 2009; Pan et al. 2011) that decrease the integrity of the system (density-dependent effects). Further investigations on later effects (> 50 years after logging) could shed light on these dynamics. Moreover, we showed that the structural changes, vines and weeds growth and alteration of the dynamics among species belonging to different layers are the main consequences of the logging and a clear explanation of the observed effects on biodiversity (Duffy et al. 2007) and carbon stocks. At the same time, the reduction of populations of large herbivorous mammals, the prevalence of wind-dispersed seeds and the increased competition between shade-tolerant species when exposed to sunlight in the forest that have been logged selectively, could be contributory factors that are collectively significant in explaining the effects documented (White 1994; Voysey et al. 1999; White and Tutin 2001).
Summarizing, this paper suggests that selective logging has several important negative effects on forest structure, dynamics, biodiversity and ecosystem services and that these effects can be truly evaluated only in the long term by analysing the evolving dynamics of repeated logging and not the mean structural values but the indices linked to the arboreal density.
The attention should be paid not just to totally destructive practices such as deforestation (clear-cutting) for alternative land uses (crops or grazing, commonly in the Amazon, or the palm oil plantations that are typical of South-east Asia), but also to the selective logging of the last virgin forests of Africa, which may be a more serious cause of forest degradation than what has been thought to date. These first results suggest that it will be crucial to increase research about the key question for forest management and conservation: is selective logging really sustainable for primary tropical forests?
References
Asner GP, Keller M, Silva JM (2004) Spatial and temporal dynamics of forest canopy gaps following selective logging in the eastern Amazon. Glob Change Biol 10:765–783
Asner GP, Knapp DE, Broadbent EN, Oliveira PJ, Keller M, Silva JN (2005) Selective logging in the Brazilian Amazon. Science 310:480–482
Avery T E, Burkhart HE (1983) Forest measurements (ed 3.). McGraw-Hill Book Company, New York
Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM (2003) Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 301:1377–1380
Barbour MG, Burk JH, Pitts WD (1980) Terrestrial plant ecology. Benjamin/Cummings
Blanc L, Echard M, Herault B, Bonal D, Marcon E, Chave J, Baraloto C (2009) Dynamics of aboveground carbon stocks in a selectively logged tropical forest. Ecol Appl 19:1397–1404
Brodie JF, Helmy OE, Brockelman WY, Maron JL (2009) Bushmeat poaching reduces the seed dispersal and population growth rate of a mammal-dispersed tree. Ecol Appl 19:854–863
Callaway RM, Mahall BE (2007) Plant ecology: family roots. Nature 448:145–147
Cannon CH, Peart DR, Leighton M, Kartawinata K (1994) The structure of lowland rainforest after selective logging in West Kalimantan, Indonesia. For Ecol Manage 67:49–68
Cannon CH, Peart DR, Leighton M (1998) Tree species diversity in commercially logged Bornean rainforest. Science 281:1366–1368
Cazzolla Gatti R (2011) Evolution is a cooperative process: the biodiversity-related niches differentiation theory (BNDT) can explain why. Theor Biol Forum 104:35–43
Chapman CA, Chapman LJ (1997) Forest Regeneration in Logged and Unlogged Forests of Kibale National Park, Uganda. Biotropica 29:396–412
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Yamakura T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99
Chave J, Coomes DA, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12:351–366
Condit R (1998) Tropical forest census plots: methods and results from Barro Colorado Island. Panama and a comparison with other plots. Springer, Berlin
Davis AL, Philips TKK (2005) Effect of deforestation on a southwest Ghana dung beetle assemblage (Coleoptera: Scarabaeidae) at the periphery of Ankasa conservation area. Environ Entomol 34:1081–1088
Dawkins HC, Philip MS (1998) Tropical moist forest silviculture and management: a history of success and failure. CABI Publishing, UK
