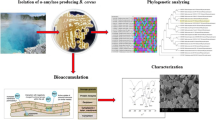
Abstract
Recovery of rare earth elements (REEs) from wastewater with Bacillus subtilis (B. subtilis) during culture is promising due to its environmental benefits. However, the effects of REEs in the culture media on B. subtilis are poorly understood. This study aims to investigate the effects of the terbium (Tb(III)), a typical rare earth element, on the cell growth, sporulation, and spore properties of B. subtilis. Tb(III) can suppress bacterial growth while enhancing spore tolerance to wet heat. Spore germination and content of dipicolinic acid (DPA) were promoted at low concentrations of Tb(III) while inhibited at a high level, but an inverse effect on initial sporulation appeared. Scanning electron microscope and energy dispersive spectrometer detection indicated that Tb(III) complexed cells or spores and certain media components simultaneously. The germination results of the spores after elution revealed that Tb(III) attached to the spore surface was a key effector of spore germination. In conclusion, Tb(III) directly or indirectly regulated both the nutrient status of the media and certain metabolic events, which in turn affected most of the properties of B. subtilis. Compared to the coat-deficient strain, the wild-type strain grew faster and was more tolerant to Tb(III), DPA, and wet heat, which in turn implied that it was more suitable for the recovery of REEs during cultivation. These findings provide fundamental insights for the recovery of rare earths during the culture process using microorganisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rare earth elements (REEs) are important strategic resources that have been widely used in photocatalysis, medical detection, and therapy, as well as agricultural material preparation due to their unique photoelectromagnetic properties (Cang et al. 2022; Wei et al. 2022; Liu et al. 2022). However, bioaccumulation or even liver damage and impaired embryonic development could be caused by long-term exposure to rare earths. (Pagano et al. 2019). In mining, rare earths are inevitably diffused with the water, which not only causes the wastage of resources but even endangers human health. Thus, environmentally friendly rare earth recovery technologies were urgently needed.
Compared to ion exchange, chemical precipitation, and membrane separation, microbial methods are more economical, eco-friendly and controllable. They can be used to treat low concentrations of rare earths (Sadovsky et al. 2016; Lima and Ottosen 2021; Shen et al. 2021). Various studies have shown that microalgae, fungi, and bacteria have a good adsorption effect on rare earths (Heilmann et al. 2021; Bergsten-Torralba et al. 2021; Shen et al. 2021). Similarly, several Bacillus species (Bacillus sp.) have also demonstrated commendable rare earth adsorption abilities. Cheng et al. reported that the adsorption of cerium by Bacillus licheniformis was up to 38.93 mg/g in aqueous solution with an initial cerium concentration of 200 mg/l (Cheng et al. 2022). Similarly, the adsorption of yttrium by Bacillus sp. ZD 1 reached 38.05 mg/g while the initial concentration of yttrium was 1.13 mM (Wang et al. 2021). However, using microbial cells as sorbents necessitated a rather stringent adsorption environment, as they were more susceptible to inactivation. Unlike cells, Bacillus spores are more resistant to external stresses such as heat, certain chemicals, and radiation (Setlow 2006). Also, they are capable of absorbing REEs. Under the same conditions, the absorption of Tb(III) by Bacillus subtilis (B. subtilis) PS4150 spores was higher than that of the cells, which was up to 94.792 µmol/g (Dong et al. 2022). Currently, the cells or spores used for adsorption of rare earths need to be prepared by purification, which results in higher costs and potentially impaired adsorption capacity. In view of that, the use of Bacillus sp. to recover rare earths during culture simplified the process and lowered costs effectively. According to our earlier research, utilizing B. subtilis PS832 during cultivation enhanced the rate of terbium Tb(III) extraction by 26.96% ~ 80.53% when compared to traditional adsorption methods at the same biomass (data not published). However, as relevant studies have been sparsely reported, numerous fundamental issues still need to be investigated for the recovery of rare earths via adsorption using Bacillus sp. during cultivation. It is essential to investigate the effects of rare earths in the culture media on the biological properties of B. subtilis and to establish criteria for strain selection, as bacterial biomass, spore resistance, and germination activity are important factors influencing REE adsorption, spore suitability, and spore reusability.
