Abstract
Vitamin K2 (menaquinone, VK2, MK) is an essential lipid-soluble vitamin that plays critical roles in inhibiting cell ferroptosis, improving blood clotting, and preventing osteoporosis. The increased global demand for VK2 has inspired interest in novel production strategies. In this review, various novel metabolic regulation strategies, including static and dynamic metabolic regulation, are summarized and discussed. Furthermore, the advantages and disadvantages of both strategies are analyzed in-depth to highlight the bottlenecks facing microbial VK2 production on an industrial scale. Finally, advanced metabolic engineering biotechnology for future microbial VK2 production will also be discussed. In summary, this review provides in-depth information and offers an outlook on metabolic engineering strategies for VK2 production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
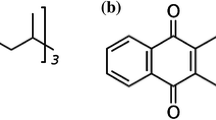
Vitamin K (VK) is an essential lipid-soluble vitamin discovered by Henrik Dam and Edward Doisy. They shared the Nobel Prize in 1943 for their work on VK (Dam 1967, 2010). VK is characterized by the presence of a 2-methyl-1,4-naphthoquinone ring. VK1 (phylloquinone, PK) and VK2 (menaquinone, MK, menadione) are the two main naturally occurring types of vitamin K (Fig. 1) and are synthesized by microorganisms. VK3, VK4, and VK5 are artificially synthesized. VK1 and VK3 can function only when converted into VK2 in the liver, after which it is absorbed with the VK2 naturally synthesized by the gastrointestinal bacteria. The chemical formula of VK2 is 2-methyl-3-alkenyl-1,4-naphthoquinone, and its molecular formula is C16H16O2·(C5H8)n. VK2 can be divided into 11 types according to the length of the isoprene side chain on C-3. These 11 types are usually expressed as MK-n, where n (4–14) refers to the number of isoprene units on C-3 (Binkley et al. 1939).
With the progress in medical research and the newly discovered functions of VK2 in inhibiting ferroptosis and reducing the risk of Parkinson’s and Alzheimer’s (Bhalerao et al. 2012; Liu et al. 2021; Mishima et al. 2022; Vos et al. 2012), many companies have realized the importance of industrializing VK2 production.
Among the VK2 homologs, MK-4 is the most common form in animals and has the widest range of physiological activities (Halder et al. 2019). The long-chain MK isoforms, such as MK-7, are found in fermented foods or produced by Bacillus subtilis. MK-4 and MK-7 are allowed in the United States as nutritional supplements for bone health (Mahdinia et al. 2017). However, the administration of MK-4 is not reflected in an increased serum concentration (Halder et al. 2019). In contrast, MK-7 is absorbed efficiently, reflecting increased serum MK-7 levels up to several days, thereby contributing to the vitamin K status (Halder et al. 2019; Lal and Berenjian 2020). US Pharmacopeia monographs have been developed to establish quality standards for MK-7 as a dietary ingredient at typically recommended levels (Marles et al. 2017). Although there are cis, trans, and cis/trans isomers of MK-7, only the all-trans form is produced naturally through fermentation and is biologically active (Lal and Berenjian 2020). Thus, enhancing the production of VK2 by environmentally friendly fermentation has been extensively studied.
Several wild-type microorganisms, such as Flavobacterium meningosepticum, Bacillus subtilis, and Lactococcus lactis, have been isolated from various environments and used for the industrial production of VK2 (Aguiar et al. 2015; Morishita et al. 1999; Tani et al. 1986; Wang et al. 2021). However, Flavobacterium meningosepticum is a conditioned pathogen, and the productivity of wild B. subtilis and Lactococcus lactis is low, below 226 mg/L (Berenjian et al. 2014). Mutation breeding has been adopted as the conventional method for enhancing the yield of VK2 during microbial fermentation. This technique includes both physical and chemical methods to induce genetic mutations and selectively modify the microorganisms (Che et al. 2018). Several factors, such as mutagen type, dosage, and time, have been optimized to induce genetic mutations in the microorganisms (Liu et al. 2015; Song et al. 2014). However, this technique’s main drawback is the difficulty in screening the positive mutants to obtain effective results. For these reasons, molecular and other efficient approaches have focused on metabolic pathways and are gradually replacing mutation breeding. Various molecular methods for effectively modifying strains, especially metabolic engineering strategies, have been applied to microbial cell factories, such as Bacillus and Escherichia coli, to produce VK2 (Gao et al. 2020; Yang et al. 2019). However, there is little literature summarizing the novel metabolic engineering strategies. This review points to the biosynthesis pathway for the production of VK2 and discusses the current limitations and future directions for microbial production of VK2. This review provides in-depth information on metabolic engineering strategies for VK2 production and also offers a perspective on metabolic engineering methods for other products that share the same intermediate metabolites or pathways.
Conventional mutation breeding strategies for MK-producing strains
To increase VK2 production, various vitamin K-producing strains have been constructed by conventional mutation breeding strategies, such as random mutagenesis (Table 1). Random mutagenesis using a chemical agent or physical treatment can be used first to construct mutant strains exhibiting desirable phenotypes (Yu et al. 2020). The well-known chemical mutagen N-methyl-N’-nitro-N-nitrosoguanidine (NTG), which causes alkylation of guanine or thymine, has been used for the construction of Bacillus and Flavobacterium mutants to overproduce VK2 (Sato et al. 2001; Song et al. 2014). Along with the chemical mutagens, various analogs of VK2 precursors, such as 1-hydroxy-2-naphthoic acid (HNA), have been used for generating mutants with increased metabolic flux in VK2 biosynthesis (Song et al. 2014; Tsukamoto et al. 2001). Diphenylamine (DPA), which inhibits the biosynthesis of the naphthoquinone ring, can also generate mutant strains overproducing VK2. Sato et al. (2001) used NTG and DPA to construct a mutant strain of B. subtilis. The resulting D200-41 strain produced 19.6 mg/L of VK2 in 500 mL in flask fermentation and 62.1 mg/L of VK2 in 5 days of static fermentation after optimizing the carbon and nitrogen sources in the growth medium. Tani et al. (1986) used NTG and HNA to construct F. meningosepticum mutants overproducing VK2. Using glycerol as a substrate, the resulting mutant strain F. meningosepticum HNA 350 − 22 produced 23 mg/L of VK2, while the wild-type F. meningosepticum IFO 12,535 strain produced 14.1 mg/L of VK2.
Physical methods for random mutagenesis, such as UV and N+ ion-beam, have also been used for generating Bacillus mutants with increased VK2 production (Song et al. 2014; Tsukamoto et al. 2001). Using UV treatment and analogs of VK2 precursors, Tsukamoto et al. (2001) attempted to generate several mutant strains based on the wild-type B. subtilis O-2 strain isolated from natto. The resulting mutant B. subtilis OUV23481 was used for making natto containing up to 1.719 g VK2 per 100 g of natto, which was 1.7 times higher than that made by the parent strain. Similarly, Song et al. (2014) used NTG and HNA with N+ ion-beam treatment to construct a mutant strain based on the wild-type B. subtilis BN2-6 strain isolated from natto. The resulting strain BN-P15-11-1 produced 2.5 mg/L of VK2, which was 166% higher than the parent strain. Further optimization of the fermentation medium increased VK2 production to 3.593 mg/L by the BN-P15-11-1 strain. Puri et al. (2015) constructed a mutant strain based on B. subtilis using 1-naphthol. In 100 mL flask fermentation for 24 h, the 1-naphthol mutant strain produced 12.5 g/mL of MK-7. In the presence of Tween-80, MK-7 production increased to 14.4 g/mL.