De Wasseige C, Defourny P (2004) Remote sensing of selective logging impact for tropical forest management. For Ecol Manage 188:161–173
Deckker M, de Graaf NRR (2003) Pioneer and climax tree regeneration following selective logging with silviculture in Suriname. For Ecol Manage 172:183–190
DeFries RS, Houghton RA, Hansen MC, Field CB, Skole D, Townshend J (2002) Carbon emissions from tropical deforestation and regrowth based on satellite observations for the 1980s and 90s. Proc Natl Acad Sci 99:14256–142611
Dixon RK, Brown S, Houghton RA, Solomon AM, Trexler MC, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263:185–190
Dranzoa C (1998) The avifauna 23 years after logging in Kibale National Park, Uganda. Biodivers Conserv 7:777–797
Drigo R, Lasserre B, Marchetti M (2009) Tropical land cover change: patterns, trends and impacts. Plant Biosyst 143:311–327
Duclos V, Boudreau S, Chapman A (2013) Shrub cover influence on seedling growth and survival following logging of a tropical forest. Biotropica 45:419–426
Dudley SA, File AL (2007) Kin recognition in an annual plant. Biol Lett 3:435–438
Duffy JE, Cardinale BJ, France KE, McIntyre PB, Thébault E, Loreau M (2007) The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol Lett 10:522–538
FAO (2010) Global Forest Resources Assessment 2010: main report. Food and Agriculture Organization of the United Nations
Farwig N, von der Bleher B, Gönna S, Böhning-Gaese K (2008) Does forest fragmentation and selective logging affect seed predators and seed predation rates of Prunus africana (Rosaceae)? Biotropica 40:218–224
Ganzhorn JU, Ganzhorn AW, Abraham JP, Andriamanarivo L, Ramananjatovo A (1990) The impact of selective logging on forest structure and tenrec populations in western Madagascar. Oecologia 84:126–133
Gascon C, Mesquita R, Higuchi N, Cabarle BJ, Hartshorn GS, Bowles IA, Rice RE, Mittermeier RA, da Fonseca GAB (1998) Logging on in the rain forests. Science 281:1453. doi:10.1126/science.281.5382.1453b
Gerwing JJ, Vidal EE (2002) Changes in Liana abundance and species diversity eight years after liana cutting and logging in an eastern amazonian forest. Conserv Biol 16:544–548
Gibson L, Lee TM, Koh LP, Brook BW, Gardner TA, Barlow J, Peres CA, Bradshaw CJA, Laurance WF, Lovejoy TE, Sodhi NS (2011) Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478:378–381
Hall J, Harris DJ, Medjibe V, Asthon PM (2003) The effects of selective logging on forest structure and tree species composition in a Central African forest: implications for management of conservation areas. For Ecol Manage 183:249–264
Healey JR, Price C, Tay J (2000) The cost of carbon retention by reduced impact logging. For Ecol Manage 139:237–255
Herbohn JL, Congdon RA (1993) Ecosystem dynamics of disturbed and undisturbed sites in North Queensland wet tropical rain forest. II: Litterfall. J Trop Ecol 9:365–380
Houghton RA, Skole DL, Nobre Carlos A, Hackler JL, Lawrence KT, Chomentowski WH (2000) Annual fluxes of carbon from deforestation and regrowth in the Brazilian Amazon. Nature 403:301–304
Iles M, Savill P, Koker G (1993) Gola Forest reserves, Sierra Leone: interim management plan. Forestry Division, Sierra Leone and UK
Imai N, Sein T, Aiba SI, Takyu M, Titin J, Kitayama K (2012) Effects of selective logging on tree species diversity and composition of Bornean tropical rain forests at different spatial scales. Plant Ecol 213:1413–1424
IUCN United Nations List of Protected Areas (2003) Gland, Switzerland and Cambridge, UK and UNEP World Conservation Monitoring Centre, Cambridge, UK
Jennings SB, Brown ND, Boshier DH, Whitmore TC, Lopes JdoCA (2001) Ecology provides a pragmatic solution to the maintenance of genetic diversity in sustainably managed tropical rain forest. For Ecol Manage 154:1–10
Johns AD (1988) Effects of “selective” timber extraction on rain forest structure and composition and some consequences for frugivores and folivores. Biotropica 20:31–37
Johns AG (1997) Timber production and biodiversity conservation in tropical rain forests. Cambridge University Press, Cambridge
Kalipeni E (2007) Tropical Forests. In: Robbins P (ed) Encyclopedia of environment and society. SAGE Publications Inc, Thousand Oaks, pp 1774–1776
Klop E, Lindsell JA, Siaka A (2008) Biodiversity of Gola Forest, Sierra Leone. Royal Society for the Protection of Birds, Conservation Society of Sierra Leone and Government of Sierra Leone, Sandy, UK, and Freetown, Sierra Leone