Currently, studies have mainly focused on the effect of REEs on bacterial growth and less on the effects of REEs in the culture media on sporulation. Previous studies have shown that microbial growth is promoted by low concentrations of rare earths but inhibited by higher concentrations, also known as “Hormesis” (Furst 1987). The growth of Bacillus sp. Bl was stimulated at low concentrations of yttrium but stopped at pH 5 when yttrium concentrations were increased to 160 mM (Liao et al. 2020). Similarly, the portion of the thermogenic curve associated with sporulation disappeared when the Sm3+ concentration was increased to 250 µg/ml, suggesting that high concentrations inhibited the formation of Bacillus thuringiensis spores (Ruming et al. 2003). Unfortunately, there has been little research into the effect of the REEs in the culture media on the properties of Bacillus sp. spores such as heat resistance and germination. Consequently, additional relevant studies are required given the relative paucity of studies on the effects of REEs in culture media on Bacillus sp., particularly the spores.
In this study, B. subtilis PS832 and PS4150 were used to investigate the effects of Tb(III) in the culture media on B. subtilis. We examined the effects of Tb(III) on the growth, sporulation, spore germination, wet heat resistance, and dipicolinic acid (DPA) content of B. subtilis. To investigate how Tb(III) acts on B. subtilis, the distribution and attachment of Tb(III) and germination after elution were also detected. This work aimed to gain insight into the role of rare earths on B. subtilis.
Materials and methods
B. subtilis strains and spore preparation
The strains used in this paper include B. subtilis PS832 (wild type) and PS4150 (tetR and spnR genes replacing the cotE and gerE genes). Both strains were provided by Dr. Peter Setlow (UConn. Health) (Ghosh et al. 2008).
Strains were inoculated in Luria-Bertani (LB) liquid culture media containing 0, 130, 800, and 2410 µM of terbium chloride hexahydrate (99.9% TbCl3·6H2O), and the spores were enriched after 72 h of incubation at 200 rpm with a temperature of 37℃. The above cultures were washed three times with sterile water, which was then processed by an ultrasonic cell crusher at 50% power for 6 min to remove the cells, and the impurities and cellular debris were removed by centrifugation at 8000 rpm for 10 min. Finally, the spore suspension with a purity of more than 98% was obtained and stored at 4℃ (Dong et al. 2019a).
Determination of bacterial growth and sporulation
Seed solution that was cultured at 180 rpm and 37℃ for 12 h was inoculated into LB liquid culture media containing 0, 130, 800, 1610, 2410 and 3210 µM of Tb(III) with an inoculum of 2% (v/v), which were then cultured at 200 rpm with a temperature of 37℃, and the optical density at 600 nm (OD600) of the culture solution was measured every 1 h until it became stable.
The inoculation and cultivation conditions for the determination of sporulation rate were the same as above. Samples were taken every 3 h for the first 12 h and then every 12 h in the next 60 h. 8~10 photographs of each sample were taken with a phase contrast microscope, and the total 3 groups of cells and spores over 100 were counted by ImageJ to calculate the rate of sporulation (Dawes and Mandelstam 1970).
Determination of spore germination
Spores with an OD600 of 1.0 were heat-shocked at 60℃ for 30 min, followed by an ice bath for 15 min, which was then incubated in 10 mM valine, 10 mM HEPES buffer (4-hydroxyethylpiperazine ethanesulfonic acid; pH 7.4) at 37℃, and the OD600 was measured every 4 min. The germination of the spore population was determined by the change of OD600, and the initial OD600 was set to 100% when the data were processed (Paidhungat and Setlow 2014).
Spores with an OD600 of 1.0 were eluted with 10 mM DPA for 5 min after centrifuging at 10,000 rpm for 2 min, which was then washed three times with sterile water. The subsequent heat-shock and incubation steps for germination were carried out as above (Dong et al. 2019b).
Determination of wet heat resistance of spores and DPA content
Spore suspensions with an OD600 of 2.0 were cooled in ice for about 15 min after being treated in water at 90℃ (PS832) or 80℃ (PS4150) for 0, 5, 10, 15, and 20 min respectively. The suspensions were inoculated on LB plates after 10-fold gradient dilution and incubated at 37℃ for 14 ~ 18 h. The number of colonies was recorded and the survival rate was calculated. In order to derive the equation of spore survival rate and incubation time, the survival rate and related incubation time were fitted with the logistic function in the Origin software, with the initial value parameter A1 and final value parameter A2 set to 1 and 0, respectively. The D value was the incubation time when the survival rate was 10%.
The fluorescence intensity of Tb-DPA complexes formed after 0, 8, 16, 24, 32, and 40 nM DPA mixed with 5 mM Tb(III) was measured by a microplate reader, and the standard curve of DPA content versus fluorescence intensity was plotted (Rosen et al. 1997; Jamroskovic et al. 2016). The fluorescence intensity of the unboiled and boiled supernatant of spore suspensions (OD600 = 1.0) was measured by a microplate reader with emission at 545 nm and excitation at 275 nm, and the total DPA content was obtained (Dong et al. 2022). After being diluted and inoculated into plates, the number of spores in the sterile aqueous suspension of each spore (OD600 = 1.0) was counted and the average DPA content of each spore was then calculated.