Other conventional mutation breeding strategies, such as high-temperature induction and analog resistance, have also been used for menaquinone production. Goodman et al. (1976) constructed the VK2-deficient mutant strain based on wild-type Bacillus licheniformis using kanamycin and shikimate. The resulting strain produced 0.3 nmol/mg of MK-7, which was lower than the titer of MK-7 produced by the wild-type strain (0.38 nmol/mg). Recently, Liu et al. (2021) used the H.β.D.R-5 mutant to create another B. amyloliquefaciens mutant having a high α-amylase activity. This was achieved through adaptive evolution with temperature-induced mutagenesis at a high growth temperature. The resulting heat-resistant mutant MK50-36 produced 57 mg/L of MK-7 in a corn starch medium over 6 days of fed-batch fermentation. Similarly, the Bacillus mutant released from feedback inhibition by aromatic amino acids effectively enhanced VK2 biosynthesis since the aromatic amino acids share their biosynthetic pathway with VK2 (Tsukamoto et al. 2001). Xu and Zhang (2017) constructed B. amyloliquefaciens H.β.D.R-5 mutant strain based on the wild-type Y-2 strain. The multi-round random mutagenesis using HNA, DPA, and β-thienylalanine (β-TA) generated analog resistance. The H.β.D.R-5 mutant produced 61.3 mg/L of MK-7 in a maize meal hydrolysate medium using a 7 L fermenter.
These results suggested that although conventional mutation breeding strategies could improve VK2 production in strains such as Bacillus and Flavobacterium mutants, the overall production was still below 95 mg/L. In addition, conventional mutation breeding strategies have low screening efficiencies from large mutant libraries. Random mutagenesis has been widely used for the reconstruction of microbial strains. However, the millions of isolates created after mutagenic treatment should be measured.
Biochemistry of VK2 biosynthesis
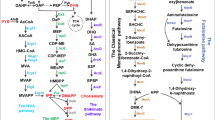
The biosynthesis pathway will be discussed first in order to better understand the metabolic engineering strategies of VK2. VK2 is composed of a main chain (2-methyl-3-alkenyl-1,4-naphthoquinone) and a side chain (polyisoprene). Therefore, polyisoprene and 1,4-dihydroxy-2-naphthoate (DHNA) are the most important intermediates in VK2 biosynthesis. The polyisoprene side chain is produced from two five-carbon (C5) universal precursors, DMAPP and isopentenyl diphosphate (IPP), through the mevalonate (MVA) or methylerythritol 4-phosphate (MEP) pathway (Kawamukai 2018) (Fig. 2). DHNA is derived from chorismate (CHA), and enters the futalosine (FL) or classical MK pathway to form the naphthoquinone headgroup (Dairi 2012; Arakawa et al. 2011). Then, the naphthoquinone is ligated with polyisoprene by MenA or MqnP to form demethylmenaquinone (DMK) (Fig. 2). MK-7 is synthesized via the methylation of DMK (Meganathan and Kwon 2011). For VK2, the polyisoprene side chain anchors naphthoquinone in the lipid membrane, while the naphthoquinone main chain is responsible for the electron transfer (Kawamukai 2018).
Metabolic pathway of VK2 Menaquinone biosynthesis is a complex process involving multiple metabolic pathways, such as glycolysis, the pentose phosphate pathway, the shikimate pathway, the MEP or MVA pathway, as well as the classical MK pathway or futalosine pathway. Enzymes are displayed in different colors in different pathways. Red, green, rose, blue and yellow indicated the enzymes involved in MEP, MVA, MK, shikimate and futalosine pathway, respectively
Intermediate metabolites:
Gly, glycerol; DHAP, dihydroxyacetone phosphate; G3P, glyceraldehyde-3-phosphate; PYR, pyruvate; DXP, 1-deoxy-D-xylose-5-phosphate; MEP, 2-C-Methyl-D-Erythritol-4-Phosphate; CDP-ME, 4-(cytidine 5′-diphospho)-2-C-methylerythritol; CDP-MEP, 2-phospho-4-(cytidine 5′-diphospho)-2-C-methylerythritol; MEcPP, 2-C-methyl-D-erythritol-2,4-cyclodiphosphate; HMBPP, 1-hydroxy-2-methyl-2-butenyl 4-diphosphate; DMAPP, dimethylallyl pyrophosphate; IPP, isopentenyl diphosphate; GPP, geranyl diphosphate; FPP, farnesyl diphosphate; HPP, heptaprenyl diphosphate; PEP, phosphoenolpyruvate; E4P, erythrose 4-phosphate; SA, shikimate acid; CHA, chorismate; SHCHC, 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate; DHNA, 1,4-dihydroxy-2-naphthoate; DMK, 2-demethylmenaquinone; FL, futalosine; DHFL, dehypoxanthinyl futalosine; MK, menaquinone; PPA, prephenate; PABA, para-aminobenzoic acid; ADC, 4-amino-4-deoxychorismate; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; MVA, mevalonate; M5P, mevalonate-5-phosphate; M5PP, mevalonate-diphosphate; HB, 4-Hydroxybutyric acid; UQ, ubiquinone
Enzymes:
GlpK, glycerol kinase; GlpD, glycerol-3-phosphate dehydrogenase; Tpi, triosephosphate isomerase; Dxs, 1-deoxyxylulose-5-phosphate synthase; Dxr, 1-deoxyxylulose-5-phosphate reductoisomerase; IspD/YacM, 2-C-methylerythritol 4-phosphate cytidylyltransferase; IspE, 4-diphosphocytidyl-2-C-methylerythritol kinase; IspF/YacN, 2-C-methylerythritol 2,4-cyclodiphosphate synthase; IspG/YgfY, 4-hydroxy-3-methylbut-2-enyl diphosphatesynthase; IspH, 4-hydroxy-3-methylbut-2-enyldiphosphate reductase; Idi, type 2 isopentenyl-diphosphate Delta-isomerase; IspA/YqiD, farnesyl diphosphate synthase; HepS/T, heptaprenyl diphosphate synthase component I/II; MenA, 1,4-dihydroxy-2-naphthoate heptaprenyltransferase; MqnA, chorismate dehydratase; MqnB, futalosine hydrolase; MqnC, dehypoxanthine futalosine cyclase; MqnD, 5,8-Dihydroxy-2-naphthoate synthase; AroG, bifunctional 3-deoxy-7-phosphoheptulonate synthase/chorismate mutase; AroB, 3-dehydroquinate synthase; AroC, 3-dehydroquinate dehydratase; AroD, shikimate dehydrog enase; AroH, chorismate mutase; AroK, shikimate kinase; AroF, chorismate synthase; MenF, isochorismate synthase; MenD, 2-succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexene-1-carboxylate synthase; MenH, demethylmenaquinone methyltransferase; MenC, o-succinylbenzoate synthase; MenE, o-succinylbenzoate-CoA ligase; MenB, 1,4-dihydroxy-2-naphthoyl-CoA synthase; MenI, 1,4-dihydroxy-2-naphthoyl-CoA hydrolase; MenG/UbiG, demethylmenaquinone methyltransferase; ThiL, thiamine monophosphate kinase; HmcM: 3-hydroxy-3-methylglutaryl-CoA synthase; MvaA, 3-hydroxy-3-methylglutaryl-CoA reductase; MvaK1, mevalonate kinase; MvaK2, phosphomevalonate kinase; MvaD, diphosphomevalonate decarboxylase; Fni, isopentenyl-diphosphate delta-isomerase; PreA, dihydropyrimidine dehydrogenase; UbiA, 4-hydroxybenzoate octaprenyltransferase
The isoprene biosynthesis pathway
The universal precursors of all isoprenoids, IPP and DMAPP, can be synthesized by two major unrelated pathways: the MVA and MEP pathways. Although most bacteria use only the MEP pathway to produce their essential isoprenoid precursors, some exceptions exist (Boucher and Doolittle 2000; Lange et al. 2000; Laupitz et al. 2004). Some bacteria, including the spirochaete Borrelia burgdorferi and the Gram-positive cocci Staphylococcus aureus and Streptococcus pneumoniae, have been confirmed to use the MVA pathway instead of the MEP pathway for IPP and DMAPP synthesis. Others, including Listeria monocytogenes and some Streptomyces strains, possess the two complete pathways (Begley et al. 2004; Boucher et al. 2001; Kuzuyama and Seto 2003; Laupitz et al. 2004).