Laporte NT, Lin TS (2003) Monitoring logging in the tropical forest of Republic of Congo with Landsat imagery. In: Geoscience and remote sensing symposium. IGARSS’03 proceedings. 2003 IEEE international, vol. 4, pp 2565–2567. IEEE, New York
Lewis SL, Lopez-Gonzalez G, Sonké B, Affum-Baffoe K, Baker TR, Ojo LO, Phillips OL, Reitsma JM, White L, Comiskey JA, Djuikouo KMN, Ewango CE, Feldpausch TR, Hamilton AC, Gloor M, Hart T, Hladik A, Lloyd J, Lovett JC, Makana JR, Malhi Y, Mbago FM, Ndangalasi HJ, Peacock J, Peh KS, Sheil D, Sunderland T, Swaine MD, Taplin J, Taylor D, Thomas SC, Votere R, Wöll H (2009) Increasing carbon storage in intact African tropical forests. Nature 457:1003–1006
Lieberman D, Lieberman M, Martin C (1987) Notes on seeds in elephant dung from Bia National Park, Ghana. Biotropica 19:365–369
Lindsell JA, Klop E (2013) Spatial and temporal variation of carbon stocks in a lowland tropical forest in West Africa. For Ecol Manage 289:10–17
Lobo J, Barrantes G, Castillo M, Quesada R, Maldonado T, Fuchs EJ, Quesada M (2007) Effects of selective logging on the abundance, regeneration and short-term survival of Caryocar costaricense (Caryocaceae) and Peltogyne purpurea (Caesalpinaceae), two endemic timber species of southern Central America. For Ecol Manage 245:88–95
Luna AC, Osumi K, Gascon AF, Lasco RD, Palijon AM, Castillo ML (1999) The community structure of cagedover tropical rain forest in Mt. Makiling Forest Reserve, Philippines. J Tropical Forest Sci 11:446–458
Maesano M, Picchio R, Lo Monaco A, Neri F, Lasserre B, Marchetti M (2013) Productivity and energy consumption in logging operation in a Cameroonian tropical forest. Ecol Eng 57:149–153. doi:10.1016/j.ecoleng.2013.04.013
Magnusson WE, de Lima OP, Reis FQ, Higuchi N, Ramos JK (1999) Logging activity and tree regeneration in an Amazonian forest. For Ecol Manage 113:67–74
Magurran A E (2004) Measuring biological diversity. Blackwell Science, Ltd, New York
Malhi Y, Grace J (2000) Tropical forests and atmospheric carbon dioxide. Trends Ecol Evol 15:332–337
Medjibe VP, Putz FE, Starkey MP, Ndouna AA, Memiaghe HR (2011) Impacts of selective logging on above-ground forest biomass in the Monts de Cristal in Gabon. For Ecol Manage 262:1799–1806
Nasi R, Cassagne B, Billand A (2006) Forest management in Central Africa: where are we? Int For Rev 8:14–20
Odum EP, Odum HT, Andrews J (1971) Fundam Ecol, vol 3. Saunders, Philadelphia
Ofori-Boateng C, Oduro W, Hillers A, Norris K, Oppong SK, Adum GB, Rödel MO (2013) Differences in the effects of selective logging on amphibian assemblages in three West African forest types. Biotropica 45:94–101
Okuda T, Suzuki M, Adachi N, Quah ES, Hussein N, Manokaran N (2003) Effect of selective logging on canopy and stand structure and tree species composition in a lowland dipterocarp forest in Peninsular Malaysia. For Ecol Manage 175:297–320
Ouédraogo DY, Beina D, Picard N, Mortier F, Baya F, Gourlet-Fleury S (2011) Thinning after selective logging facilitates floristic composition recovery in a tropical rain forest of Central Africa. For Ecol Manage 262:2176–2186
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Hayes D (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993
Perry L, Thelen GC, Ridenour WM, Weir TL, Callaway RM (2005) Dual role for an allelochemical: (±)-catechin from Centaurea maculosa root exudates regulates conspecific seedling establishment. J Ecol 96:1126–1135
Picard N, Gourlet-Fleury S, Forni É (2012) Estimating damage from selective logging and implications for tropical forest management. Can J For Res 42:605–613
Pinard MA, Cropper WP (2000) Simulated effects of logging on carbon storage in dipterocarp forest. J Appl Ecol 37:267–283
Plumptre AJ (1996) Changes following sixty years of selective timber harvesting in the Budongo Forest Reserve, Uganda. For Ecol Manage 89:101–113
Plumptre AJ, Reynolds V (1994) The effect of selective logging on the primate populations in the Budongo Forest Reserve, Uganda. J Appl Ecol 31:631–641
Press WH, Teukolsky SA, Vetterling WT, Flannery BP (2007) Numerica Recipes, III edn. Cambridge University Press, Cambridge