Determination of Tb(III) distribution and its attachment on the spore surface
Seed solution that was cultured at 250 rpm and 37℃ for 5 h was inoculated into LB liquid culture media containing 0, 130, 800, and 2410 µM of Tb(III) with an inoculum of 2% (v/v), which was then cultured at 200 rpm with a temperature of 37℃. Samples were taken every 3 h during the first 12 h and every 12 h for the last 60 h of the culture. Fluorescence intensity was measured after the mother liquor or supernatant was incubated with 1 mM DPA and 50 mM HEPES (pH 7.4).
The spore suspension (OD600 = 2.0) was eluted by 10 mM DPA for 5 min, and the supernatant was obtained by centrifugation at 10,000 rpm for 2 min. The subsequent fluorescence assay was carried out as above.
Precipitates were collected by centrifugation, washed three times with sterile water, and freeze-dried in a vacuum. After gold spraying, micrographs and energy spectrum scans of the samples were observed using SEM (MLA650F, FEI, Hillsboro, OR, USA).
Results
Effects of Tb(III) in the culture media on bacterial growth and sporulation
Since cell growth was related to the amount and efficiency of rare earth recovery, the growth of PS832 and PS4150 strains was determined using the spectrophotometric method (Fig. 1). The growth of both strains was inhibited by the Tb(III) in the media, and the inhibition effect of Tb(III) on cell growth was enhanced as the concentration of Tb(III) increased (Fig. 1a-b). Furthermore, in the absence of Tb(III), both strains reached a stationary phase after 14 h of incubation. Whereas, the OD600 of PS832 was significantly higher than that of PS4150, implying that PS832 had a faster growth rate and greater yield.
Bacterial growth and sporulation rate at different concentrations of Tb(III). Bacterial growth of PS832 (a) and PS4150 (b) at 0, 130, 800, 1610, 2410, and 3210 µM Tb(III). The sporulation rates of PS832 (c) and PS4150 (d) at 0, 130, 800 and 2410 µM Tb(III) were determined by microscopic counting according to the methods described in the Materials and Methods. The two points that correspond with the red dashed line in the enlarged figures within Fig.(c-d) are significantly different according to ANOVA (P ≤ 0.05)
According to previous methods, a water bath at 80℃ for 10 min was sufficient to distinguish spores from cells (Akanuma et al. 2013). However, the survival rate of PS4150 spores obtained from LB liquid medium in the water bath at 80℃ for 10 min was only about 20% (data not shown). Consequently, compared to that without Tb(III), the sporulation rates for both strains after 12 h of incubation at 130 µM were marginally lower, while both were higher at 2410 µM (Fig. 1c-d). Initial spore formation (12 h) was inhibited at low concentration of Tb(III) and stimulated at high concentration.
Effects of the Tb(III) in the culture media on spore germination
Spore germination is a critical property of spores. It is essential for the survival of B. subtilis and the determination of the reusability of the strain after the REE recovery. Compared to the group without Tb(III), the germination rate of PS832 spores was higher at 130 and 800 µM, while that of PS4150 was lower (Fig. 2). Both strains experienced inhibition of germination under 2410 µM of Tb(III), but this effect was more pronounced in PS4150. The presence of the Tb(III) in culture media resulted in hormetic effects on the spore germination of PS832. However, only inhibitory effects on PS4150 germination were observed with Tb(III) in the culture media, and this effect was intensified at higher concentrations of Tb(III). These findings demonstrated that PS4150 spores were apparently more sensitive to Tb(III).
The germination of spores before and after elution. Spores of PS832 (a) and PS4150 (b), either untreated or eluted (eluted by 10 mM DPA for 5 min) obtained at 0, 130, 800, and 2410 µM Tb(III), were then incubated with 10 mM L-valine and 10 mM HEPES (pH 7.4) at 37℃. Every 4 min, the optical density at 600 nm (OD600) was measured, following the guidelines provided in the Methods
Further investigations of how the effect of the Tb(III) in culture media on spore germination occurs was carried out by examining the germination of spores after elution with DPA (Fig. 2). In the absence of Tb(III), both strains showed inhibited germination after DPA elution, but PS4150 spores were significantly more inhibited than PS832 spores. Interestingly, the promotion or inhibition effect of Tb(III) on PS832 spore germination was significantly relieved after elution. In contrast, the suppressive impact on the germination of PS4150 spores was significantly enhanced after elution. According to these findings, spore germination was mostly influenced by the amount of Tb(III) in the culture media adhered to the spore surface, and PS4150 spores were more susceptible to exogenous DPA than PS832 spores.