In the first steps of the MVA pathway, 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) is produced from the sequential condensation of three molecules of acetyl-CoA catalyzed by the enzymes acetoacetyl-CoA thiolase (AACT) and HMG-CoA synthase (HMGS). HMG-CoA reductase (HMGR) catalyzes the irreversible conversion of HMG-CoA into MVA in the first committed step of the pathway. Then, MVA is sequentially phosphorylated and decarboxylated to generate IPP by the enzymes mevalonate kinase (MVK), 5-phosphomevalonate kinase (PMVK), and 5-diphosphomevalonate decarboxylase (DPMD). The activity of an IPP/DMAPP isomerase (IDI) enzyme is required to form DMAPP from IPP.
The MEP pathway has been best characterized in E. coli, a model bacterium that lacks the MVA pathway (Rohmer 2008; Eisenreich et al. 2001). It starts with the condensation of (hydroxyethyl) thiamin derived from pyruvate with the C-1 aldehyde group of D-glyceraldehyde 3-phosphate. The resulting 1-deoxy-D-xylulose 5-phosphate (DXP) is produced in a reaction catalyzed by the enzyme DXP synthase (DXS). In the second step, DXP reductoisomerase (DXR)/IspC catalyzes the intramolecular rearrangement and reduction of DXP to produce MEP. The sequential action of the enzymes MEP cytidylyltransferase (MCT)/IspD, [4-(cytidine 5-diphospho)-2-C-methyl-D-erythritol kinase (CMK)/IspE, 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase (MDS)/IspF, and 4-hydroxy-3-methylbut-2-enyl diphosphate (HMBPP) synthase/IspG transforms MEP into HMBPP. Finally, the enzyme HDR (HMBPP reductase)/IspH catalyzes the simultaneous formation of IPP and DMAPP in an approximate 5:1 proportion. For the biosynthesis of MK-7, heptaprenyl diphosphate (HPP) with seven isoprene units is needed.
The DHNA biosynthesis pathway
Glyceraldehyde-3-phosphate enters the pentose phosphate (HMP) pathway to yield the important intermediate of 4-phosphate-erythritol, which can be used to synthesize shikimic acid (SA) by a series of ligation, dehydration, and dehydrogenation reactions. SA is the starting point of VK2 biosynthesis and is used to form the quinone skeleton of DHNA through six enzymes encoded by menFDHCEB genes (Dairi 2012; Meganathan 2001). The HPP unit is transferred to the carboxyl group of DHNA by 1,4-dihydroxy-2-naphthoate heptaprenyl transferase encoded by menA. VK2 is then finally formed through methylation by UbiE/MenG (Dairi 2012; Meganathan and Kwon 2011).
In addition to the classical MK pathway from SA, the futalosine pathway is an alternative pathway for VK2 biosynthesis. The futalosine pathway encompasses seven enzymes encoded by the men gene cluster in Bacillus spp. and E. coli (Hiratsuka et al. 2008). The discovery of the futalosine pathway was due to the men gene cluster in the genome of Streptomyces. However, it has not been found in some pathogenic species, such as Helicobacter pylori and Campylobacter jejuni. DHNA and additional genes and enzymes for VK2 biosynthesis exist in S. coelicolor A3 (Joshi et al. 2018; Hiratsuka et al. 2009). In the futalosine pathway, CHA is converted to futalosine (Arakawa et al. 2011) and finally forms DHNA by four enzymes encoded by the mqnABCD gene cluster (Kim et al. 2014). Polyprenyl diphosphate is then attached by MqnP to form VK2 (Cotrim et al. 2017). In addition, a modified futalosine pathway, which starts from 6-amino-6-deoxyfutalosine instead of futalosine as the first step, has been found in Campylobacter jejuni (Xu et al. 2011). In most microorganisms, the mqn genes encoding the futalosine pathway are scattered throughout the genome (Arakawa et al. 2011; Dairi 2012; Joshi et al. 2018). The reason why some microorganisms use the new pathway to synthesize VK2 is still unclear, but new ideas for drug development have been provided based on this pathway (Choi et al. 2016; Paudel et al. 2016). In particular, studying inhibitors targeting this pathway is a hot topic for the development of new antibiotics because the futalosine-dependent VK2 biosynthesis pathway is absent in humans (Shimizu et al. 2018; Tanaka et al. 2011).
Static metabolic engineering of the SA pathway
Static regulation of target metabolic pathways is a traditional regulatory strategy to improve the synthesis efficiency of target compounds in microbial cell factories. Static regulation refers to the direct upregulation, downregulation, or knockout of genes in a metabolic pathway in order to maximize the metabolic flow towards the product (Fig. 3A). Low expression levels of key enzymes involved in VK2 biosynthesis may be the rate-limiting steps for VK2 production in different strains. Therefore, the challenge includes determining which enzymes are critical and how to regulate the expression of these enzymes.
Overview of static (A) and dynamic (B) metabolic regulation. B: ① is input signal, which is Including chemical molecules, light control, temperature control, metabolite, etc. ② is signal responsive protein. ③ can be a directly regulated gene pathway, or a cascade control of repressor protein expression pathway to achieve bidirectional regulation or more complex regulatory networks
In the pathways described above, the most important intermediates in VK2 biosynthesis are chorismate and isoprene. Chorismate enters the classical MK pathway or futalosine pathway to form the naphthoquinone headgroup (Fig. 2) (Johnston and Bulloch 2020). The SA pathway connects the central carbon metabolism with the biosynthesis of chorismate, which is a key precursor for the production of aromatic amino acids and a large number of other aromatic compounds in microorganisms, including VK2 (Lee and Wendisch 2017; Jiang and Zhang 2016). In E. coli, three different 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase (DAHPS) isoenzymes encoded by the aroGFH genes contribute to the total DAHPS activity and are subject to allosteric control by l-phenylalanine, l-tyrosine, and l-tryptophan, respectively (Ikeda 2006; Kim et al. 2018; Liu et al. 2019b). Through structural analysis of mutant enzymes that are not sensitive to feedback, certain specific amino acid residues involved in the allosteric site have been identified, and feedback-resistant (fbr) variants of aroG and aroF have been developed (Ikeda 2006; Chen and Zeng 2017; Liu et al. 2019a). Hence, eliminating feedback inhibition of the key enzymes obtained by introducing site-directed mutations is usually the first and most important step for constructing a high-producing strain.