Preston FW (1962) The canonical distribution of commonness and rarity: Part I. Ecology 43:185–215, 431–432
Rametsteiner E, Simula M (2003) Forest certification - an instrument to promote sustainable forest management? J Environ Manage 67:87–98
Santos KD, Kinoshita LS, Rezende AA (2009) Species composition of climbers in seasonal semideciduous forest fragments of Southeastern Brazil. Biota Neotropica 9:175–188
Schleuning M, Farwig N, Peters MK, Bergsdorf T, Bleher B, Brandl R, Böhning-Gaese K (2011) Forest fragmentation and selective logging have inconsistent effects on multiple animal-mediated ecosystem processes in a tropical forest. PLoS ONE 6:e27785
Schnitzer SA, Dalling JW, Carson WP (2000) The impact of lianas on tree regeneration in tropical forest canopy gaps: evidence for an alternative pathway of gap-phase regeneration. J Ecol 88:655–666
Shearman P, Bryan J, Laurance WF (2012) Are we approaching ‘peak timber’ in the tropics? Biol Conserv 151:17–21
Shmida A (1984) Whittaker’s plant diversity sampling method. Isr J Bot 33:41–46
Short JC (1983) Density and seasonal movements of forest elephant (Loxodonta Africana cyclotis, Matschie) in Bia National Park, Ghana. Afr J Ecol 21:175–184
Silva JNM, Carvalho JOP, Lopes JdoCA, Costa DH, Oliveira LC, Vanclay JK, Skovsgaard JP (1995) Growth and yield of a tropical rain Forest in the Brazilian Amazon 13 years after logging. For Ecol Manage 71:267–274
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tgnor M, Miller HL (2007) IPCC, 2007: climate change 2007: the physical science basis. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. SD Solomon (Ed)
Terborg J, Wright J (1994) Effects of Mammalian Herbivores on plant recruitment in two neotropical forest. Ecology 75:1829–1833
Thompson I, Mackey B, McNulty S, Mosseler A (2009) Forest resilience, biodiversity, and climate change. A synthesis of the biodiversity/resilience/stability relationship in forest ecosystems. Secretariat of the Convention on Biological Diversity, Montreal. Technical Series no. 43
Thorpe AS, Thelen GC, Diaconu A, Callaway RM (2009) Root exudates are allelopathic in invaded community but not in native community: field evidence for the novel weapons hypothesis. J Ecol 97:641–645
Verissimo A, Barreto P, Mattos M (1992) Logging impacts and prospects for sustainable forest management in an old Amazonian frontier: the case of Paragominas. For Ecol Manage 55:169–184
Villela DM, Nascimento MT, Aragão LEO, Da Gama DM (2006) Effect of selective logging on forest structure and nutrient cycling in a seasonally dry Brazilian Atlantic forest. J Biogeogr 33:506–516
Voysey BC, McDonald KE, Rogers ME, Tutin CE, Parnell RJ (1999) Gorillas and seed dispersal in the Lope Reserve, Gabon. I: Gorilla acquisition by trees. J Trop Ecol 15:23–38
White LJT (1994) The effects of commercial mechanised selective logging on a transect in lowland rainforest in the Lopé Reserve. Gabon Ecol 10:313–322. doi:10.1017/S0266467400007987
White LJT, Tutin C (2001) Why chimpanzees and gorillas respond differently to logging: a cautionary tale from Gabon. In: Webber W, White LJT, Vedder A, Naughton-Treves L (eds) Cconservation: an interdisciplinary perspective. Yale University Press, New Haven, pp 449–462
Zanne AE, Lopez-Gonzalez G, Coomes DA, Ilic J, Jansen S, Lewis SL, Miller RB, Swenson NG, Wiemann MC, Chave J (2009) Data from: towards a worldwide wood economics spectrum. Dryad Digit Reposit. doi:10.5061/dryad.234
Acknowledgments
This work was supported by the European Research Council (ERC) Grant for Africa GHG project. We thank Bia National Park and Ankasa Forest Reserve guides and operators, Ghana and Cameroon Forestry Commissions, Mr. Ntim Gyakari and Mr Seth Nuamah for thier invaluable work on the taxonomy of West African tree species, the Gola Forest Programme and the Ministry of Agriculture, Forestry and Food Security of Sierra Leone and the Gabonese Herbarium staff.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Cazzolla Gatti, R., Castaldi, S., Lindsell, J.A. et al. The impact of selective logging and clearcutting on forest structure, tree diversity and above-ground biomass of African tropical forests. Ecol Res 30, 119–132 (2015). https://doi.org/10.1007/s11284-014-1217-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-014-1217-3