Effects of Tb(III) in the culture media on the wet heat resistance and DPA content of spores
Wet heat resistance is a typical feature of spores, which is important for the adaptation of strains to environments with different temperatures. Previous studies have shown that spores obtained from liquid media are significantly less tolerant to wet heat than those from agar plates (Rose et al. 2007). However, it was still unknown whether the wet heat resistance of spores would be enhanced by the addition of rare earths in the culture media. Therefore, the effects of Tb(III) on the wet heat resistance of PS832 and PS4150 spores at 90℃ and 80℃ respectively were investigated to delve into the change in wet heat resistance of spores (Fig. 3a-b). According to the difference in the survival rate of various PS832 spores after incubation at 90℃ for 5 min, it was found that the wet heat resistance of PS832 spores was significantly enhanced by the Tb(III), especially at high concentration (Fig. 3a). However, there was no significant difference observed in the wet heat resistance between PS4150 spores generated in the Tb(III)-supplemented media and the control media (Fig. 3b). The results are consistent with the conclusion obtained from the D values in Table 1 (Table 1). In addition, the wet heat resistance of PS4150 spores was significantly lower than that of PS832 spores (data not shown). The presence of Tb(III) in the culture media improved the wet heat resistance of spores. However, this effect might be hidden by a defective coat, since the intact coat is crucial for the wet heat resistance of the spore.
The wet heat resistance of spores, and the standard curve of the fluorescence of Tb-DPA complexes with different amounts of DPA. PS832 (a) and PS4150 (b) spores, obtained from culturing in medium containing varying concentrations of Tb(III) (0, 130, 800 and 2410 µM), were exposed to water baths at 90 and 80℃ for 0, 5, 10, 15, and 20 min, respectively. The quantity of spores that remained was subsequently determined through plate counting, and the resultant spore survival rate was calculated. (c) Standard curve of DPA content and fluorescence intensity, whose equation is \(y=996.47x\) (R²=0.9948)
DPA accounted for approximately 25% of the core dry weight of the spore, which is important in maintaining the resistance and stability of the spore (Setlow et al. 2006; Setlow 2006; Magge et al. 2008). In this study, the average DPA content of spores was determined (Table 1, and Fig. 3c). Compared to the control group, the DPA content of the spores of both strains was slightly higher at 130 µM, while both were lower at 2410 µM. Furthermore, the decrease in DPA content of PS4150 spores in the presence of Tb(III) was comparatively greater than that of PS832 spores. Additionally, the DPA content of PS4150 spores was significantly lower than that of PS832 spores at each concentration of Tb(III). All these results suggested that Tb(III) in the culture media exhibited hormetic effects on the DPA content of spores and the intact spore coat is critical in maintaining the DPA content of spores.
Distribution of Tb(III) in the culture media and its attachment on the spore surface
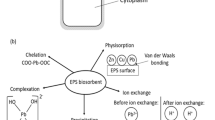
Unlike in an aqueous solution, the effects of the REEs in the culture media on cells or spores were more complex due to the presence of bacterial metabolism, media, and REEs. The distribution of Tb(III) and its attachment on the spore surface of PS832 during culture was investigated using fluorescence and SEM-EDS (Figs. 4 and 5, and Fig. 6). The fluorescence intensity of the cultures was proportional to the amount of Tb(III) added, but much higher than that of the supernatant (Fig. 4a). However, within the first 12 h, there was a slight decrease in the fluorescence intensity of the supernatant at 800 and 2410 µM. However, no significant change was observed at 130 µM. Notably, the fluorescence intensity of the supernatant at each concentration of Tb(III) increased gradually from 24 to 72 h, while the total biomass remained stable during this period (Fig. 4b). These results suggested that certain components of the culture media were able to form complexes with Tb(III), which was consistent with the results of SEM-EDS (Figs. 5 and 6a). However, such complexes were gradually lifted with the consumption of media.