In a study of modular pathway engineering to promote MK-7 production, Yang et al. (2019) found that the overexpression of aroADE in B. subtilis inhibited the biosynthesis of MK-7 despite the transcriptional levels of these genes being significantly increased (> 300-fold). It was suggested that increased production of aromatic amino acids resulted in feedback inhibition of the shikimate pathway, ultimately inhibiting the production of MK-7. Cui et al. (2019) overexpressed aroAK with the feedback inhibition-resistant aroGfbr from E. coli, resulting in a two-fold increase in MK-7 production compared to the wild type (Table 2).
We can also learn from studies on the production of aromatic chemicals and derivatives such as p-aminobenzoate, salicylate, cis, cis-muconic acid (MA), 4-hydroxycoumarin (4-HC), and 4-hydroxybenzoic acid (Kim et al. 2020; Choi et al. 2020; Rekhter et al. 2019; Noda and Kondo 2017). To improve microbial biosynthesis of 4-hydroxycoumarin, Lin et al. (2013) constructed a chorismate-boosting plasmid pCS-APTA (overexpressing aroL, ppsA, tktA, and aroGfbr), which led to the production of 283.9 mg/L 4HC, a 37% increase compared with its original strain. Additionally, strategies for synthesizing chorismate derivatives are often based on the release of pyruvate competition. Therefore, using metabolic engineering strategies involving the pyruvate recycling system combined with improved chorismate supply can promote cell growth, leading to high production and yield of chorismate derivatives.
Static metabolic engineering of polyisoprene biosynthesis
Polyisoprene biosynthesis plays an important role in the production of isoprenoid compounds, of which there are more than 50,000 in nature (Wang et al. 2017; Frank and Groll 2017). Isoprenoids perform a wide variety of important biological functions, including electron transport, growth regulation, antioxidation, and hormonal signaling (Tetali 2019; Zada et al. 2018; Wang et al. 2017; Kuzuyama 2017). Pathway engineering of polyisoprene biosynthesis is also commonly used to improve VK2 production. The polyisoprene tail, which forms the side chain of VK2, is produced from two five-carbon (C5) universal precursors, IPP and DMAPP, through the MVA or MEP pathway (Kawamukai 2018). The five-carbon monomer IPP and its isomer DMAPP then form long isoprene side chains, such as geranyl pyrophosphate (GPP), farnesyl pyrophosphate (FPP), geranylgeranyl pyrophosphate (GGPP), and heptaprenyl diphosphate (HPP), by consecutive condensate (Fig. 2) (Joshi et al. 2018). The overexpression of ispD, ispF, ispH, and ispG increased the production of MK-7 from B. subtilis BS20MEP (Chen et al. 2020), and an 11-fold increase in MK-7 production was realized by expressing the Pspac-MenA-DxS-Dxr-Idi cassette (Ma et al. 2019b). Moreover, overexpressing hepS encoding heptaprenyl pyrophosphate synthase led to a greater increase than by other enzymes in Bacillus amyloliquefaciens Y-2. Sequentially overexpressing ispDFHG, dxs, and dxr in the BS20 strain increased the MK-7 titer to 415 ± 3.2 mg/L (Chen et al. 2020), providing information on the different rate-limiting steps in different MK-7 producers. The supply of heptaprenyl-PP was improved by engineering the MEP pathway to overexpress dxs, dxr, ispD (yacM), and ispF (yacN). However, overexpression of the other three pathway genes ispE, ispH (yqfP), and ispA (yqiD) resulted in decreased production of MK-7 (Yang et al. 2019). This indicated that ispE overexpression might lead to an imbalance in the MEP pathway.
The MVA pathway does not exist in most prokaryotes, but a heterologous MVA pathway can be constructed in bacterial hosts to provide more IPP and DMAPP (Li and Wang 2016). Overexpression of the heterogeneous MVA module genes (mvaK1, mvaK2, mvaD, mvaS, and mvaA), combined with knocking out hepT and simultaneously overexpressing dxs, dxr, and ispD-ispF in the MEP module, increased the MK-4 yield to 90.1 ± 1.7 mg/L, which was 11.1-fold compared with the parental strain (Table 2) (Yuan et al. 2020). Introducing heptaprenyl pyrophosphate synthetase (HepPPS) from B. subtilis and optimizing enzyme expression in the MVA pathway in E. coli increased the titer of MK-7 to 2.3 µM, which was 22-fold higher than that of the original strain (Gao et al. 2020). Introducing the MVA pathway genes in E. meningoseptica sp. F2 into E. coli by co-culturing E. meningoseptica sp. F2 and E. coli H01 (CO2 system) finally produced 25.51 ± 1.25 mg/L of MK-n (Yang et al. 2022). Because the MEP pathway requires three ATP and three NADPH while the MVA pathway requires three ATP and two NADPH (Partow et al. 2012; Liu et al. (2019a) overexpressed the gapC gene from C. acetobutylicum ATCC 824 to balance the cofactor NADPH and increased isoprene production.
VK2 has various subtypes with different numbers of isoprene units called MK-n (Mahdinia et al. 2017). The number of isoprene units produced differs in different microorganisms (Tables 1 and 2). For example, bacteria such as B. subtilis synthesize MK-7, E. coli synthesize ubiquinone (UQ)-8 and MK-8, while yeasts such as Saccharomyces cerevisiae and Schizosaccharomyces pombe produce UQ-6 and UQ-10, respectively (Kawamukai 2018). Researchers have explored which enzymes and motifs are responsible for the chain length. Two common DDXXD motifs are found in the amino acid sequence of trans-prenyltransferases in different VK2-producing strains. The first motif is responsible for binding with farnesyl diphosphate (FPP), while the second is responsible for binding with IPP (Guo et al. 2004; Koyama et al. 1996; Marrero et al. 1992). Moreover, the amino acid located in the fifth position before the first DDXXD is alanine in H. influenzae OPPS, E. meningoseptica OPPS (EmOPPS), B. subtilis HepPPS, and E. coli OPPS and is important in determining the side chain length (Han et al. 2015). Pentaisoprene and hexameric isoprene diphosphate are the products of EmOPPS with IPP and FPP as substrates, while octaprenyl diphosphate is generated by catalyzing consecutive condensation reactions of FPP with five molecules of IPP (Guo et al. 2004; Tonhosolo et al. 2005). To verify that the trans-prenyltransferases in different microorganisms are critical for deciding the number of isoprene units, heterogeneous heptaprenyl pyrophosphate synthetase (HepPPS) of B. subtilis was transferred into E. coli. Because E. coli contains octaprenyl diphosphate (OctPP) synthase (IspB) and does not contain HepPPS, it synthesizes mainly MK-8 under micro-anaerobic conditions (Kong and Lee 2011). Interestingly, MK-7 production was first achieved in engineered E. coli by the overexpression of B. subtilis-derived HepPPS (BsHepPPS) (Gao et al. 2020).
Static metabolic engineering of MK biosynthesis
MK biosynthesis refers to the biosynthetic process from CHA to VK2. In B. subtilis, MK biosynthesis proceeds via nine enzymatic reactions that are encoded by the menFDHBEC operon, the hepS-menG-hepT operon, menA, menI, and menG/ubiG genes. The above cistrons have been overexpressed in B. subtilis 168 using strong promoters to increase the copy number. Only the step reaction by MenA (the prenylation of DHNA and polyisoprene to DMK) increased the titer of MK-7 by 42 mg/L compared with the original strain (Cui et al. 2021; Yang et al. 2019). A similar increase has also been observed by overexpressing only menA in B. subtilis 168 (Xu et al. 2017). Although the futalosine-dependent pathway has been widely investigated as a target for the development of new herbicides and antibiotics (Zhi et al. 2014), there have been no studies on the metabolic engineering of the FL pathway to increase VK2 production. Therefore, combining these two MK biosynthesis pathways may be a promising strategy for the biosynthesis of VK2.
Combination of static metabolic engineering techniques for the entire metabolic module
Enhancing the precursor supply and eliminating byproduct synthesis pathways are commonly used to improve strain performance. UQ is an important membrane component and shares the same isoprene metabolic pathway with VK2. Inactivating 4-hydroxybenzoate octaprenyl transferase (the prenylation of 4-hydroxybenzoate and polyisoprene to 3-polyprenly-4-hydroxybenzoate) by site-directed mutagenesis increased the VK2 content in E. meningoseptica by 130% (Liu et al. 2017). Furthermore, co-expressing dxr and menA and supplementing the medium with substrate precursors such as sodium pyruvate and SA resulted in an 11-times increase in VK2 content (Liu et al. 2018).
Modular pathway engineering is another effective method for improving the biosynthesis of VK2. Using B. subtilis 168 as the chassis, a 2.1-fold and 82% increase was obtained by overexpressing menA in the MK pathway and overexpressing dxs, dxr, yacM, yavN, and glpD and deleting dhbB in the MEP pathway. However, enhancing the SA pathway affected VK2 biosynthesis negatively because of feedback inhibition by CHA. MK-7 production reached 69.5 mg/L in the final mutant, representing a more than 20-fold increase compared with the starting strain (Yang et al. 2019).
A power imbalance hindered the redox metabolism to facilitate the accumulation of the desired MK-7 production in B. subtilis. After overexpressing the rate-limiting enzymes DXS, Fni, DXR, MenF, AroA, and MenA during MK-7 synthesis, the redox metabolism could be rebalanced by expressing Pos5P (the key enzyme for NADPH regeneration), which aided the conversion of NADH to NADPH (Ding et al. 2022). Recently, comparative transcriptomics revealed that cell membranes and electron transfer are important factors in promoting MK-7 synthesis. Overexpressing the cell membrane proteins tatAD-CD and menaquinol-cytochrome C reductase qcrA-C increased the titer of MK-7 significantly from 200 to 310 mg/L in a 15-L bioreactor (Cui et al. 2020).
The intermediate metabolites in the VK2 biosynthesis pathway also participate in the biosynthesis of various other chemicals, including some well-studied terpenoids and aromatic acids (Ikeda et al. 2006).
Dynamic metabolic engineering regulation
The static metabolic engineering regulation based on gene knockout and overexpression in metabolic pathways has been good for constructing cell factories. However, gene overexpression in metabolic pathways generally leads to the accumulation of toxic intermediate metabolites, while the downregulation and knockout of genes can lead to a lack of important metabolites required for cell growth. Therefore, static metabolic engineering regulation may ultimately overload or destroy the normal metabolic network and cause metabolic imbalances. For VK2 synthesis, the accumulation of the toxic metabolic intermediate 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate (HMBPP) presumably inhibits cell growth (Li et al. 2017). Moreover, the synthesis of VK2 requires more than 30 steps of enzymatic catalysis, making it difficult to increase the total yield by regulating the expression of only a few genes (Fig. 2). Therefore, dynamic regulation was introduced to improve VK2 synthesis.
Dynamic regulation refers to the use of specific biological recognition elements to regulate the expression levels of downstream genes in response to changes in the internal or external environment of cells. The result is that the expression levels of downstream genes change with changes in the environment (Fig. 3B). With the capability of adapting to complicated extracellular or intracellular environments, engineered dynamic regulation systems are valuable for fine-tuning metabolic flux (Anesiadis et al. 2018; Xu 2018). The quorum-sensing (QS) system can regulate gene expression according to changes in cell density (Lv et al. 2019). The QS system is not dependent on inducers, interventions, or metabolic pathways but rewires the control processes to depend on cell density (Lyon et al. 2004). For instance, to avoid the toxicity of heterogeneous pathways on cells, an Esa QS circuit with activation and inhibition functions was put forward to produce metabolites without inducers. With activation of the QS system and dynamic regulation of the biosynthetic pathway by Esa-PesaR, the titer of 4-hydroxyphenylacetic acid increased by 46.4% compared with the static control pathway in E. coli (Shen et al. 2019). Recently, some dynamic pathway regulation strategies have been successfully applied to improve the synthesis of VK2 (Fig. 4). Using site-directed mutagenesis of SinR, a constitutively expressed transcriptional regulator identified as a master regulator of biofilm formation, Wu et al. (2021) maximized the yield of MK-7 to 102.56 ± 2.84 mg/L while achieving a balance between product synthesis and cell growth. Cui et al. (2019) designed a bifunctional and modular Phr60-Rap60-Spo0A QS system. In this system, the transcription factor Spo0A is regulated by the population response signaling molecule Phr and Rap. Rap60 can be inhibited by the signaling molecule Phr60 that responds to cell density. Rap60 not only inhibits the phosphorylation level of Spo0A but also inhibits the activity of histidine kinase KinA. Through the action of KinA-E and two phosphate transfer proteins Spo0F and Spo0B, Spo0A is phosphorylated and then regulates the expression of related target genes. Based on the above principle, researchers constructed a population response regulation system for the dynamic regulation of the MK-7 pathway that led to a 40-fold improvement in MK-7 production from 9 to 360 mg/L. Yuan et al. (2021) developed a modular PhrQ-RapQ-ComA QS system based on the promoter PA11, which is upregulated by phosphorylated ComA (ComA-P). PA11 was employed as a promoter, and with the expression of the three genes ispH, crtE, and menA in the strain, the highest yields for MK-4 were obtained. The dynamic adjustment approach increased the yield of MK-4 in a shake flask from 120.1 ± 0.6 to 178.9 ± 2.8 mg/L and reached 217 ± 4.1 mg/L in a 3-L bioreactor, verifying the effectiveness of the dynamic pathway regulation strategy.
Schematic design for dynamic fine-tuning of VK2 synthesis. (A) PhrQ-RapQ-X system based dynamic regulation of critical genes in the MK synthesis pathway, X represented transcription factor. (B) Analysis of the promoter intensities. (C) Mutation screening of promoters. (D) PhrQ-RapQ effects on X-P regulation system in B. subtilis. (E) PhrQ-RapQ-X system based dynamic regulation of critical genes in the MK synthesis pathway
Future studies
Besides static and dynamic metabolic engineering regulation, more advanced metabolic engineering biotechnologies will be tried to enhance production, accelerate industrialization, and reduce the cost of VK2 production.
First, some advanced metabolic engineering strategies, such as the combination of lipid and systemic metabolic engineering methods, will be tried in the future. Terpenes, a class of hydrophobic substances, often subject significant pressure on cells due to their lipotoxicity when synthesized through cell factories. As special organelles, lipid droplets can store lipophilic substances, which could solve this problem. Hong et al. (2019) first utilized this strategy to efficiently synthesize lycopene. By increasing the number and size of the yeast lipid droplets storing lycopene, the yeast metabolite lycopene accumulated. The high density fermentation yield of lycopene reached 2.37 g/L. In addition, the yield of α-amyrin, a triterpenoid, increased by 11-fold when lipid engineering and metabolic engineering were used with Saccharomyces cerevisiae (Yu et al. 2020). Therefore, as VK2 is a terpene, combining lipid and systemic metabolic engineering could be used in static and dynamic metabolic engineering regulation strategies.