The distribution of Tb(III) during the cultivation of PS832. Fluorescence intensity of Tb-DPA in medium (a) and supernatant (b) was measured after PS832 incubation in the culture media containing 0, 130, 800, and 2410 µM of Tb(III) for different times (0, 3, 6, 9, 12, 24, 36, 48, 60, and 72 h), all of which were determined as described in the Methods
SEM micrographs of cells or spores of PS832 at different concentrations of Tb(III) and culture times. The surface morphology of cells or spores at 0 or 2410 µM of Tb(III) and cultured for 12, 24, 36, or 48 h was observed using SEM, as described in Methods. Tb(III) complexes were observed in the vicinity of the cells or spores as noted in the red circle
Elemental mass percentage of different point types of PS832, and the mean amount of Tb(III) attached to the spores. (a) Elemental mass percentage of C, O, and Tb. X in “X-Y” in the horizontal axis represents the culture time (12, 24, 36, and 48 h), and Y represents the point type (A represents the surface of cells or spores cultured at 0 µM Tb(III), while B and C represent the surface of cells or spores and complex obtained at 2410 µM Tb(III) respectively). (b) The mean attachment of Tb(III) on the surface of PS832 and PS4150 spores obtained by incubation in the culture media containing 0, 130, 800, and 2410 µM of Tb(III) was determined as described in Materials and Methods
Spores are the predominant form of B. subtilis during the post-culture phase when the rate of spore formation is stable and Tb(III) is predominantly attached to spores. The fluorometric method in Materials and methods was used to determine the Tb(III) attachment on the spore surface (Fig. 6b). A significant increase in the mean Tb(III) attachment to the spore surface of both strains was observed as the Tb(III) concentration increased to 2410 µM. It was consistent with the adsorption of rare earths in an aqueous solution. However, the amount of Tb(III) adhered to the surface of PS4150 was higher than that of PS832 at each concentration of Tb(III) (Fig. 6b). It was in accordance with past research that PS4150 spores exhibited greater capacity for Tb(III) adsorption compared to PS832 spores (Dong et al. 2022).
Discussion
The work in this paper confirmed that either cells or spores were capable of adsorbing Tb(III) during culture, although PS4150 spores exhibited a higher adsorption capacity than PS832, which could be linked to the number of adsorption sites (groups), given that related groups such as phosphate, amino, and carboxyl groups were involved in Tb(III) adsorption, which was consistent with recent findings (Dong et al. 2022). However, unlike traditional adsorption, adsorption in culture was intricate because of the existence of media and bacterial metabolism (Fig. 7). Nevertheless, experimental evidence demonstrates the great promise of REE adsorption for recovery during culture. On the one hand, the adsorption of rare earths in culture exhibited a significant increase of 26.96% ~ 80.53% compared to the usual adsorption technique with similar biomass (Data not shown). On the other hand, the wastewater with rare earths used for practical treatment usually contains other metal ions, which may provide nutrients for the growth and sporulation of B. subtilis. However, further studies on application protocols and subsequent elution are still needed for the practical application of this approach.
Distribution diagram of Tb(III) during the culture of B. subtilis. Tb(III) in the culture media complexed both cells or spores and certain media components. With the culture, the bacteria began to utilize the media components that complexed with Tb(III), which allowed the release of free Tb(III) ions. In addition, Tb(III) might also enter the cells and influence or even participate in the related metabolism
The work in this paper confirmed the inhibitory effect of at least one of the rare earths, Tb(III), in the culture media on the growth of B. subtilis. The result revealed some new findings compared with previous studies (Liao et al. 2020). Tb(III) might have the ability to regulate the activity of some enzymes, which was similar to how scandium stimulated the production of B. subtilis amylase at the transcriptional level (Inaoka and Ochi 2011). Although its mechanism and enzyme species are unidentified now, Tb(III) might replace other metals at the enzyme site, given its higher valence state and similar ionic radius to calcium. In contrast, the effect of Tb(III) in the culture media on the initial sporulation was reversed. Although the exact mechanism is still unclear, we know that sporulation is regulated by the phosphorylation of Spo0A, which is initiated by the autophosphorylation of histidine kinase in response to external stimuli (Tan and Ramamurthi 2014). Thus, Tb(III) in the culture media was relatively weakly stressed at low concentrations as it was likely to act as a nutrient element. However, at higher concentrations, it became a stressor to inhibit the activity of relevant enzymes, resulting in a relatively strong nutrient stress environment. Since B. subtilis usually forms spores under nutrient stress, it is likely that the autophosphorylation of histidine kinase was modulated by Tb(III) in the culture media that worked indirectly on the nutrient environment, which further affected initial B. subtilis sporulation (Driks 2002).