Second, with the development of synthetic biology, genetic modification tools have been developed for non-model microorganisms for reconstructing the biosynthetic pathway. This would also be interesting for VK2 production. Plasmid introduction or homologous recombination using antibiotic-resistance and counter-selectable markers was commonly used to modify the VK2 metabolic pathway (Ma et al. 2019a; Yang et al. 2019). However, there were many problems, including the instability of the plasmid, low transformation efficiency, and metabolic burden on the host cell. Therefore, advanced gene editing should be explored in further studies.
In recent years, CRISPR kits have become suitable for B. subtilis gene editing. For example, after inserting the gRNA cassette into multiple gRNA delivery vectors, linearizing all gRNA delivery vectors, and transforming B. subtilis, the efficiency of single gene mutations was 100%, and the efficiency of double gene mutations was 85% (Westbrook et al. 2016). A CRISPR-Cas9 vector was used to introduce two large deletions in the B. subtilis 168 chromosome. The problems of the counterselection methods were overcome by this single-plasmid system (Altenbuchner et al. 2016). Furthermore, García-Moyano et al. (2020) designed a vector compatible with high-throughput fragment exchange cloning for the heterologous expression in Bacillus and E. coli.
In addition, gene-editing technology has been further developed based on CRISPR-Cas9 technology. A CRISPR-Cpf1-based toolkit employing a type V Cas protein has been designed. Genes and gene clusters, such as sacA, ganA, ligD, ligV, and bac operon, can be precisely deleted with high editing efficiency using this platform (Hao et al. 2020). Based on CRISPR gene editing technology, the production of plipastatins, riboflavin, and amorphadiene has been successfully increased in B. subtilis (Ahmed-Hocine et al. 2020; Zou et al. 2020; Song et al. 2021). Thus, we believe that the CRISPR-Cas9 system will emerge as one of the most efficient gene-editing tools for VK2 biosynthesis and production.
Conclusions
This review aimed to provide an overview of the metabolic engineering strategies for the microbial production of VK2 based on the engineering of static biosynthesis and dynamic regulation. More importantly, we explored some advanced synthesis methods of other terpenes similar to VK2, which gives us more insight into the available strain engineering strategies. Using more biotechnological methods, such as lipid engineering, systemic metabolic engineering, and CRISPR gene editing technology, will increase productivity and reduce the cost of microbial VK2 fermentation to realize its industrial production.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aguiar TQ, Silva R, Domingues L (2015) Ashbya gossypii beyond industrial riboflavin production: a historical perspective and emerging biotechnological applications. Biotechnol Adv 33:1774–1786
Ahmed-Hocine B, Marius B, Georg S, Matthias M (2020) Rational engineering of transcriptional riboswitches leads to enhanced metabolite levels in Bacillus subtilis. Metab Eng 61
Altenbuchner J (2016) Editing of the Bacillus subtilis genome by the Crispr-cas9 system. Appl Environ Microbiol 82(17):5421–5427
Anesiadis N, Cluett WR, Mahadevan R (2018) Dynamic metabolic engineering for increasing bioprocess productivity. Metab Eng 10:255–266
Arakawa C, Kuratsu M, Furihata K, Hiratsuka T, Itoh N, Seto H, Dairi T (2011) Diversity of the early step of the futalosine pathway. Antimicrob Agents Chemother 55(2):913
Begley M, Gahan CG, Kollas AK, Hintz M, Hill C, Jomaa H, Eberl M (2004) The interplay between classical and alternative isoprenoid biosynthesis controls γδ T cell bioactivity of Listeria monocytogenes. FEBS Lett 561:99–104
Berenjian A, Mahanama R, Talbot A, Regtop H, Kavanagh J, Dehghani F (2014) Designing of an intensification process for biosynthesis and recovery of menaquinone-7. Appl Biochem Biotechnol 172:1347–1357
Bhalerao S, Clandinin TR (2012) Vitamin K2 takes charge. Science 336:1241–1242
Binkley SB, Maccorquodale DW, Thayer A, Doisy EA (1939) The isolation of vitamin K1. J Biol Chem 130:219–234
Boucher Y, Doolittle WF (2000) The role of lateral gene transfer in the evolution of isoprenoid biosynthesis pathways. Mol Microbiol 37:703–716
Boucher Y, Huber H, L’Haridon S, Stetter KO, Doolittle WF (2001) Bacterial origin for the isoprenoid biosynthesis enzyme HMG-CoA reductase of the archaeal orders Thermoplasmatales and Archaeoglobales. Mol Biol Evol 18:1378–1388
Che J, Liu B, Liu G, Chen Q, Huang D (2018) Induced mutation breeding of Brevibacillus Brevis FJAT-0809-GLX for improving ethylparaben production and its application in the biocontrol of Lasiodiplodia theobromae. Postharvest Biol Technol 146:60–67
Chen L, Zeng AP (2017) Rational design and metabolic analysis of Escherichia coli for effective production of l-tryptophan at high concentration. Appl Microbiol Biotechnol 101:559–568
Chen T, Xia H, Cui S, Lv X, Li X, Liu Y, Li J, Du G, Liu L (2020) Combinatorial methylerythritol phosphate pathway engineering and process optimization for increased menaquinone-7 synthesis in Bacillus subtilis. J Microbiol Biotechnol 30:762–769
Choi SR, Larson MA, Hinrichs SH, Bartling AM, Frandsen J, Narayanasamy P (2016) Discovery of bicyclic inhibitors against menaquinone biosynthesis. Future Med Chem 8(1):11–16
Choi S, Lee HN, Park E, Lee SJ, Kim ES (2020) Recent advances in microbial production of cis, cis-muconic acid. Biomolecules 10:1238
Cotrim CA, Weidner A, Strehmel N, Bisol TB, Meyer D, Brandt W, Wessjohann PLA, Stubbs PMT (2017) A distinct aromatic prenyltransferase associated with the futalosine pathway. Chemistryselect 2(29):9319–9325
Cui S, Lv X, Wu Y, Li J, Du G, Ledesma-Amaro R, Liu L (2019) Engineering a bifunctional Phr60-Rap60-Spo0A quorum-sensing molecular switch for dynamic fine-tuning of menaquinone-7 synthesis in Bacillus subtilis. ACS Synth Biol 8:1826–1837
Cui S, Xia H, Chen T, Gu Y, Lv X, Liu YF, Li JH, Du GC, Liu L (2020) Cell membrane and electron transfer engineering for improved synthesis of menaquinone-7 in Bacillus subtilis. iScience 23(3):100918
Dairi T (2012) Menaquinone biosyntheses in microorganisms. Meth Enzymol 515:107–122
Dam H (1967) Historical survey and introduction. Vitam Horm 24:295–306
Dam H (2010) The antihaemorrhagic vitamin of the chick. Nutr Rev 31(4):121–121
Ding XM, Zheng ZM, Zhao GH, Wang L, Wang H, Yang Q, Zhang MX, Li LY, Wang P (2022) Bottom–up synthetic biology approach for improving the efficiency of menaquinone–7 synthesis in Bacillus subtilis. Microb Cell Fact 21:101
Eisenreich W, Rohdich F, Bacher A (2001) Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci 6:78–84
Frank A, Groll M (2017) The methylerythritol phosphate pathway to isoprenoids. Chem Rev 117:5675–5703
Gao Q, Chen H, Wang W, Huang J, Tao Y, Lin B (2020) Menaquinone-7 production in engineered Escherichia coli. World J Microbiol Biotechnol 36(9):132
Gao Q, Chen H, Wang G, Yang W, Zhong X, Liu J, Huo X, Liu W, Huang J, Tao Y, Lin B (2021) Highly efficient production of menaquinone-7 from glucose by metabolically engineered Escherichia coli. ACS Synth Biol 10(4):756–765
García-Moyano A, Larsen Ø, Gaykawad S, Christakou E, Boccadoro C, Puntervoll P (2020) Fragment exchange plasmid tools for CRISPR/Cas9-mediated gene integration and protease production in Bacillus subtilis. Appl Environ Microbiol 87(1)
Goodman SR, Marrs BL, Narconis RJ, Olson RE (1976) Isolation and description of a menaquinone mutant from Bacillus licheniformis. J Bacteriol 125:282–289
Guo RT, Kuo CJ, Ko TP, Chou CC, Liang PH, Wang AH (2004) A molecular ruler for chain elongation catalyzed by octaprenyl pyrophosphate synthase and its structure-based engineering to produce unprecedented long chain trans-prenyl products. Biochemistry 43:7678–7686
Halder M, Petsophonsakul P, Akbulut AC, Pavlic A, Bohan F, Anderson E, Maresz K, Kramann R, Schurgers L (2019) Vitamin K: double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and Disease. Int J Mol Sci 20:896
Han X, Chen CC, Kuo CJ, Huang CH, Zheng Y, Ko TP (2015) Crystal structures of ligand-bound octaprenyl pyrophosphate synthase from Escherichia coli reveal the catalytic and chain-length determining mechanisms. Proteins 83:37–45
Hao W, Suo F, Lin Q, Chen Qi, Zhou L, Liu Z (2020) Design and construction of portable CRISPR-cpf1-mediated genome editing in Bacillus subtilis 168 oriented toward multiple utilities. Front Bioeng Biotechnol 8:524676
Hiratsuka T, Furihata K, Ishikawa J, Yamashita H, Itoh N, Seto H, Dairi T (2008) An alternative menaquinone biosynthetic pathway operating in microorganisms. Science 321(5896):1670–1673
Hiratsuka T, Itoh N, Seto H, Dairi T (2009) Enzymatic properties of futalosine hydrolase, an enzyme essential to a newly identified menaquinone biosynthetic pathway. Biosci Biotechnol Biochem 73(5):1137–1141
Hong J, Park SH, Kim S, Kim SW, Hahn JS (2019) Efficient production of lycopene in Saccharomyces cerevisiae by enzyme engineering and increasing membrane flexibility and NADPH production. Appl Microbiol Biotechnol 103:211–223
Ikeda M (2006) Towards bacterial strains overproducing l-tryptophan and other aromatics by metabolic engineering. Appl Microbiol Biot 69:615–626
Jiang M, Zhang H (2016) Engineering the shikimate pathway for biosynthesis of molecules with pharmaceutical activities in E. Coli. Curr Opin Biotechnol 42:1–6
Johnston JM, Bulloch EM (2020) Advances in menaquinone biosynthesis: sublocalisation and allosteric regulation. Curr Opin Struct Biol 65:33–41
Joshi S, Fedoseyenko D, Mahanta N, Manion H, Naseem S, Dairi T, Begley TP (2018) Novel enzymology in futalosine-dependent menaquinone biosynthesis. Curr Opin Chem Biol 47:134–141
Kawamukai M (2018) Biosynthesis and applications of prenylquinones. Biosci Biotechnol Biochem 82:963–977
Kim RQ, Offen WA, Davies GJ, Stubbs KA (2014) Structural enzymology of Helicobacter pylori methylthioadenosine nucleosidase in the futalosine pathway. Acta Crystallogr 70(1):177–185
Kim B, Binkley R, Kim HU, Lee SY (2018) Metabolic engineering of Escherichia coli for the enhanced production of l-tyrosine. Biotechnol Bioeng 115:2554–2564
Kim H, Kim SY, Sim GY, Ahn JH (2020) Synthesis of 4-hydroxybenzoic acid derivatives in Escherichia coli. J Agric Food Chem 68:9743–9749
Kong MK, Lee PC (2011) Metabolic engineering of menaquinone-8 pathway of Escherichia coli as a microbial platform for vitamin K production. Biotechnol Bioeng 108:1997–2002
Koyama T, Tajima M, Sano H, Doi T, Koike-Takeshita A, Obata S (1996) Identification of significant residues in the substrate binding site of Bacillus stearothermophilus farnesyl diphosphate synthase. Biochemistry 35:9533–9538
Kuzuyama T (2017) Biosynthetic studies on terpenoids produced by Streptomyces. J Antibiot (Tokyo) 70:811–818
Kuzuyama T, Seto H (2003) Diversity of the biosynthesis of the isoprene units. Nat Prod Rep 20:171–183
Lal N, Berenjian A (2020) Cis and trans isomers of the vitamin menaquinone-7: which one is biologically significant? Appl Microbiol Biotechnol 104:2765–2776
Lange BM, Rujan T, Martin W, Croteau R (2000) Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc Natl Acad Sci USA 97:13172–13177
Laupitz R, Hecht S, Amslinger S, Zepeck F, Kaiser J, Richter G, Schramek N, Steinbacher S, Huber R, Arigoni D (2004) Biochemical characterization of Bacillus subtilis type II isopentenyl diphosphate isomerase, and phylogenetic distribution of isoprenoid biosynthesis pathways. Eur J Biochem 271:2658–2669
Lee JH, Wendisch VF (2017) Biotechnological production of aromatic compounds of the extended shikimate pathway from renewable biomass. J Biotechnol 257:211–221
Li Y, Wang G (2016) Strategies of isoprenoids production in engineered bacteria. J Appl Microbiol 121:932–940
Li Q, Fan F, Gao X, Yang C, Bi C, Tang J, Liu T, Zhang X (2017) Balanced activation of IspG and IspH to eliminate MEP intermediate accumulation and improve isoprenoids production in Escherichia coli. Metab Eng 44:13–21
Lin Y, Shen X, Yuan Q, Yan Y (2013) Microbial biosynthesis of the anticoagulant precursor 4-hydroxycoumarin. Nat Commun 4:2603
Liu Y, Wang L, Zheng ZM, Qiu HW, Wang P, Zhao GH, Gong GH, Song JY, Dai J (2015) Improvement of vitamin K2 production by Escherichia sp. with nitrogen ion beam implantation induction. Plasma Sci Technol 17(2):159–166
Liu Y, Ding XM, Xue ZL, Hu LX, Cheng Q, Chen MH, Su Y, Zhu B, Xu P (2017) Site-directed mutagenesis of UbiA to promote menaquinone biosynthesis in Elizabethkingia meningoseptica. Process Biochem 58:186–192
Liu Y, Yang ZM, Xue ZL, Qian SH, Wang Z, Hu LX, Wang J, Zhu H, Ding XM, Yu F (2018) Influence of site-directed mutagenesis of UbiA, overexpression of dxr, menA and ubiE, and supplementation with precursors on menaquinone production in Elizabethkingia meningoseptica. Process Biochem 68:64–72
Liu CL, Dong HG, Zhan J, Liu X, Yang Y (2019a) Multi-modular engineering for renewable production of isoprene via mevalonate pathway in Escherichia coli. J Appl Microbiol 126(4):1128–1139