The work in this paper also led to some conclusions regarding the effect of Tb(III) in the culture media on the properties of Bacillus spores: First of all, the impact of Tb(III) in the culture media on the spore germination of PS832 were hormetic effects, while that of PS4150 was only inhibited. The former was in line with our previous findings, while the latter was consistent with the spore germination of coat-deficient spores that was strongly inhibited by Tb(III) (Yi et al. 2011). Initially, it was assumed that the direct effect of Tb(III) attached to the spores and the indirect effect of Tb(III) during incubation on germination would coexist and be dominated by the latter. However, the results of germination after elution showed that the effect of Tb(III) in the culture media on germination was dominated by the direct effect of Tb(III), although the indirect effect did exist. As previously investigated, the DPA release channel of the coat-deficient PS4150 spores could probably be blocked by Tb(III) (Yi et al. 2011). However, hormetic effects were exhibited by Tb(III) on PS832 spore germination and the remission effect after elution was in the mid to late stages of germination. It appeared that the activity of cortex-lytic enzymes (CLEs) located on the surface of the coat might have been directly influenced by the Tb(III) attached to the spore surface, which needed to be further explored by using different germinants. Second, the resistance of PS832 spores to wet heat was enhanced by the Tb(III) in the culture media, while the wet heat resistance of PS4150 spores was not affected by Tb(III). According to recent studies, there was no significant difference in the wet heat resistance of B. subtilis spores after being loaded with Tb(III) (Dong et al. 2019b). Therefore, unlike the germination of spores, the wet heat resistance of spores should be mainly influenced by the indirect effects of Tb(III) in the culture media. However, the mechanism was not yet clear, as numerous factors were involved in the wet heat resistance of spores (Setlow 1994). The spore mineralization was increased by Tb(III) in the culture media, which might have further influenced the core water content and inner membrane fluidity, as the addition of metal ions to the culture medium is one way of regulating the spore mineralization (Marquis and Shin 1994). In addition, low concentrations of Tb(III) probably acted as a nutrient or stimulated the enzyme activity to improve the tightness of spores; while high concentrations of Tb(III) might act as an environmental factor that would likely to induce the thickening of the coat. Notably, all of the enhanced effects on PS4150 spores might have been masked by defects in the coat, given the critical role of the intact coat in the wet heat resistance of spores (Ghosh et al. 2008). Furthermore, hormetic effects were also observed by Tb(III) in the culture media on the DPA content of the spores. Although the exact mechanism has not yet been investigated, previous studies have shown that DPA is synthesized in the mother cells by dihydrodipyridylic acid, catalyzed by DPA synthase, and then imported into the forespore by the combined action of the SpoVV protein and a membrane complex consisting of C, D, and Eb (encoded by the spoVA transposon) (Errington 1993; Ramirez-Guadiana et al. 2017; Gao et al. 2022). Thus, the substrate supply, associated with the enzyme activity, and DPA input were potentially influenced by Tb(III) in the culture media. Indeed, the supply of substrate was not the major factor in view of the similar DPA content of spores at 130 and 800 µM Tb(III). Consequently, it is likely that the activity of related enzymes such as DPA synthase was affected by Tb(III) based on the results of Tb(III) effects on DPA content in spores. Moreover, the blockage of DPA input channels might also be caused by high concentrations of Tb(III) for its high concentration and similar ionic radius to Ca2+.
In this study, some results were difficult to interpret. The germination of B. subtilis spores was inhibited using L-valine as their germinant and being eluted by DPA, particularly that of the coat-deficient spores. The concentration of DPA used was 10 mM, somewhat higher than the concentration of DPA within the osmotic-sensitive coat-deficient spores (estimated to be about 4.58 mM), resulting in the infiltration of exogenous DPA. On the one hand, the DPA release channel of spore germination might have been blocked by DPA during the elution, which was exacerbated in the presence of Tb(III), since the DPA channel might also be blocked by Tb(III) as analyzed by Yi et al. (Yi et al. 2011). On the other hand, germinate-related CLEs and germination receptors (e.g. Ger A) might be impaired by excess exogenous DPA during elution.
Conclusion
In conclusion, the role of Tb(III), a typical rare earth element, in the culture media on the biological properties of B. subtilis, such as growth, sporulation, and spore germination, has been investigated. Further research into the distribution and attachment of Tb(III), and the germination of eluted spores has enhanced our comprehension of how Tb(III) affects B. subtilis cells and spores during cultivation. Finally, we analyzed the distinctions between the effects of Tb(III) on PS832 and PS4150, which can help to interpret the mechanism of the Tb(III) effect on B. subtilis and improve the selection criteria for strains used to recover REEs during culture. Such results are fundamental for the practical application of the method to recover REEs via microorganism adsorption during culture.
Data availability
No datasets were generated or analysed during the current study.
References
Akanuma G, Suzuki S, Yano K et al (2013) Single mutations introduced in the essential ribosomal proteins L3 and S10 cause a sporulation defect in Bacillus subtilis. J Gen Appl Microbiol 59:105–117. https://doi.org/10.2323/jgam.59.105
Bergsten-Torralba LR, Nascimento CRS, Buss DF, Giese EC (2021) Kinetics and equilibrium study for the biosorption of lanthanum by Penicillium simplicissimum INCQS 40,211. 3 Biotech 11:460. https://doi.org/10.1007/s13205-021-03004-2
Cang L, Qian Z, Wang J et al (2022) Applications and functions of rare-earth ions in perovskite solar cells. Chin Phys B 31:038402. https://doi.org/10.1088/1674-1056/ac373a
Cheng Y, Zhang T, Zhang L et al (2022) Resource recovery: Adsorption and biomineralization of cerium by Bacillus licheniformis. J Hazard Mater 426:127844. https://doi.org/10.1016/j.jhazmat.2021.127844
Dawes IW, Mandelstam J (1970) Sporulation of Bacillus subtilis in continuous culture. J Bacteriol 103:529–535. https://doi.org/10.1128/jb.103.3.529-535.1970
Dong W, Green J, Korza G, Setlow P (2019a) Killing of spores of Bacillus species by cetyltrimethylammonium bromide. J Appl Microbiol 126:1391–1401. https://doi.org/10.1111/jam.14242
Dong W, Li S, Camilleri E et al (2019b) Accumulation and Release of Rare Earth ions by Spores of Bacillus Species and the location of these ions in spores. Appl Environ Microbiol 85:e00956–e00919. https://doi.org/10.1128/AEM.00956-19
Dong W, Wang H, Ning Z et al (2022) Bioadsorption of Terbium(III) by spores of Bacillus subtilis. Minerals 12:866. https://doi.org/10.3390/min12070866
Driks A (2002) Overview: development in bacteria: spore formation in Bacillus subtilis. Cell Mol Life Sci 59:389–391. https://doi.org/10.1007/s00018-002-8430-x
Errington J (1993) Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev 57:33
Furst A (1987) Hormetic effects in Pharmacology: pharmacological inversions as prototypes for Hormesis. Health Phys 52:527
Gao Y, Barajas-Ornelas RDC, Amon JD et al (2022) The SpoVA membrane complex is required for dipicolinic acid import during sporulation and export during germination. Genes Dev 36:634–646. https://doi.org/10.1101/gad.349488.122
Ghosh S, Setlow B, Wahome PG et al (2008) Characterization of spores of Bacillus subtilis that lack most coat layers. J Bacteriol 190:6741–6748. https://doi.org/10.1128/JB.00896-08
Heilmann M, Breiter R, Becker AM (2021) Towards rare earth element recovery from wastewaters: biosorption using phototrophic organisms. Appl Microbiol Biotechnol 105:5229–5239. https://doi.org/10.1007/s00253-021-11386-9
Inaoka T, Ochi K (2011) Scandium stimulates the production of Amylase and Bacilysin in Bacillus subtilis. Appl Environ Microbiol 77:8181–8183. https://doi.org/10.1128/AEM.06205-11
Jamroskovic J, Chromikova Z, List C et al (2016) Variability in DPA and Calcium Content in the spores of Clostridium species. Front Microbiol 7:1791. https://doi.org/10.3389/fmicb.2016.01791
Liao Y, Wang X, Yin L et al (2020) Screening, identification and characteristics of Yttrium -resistant Bacteria in the Rare Earth Mine tailings of Southern Jiangxi, China. Fresenius Environ Bull 29:6906–6913
Lima AT, Ottosen L (2021) Recovering rare earth elements from contaminated soils: critical overview of current remediation technologies. Chemosphere 265:129163. https://doi.org/10.1016/j.chemosphere.2020.129163
Liu Y, Gui Z, Liu J (2022) Research Progress of Light Wavelength Conversion materials and their applications in Functional Agricultural films. Polymers 14:851. https://doi.org/10.3390/polym14050851
Magge A, Granger AC, Wahome PG et al (2008) Role of Dipicolinic Acid in the Germination, Stability, and viability of spores of Bacillus subtilis. J Bacteriol 190:4798–4807. https://doi.org/10.1128/JB.00477-08
Marquis RE, Shin SY (1994) Mineralization and responses of bacterial spores to heat and oxidative agents. FEMS Microbiol Rev 14:375–379. https://doi.org/10.1111/j.1574-6976.1994.tb00111.x
Pagano G, Thomas PJ, Di Nunzio A, Trifuoggi M (2019) Human exposures to rare earth elements: present knowledge and research prospects. Environ Res 171:493–500. https://doi.org/10.1016/j.envres.2019.02.004
Paidhungat M, Setlow P (2014) Spore germination and outgrowth. In: Sonenshein AL, Hoch JA, Losick R (eds) Bacillus subtilis and its Closest relatives. ASM Press, Washington, DC, USA, pp 537–548
Ramirez-Guadiana FH, Meeske AJ, Rodrigues CDA et al (2017) A two-step transport pathway allows the mother cell to nurture the developing spore in Bacillus subtilis. Plos Genet 13:e1007015. https://doi.org/10.1371/journal.pgen.1007015
Rose R, Setlow B, Monroe A et al (2007) Comparison of the properties of Bacillus subtilis spores made in liquid or on agar plates. J Appl Microbiol 103:691–699. https://doi.org/10.1111/j.1365-2672.2007.03297.x
Rosen DL, Sharpless C, McGown LB (1997) Bacterial spore detection and determination by Use of Terbium Dipicolinate Photoluminescence. Anal Chem 69:1082–1085. https://doi.org/10.1021/ac960939w
Ruming Z, Yi L, Wenhua L et al (2003) Effect of Sm3+ ion on growth of Bacillus thuringiensis by Microcalorimetry. BTER 95:269–278. https://doi.org/10.1385/BTER:95:3:269
Sadovsky D, Brenner A, Astrachan B et al (2016) Biosorption potential of cerium ions using Spirulina biomass. J Rare Earths 34:644–652. https://doi.org/10.1016/S1002-0721(16)60074-1
Setlow P (1994) Mechanisms which contribute to the long-term survival of spores of Bacillus species. J Appl Bacteriol 76. https://doi.org/10.1111/j.1365-2672.1994.tb04357.x. :49S-60S
Setlow P (2006) Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525. https://doi.org/10.1111/j.1365-2672.2005.02736.x
Setlow B, Atluri S, Kitchel R et al (2006) Role of Dipicolinic Acid in Resistance and Stability of spores of Bacillus subtilis with or without DNA-Protective α/β-Type small acid-soluble proteins. J Bacteriol 188:3740–3747. https://doi.org/10.1128/JB.00212-06
Shen J, Liang C, Zhong J et al (2021) Adsorption behavior and mechanism of Serratia marcescens for Eu(III) in rare earth wastewater. Environ Sci Pollut Res 28:56915–56926. https://doi.org/10.1007/s11356-021-14668-x
Tan IS, Ramamurthi KS (2014) Spore formation in Bacillus subtilis: Bacillus subtilis sporulation. Environ Microbiol Rep 6:212–225. https://doi.org/10.1111/1758-2229.12130
Wang W, Xu Y, Yan R, Zhang Z (2021) New insights into Ion Adsorption Type Rare-Earths Mining-Bacterial Adsorption of Yttrium Integrated with Ammonia Nitrogen removal by a Fungus. Sustainability 13:9460. https://doi.org/10.3390/su13169460
Wei Z, Liu Y, Li B et al (2022) Rare-earth based materials: an effective toolbox for brain imaging, therapy, monitoring and neuromodulation. Light-Sci Appl 11:175. https://doi.org/10.1038/s41377-022-00864-y
Yi X, Bond C, Sarker MR, Setlow P (2011) Efficient inhibition of germination of Coat-deficient bacterial spores by Multivalent Metal Cations, including Terbium (Tb3+). Appl Environ Microbiol 77:5536–5539. https://doi.org/10.1128/AEM.00577-11
Acknowledgements
The authors gratefully acknowledge Peter Setlow for his suggestions on this work. This work was supported by the Jiangxi Provincial Natural Science Foundation (No. 20212ACB213004), the Youth Jinggang Scholars Program in Jiangxi Province (No. QNJG2020050), the Science and Technology Program of Ganzhou (No. 202101095076), and the Science and Technology Program of Yichun (No. 2023ZDJCYJ04).
Author information
Authors and Affiliations
Contributions
Z.N.: experiment, data analysis, manuscript drafting, and editing; W.D.: methodology, manuscript drafting and editing, and review; Z.B.: experiment and manuscript editing; H.H.: methodology and experiment; K.H.: methodology and experiment.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ning, Z., Dong, W., Bian, Z. et al. Insight into effects of terbium on cell growth, sporulation and spore properties of Bacillus subtilis. World J Microbiol Biotechnol 40, 79 (2024). https://doi.org/10.1007/s11274-024-03904-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-024-03904-4