Liu X, Niu H, Li Q, Gu P (2019b) Metabolic engineering for the production of l-phenylalanine in Escherichia coli. Biotech 9:85
Liu SX, Li S, Shen GM, Sukumar N, Krezel AM, Li WK (2021) Structural basis of antagonizing the VK catalytic cycle for anticoagulation. Science 371:652401
Lv Y, Qian S, Du G, Chen J, Zhou J, Xu P (2019) Coupling feedback genetic circuits with growth phenotype for dynamic population control and intelligent bioproduction. Metab Eng 54:109–116
Lyon GJ, Novick RP (2004) Peptide signaling in Staphylococcus aureus and other Gram-positive bacteria. Peptides 25:1389–1403
Ma XC, Zhu SY, Luo MM, Hu XC, Peng C, Huang H, Ren LJ (2019a) Intracellular response of Bacillus natto in response to different oxygen supply and its influence on menaquinone-7 biosynthesis. Bioprocess Biosyst Eng 42:817–828
Ma YW, McClure DD, Somerville MV, Proschogo NW, Dehghani F, KavanaghJM, Coleman NV (2019b) Metabolic engineering of the MEP pathway in Bacillus subtilis for increased biosynthesis of menaquinone-7. ACS Synth Biol 8(7):1620–1630
Mahdinia E, Demirci A, Berenjian A (2017) Production and application of menaquinone-7 (vitamin K2): a new perspective. World J Microbiol Biotechnol 33:2
Marles RJ, Roe AL, Oketch-Rabah HA (2017) US pharmacopeial convention safety evaluation of menaquinone-7, a form of vitamin K. Nutr Rev 75:553–578
Marrero PF, Poulter CD, Edwards PA (1992) Effects of site-directed mutagenesis of the highly conserved aspartate residues in domain II of farnesyl diphosphate synthase activity. J Biol Chem 267:21873–21878
Meganathan R (2001) Menaquinone and ubiquinone biosynthesis. Biochemistry 40(29):8641–8641
Meganathan R, Kwon O (2011) Biosynthesis of menaquinone (vitamin K2) and ubiquinone (Coenzyme Q). EcoSal Plus 3(2):173–218
Mishima E, Ito J, Wu ZJ, Nakamura T, Wahida A, Doll S, Tonnus W, Nepachalovich P, Eggenhofer E, Aldrovandi M, Henkelmann B, Yamada K, Wanninger J, Zilka O, Sato E, Feederle R, Hass D, Maida A, Mourão ASD, Linkermann A, Geissler EK, Nakagawa K, Abe T, Fedorova M, Proneth B, Pratt DA, Conrad M (2022) A non-canonical VK cycle is a potent ferroptosis suppressor. Nature 608:778–783
Morishita T, Tamura N, Makino T, Kudo S (1999) Production of menaquinones by lactic acid bacteria. J Dairy Sci 82:1897–1903
Noda S, Kondo A (2017) Recent advances in microbial production of aromatic chemicals and derivatives. Trends Biotechnol 35:785–796
Partow S, Siewers V, Daviet L, Schalk M, Nielsen J (2012) Reconstruction and evaluation of the synthetic bacterial MEP pathway in Saccharomyces cerevisiae. PLoS ONE 7(12):e52498
Paudel A, Hamamoto H, Panthee S, Sekimizu K (2016) Menaquinone as a potential target of antibacterial agents. Drug Discov Ther 10(3):123–128
Puri A, Iqubal M, Zafar R, Panda BP (2015) Influence of physical, chemical and inducer treatments on menaquinone-7 biosynthesis by Bacillus subtilis MTCC 2756. Songklanakarin J Sci Technol 37:283–289
Rekhter D, Ludke D, Ding Y, Feussner K, Zienkiewicz K, Lipka V, Wiermer M, Zhang Y, Feussner I (2019) Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 365:498–502
Rohmer M (2008) From molecular fossils of bacterial hopanoids to the formation of isoprene units: discovery and elucidation of the methylerythritol phosphate pathway. Lipids 43:1095–1107
Sato T, Yamada Y, Ohtani Y, Mitsui N, Murasawa H, Araki S (2001) Production of menaquinone (vitamin K2)-7 by Bacillus subtilis. J Biosci Bioeng 91:16–20
Shen YP, Fong LS, Yan ZB, Liu JZ (2019) Combining directed evolution of pathway enzymes and dynamic pathway regulation using a quorum-sensing circuit to improve the production of 4-hydroxyphenylacetic acid in Escherichia coli. Biotechnol Biofuels 12:94
Shimizu Y, Ogasawara Y, Matsumoto A, Dairi T (2018) Aplasmomycin and boromycin are specific inhibitors of the futalosine pathway. J Antibiot 71:968–970
Song J, Liu H, Wang L, Dai J, Zheng Z (2014) Enhanced production of vitamin K2 from Bacillus subtilis (natto) by mutation and optimization of the fermentation medium. Braz Arch Biol Technol 57:606–612
Song Y, He S, Abdallah II, Jopkiewicz A, Setroikromo R, van Merkerk R (2021) Engineering of multiple modules to improve amorphadiene production in Bacillus subtilis using Crispr-Cas9. J Agric Food Chem 69(16):4785–4794
Tanaka R, Kunisada T, Kushida N, Yamada K, Ikeda S, Noike M, Ono Y, Itoh N, Takami H, Seto H (2011) Branched fatty acids inhibit the biosynthesis of menaquinone in Helicobacter pylori. J Antibiot 64(1):151–153
Tani Y, Asahi S, Yamada H (1986) Menaquinone (vitamin K2)-6 production by mutants of Flavobacterium meningosepticum. J Nutr Sci Vitaminol 32:137–145
Tetali SD (2019) Terpenes and isoprenoids: a wealth of compounds for global use. Planta 249:1–8
Tonhosolo R, D’Alexandri FL, Genta FA, Wunderlich G, Gozzo FC, Eberlin MN (2005) Identification, molecular cloning and functional characterization of an octaprenyl pyrophosphate synthase in intra-erythrocytic stages of Plasmodium Falciparum. Biochem J 392:117–126
Tsukamoto Y, Kasai M, Kakuda H (2001) Construction of a Bacillus subtilis (natto) with high productivity of vitamin K2 (menaquinone-7) by analog resistance. Biosci Biotechnol Biochem 65:2007–2015
Vos M, Esposito G, Edirisinghe JN, Vilain S, Haddad DM, Slabbaert JR, Meensel SV, Schaap O, Strooper BD, Meganathan R, Morais VA, Verstreken P (2012) Vitamin K2 is a mitochondrial electron carrier that rescues Pink1 deficiency. Science 336:1306–1310
Wang C, Zada B, Wei G, Kim SW (2017) Metabolic engineering and synthetic biology approaches driving isoprenoid production in Escherichia coli. Bioresour Technol 241:430–438
Wang Y, Liu L, Jin Z, Zhang D (2021) Microbial cell factories for green production of vitamins. Front Bioeng Biotechnol 9:661562
Westbrook AW, Moo-Young M, Chou CP (2016) Development of a Crispr-Cas9 tool kit for comprehensive engineering of Bacillus subtilis. Appl Environ Microbiol 82:4876–4895
Wu J, Li W, Zhao SG, Qian SH, Wang Z, Zhou MJ, Hu WS, Wang J, Hu LX, Liu Y, Xue ZL (2021) Site-directed mutagenesis of the quorum sensing transcriptional regulator SinR affects the biosynthesis of menaquinone in Bacillus subtilis. Microb Cell Fact 20(1):1–19
Xu P (2018) Production of chemicals using dynamic control of metabolic fluxes. Curr Opin Biotechnol 53:12–19
Xu JZ, Zhang W (2017) Menaquinone-7 production from maize meal hydrolysate by Bacillus isolates with diphenylamine and analogue resistance. J Zhejiang Univ Sci B 18:462–473
Xu L, Dmitry A, Gaynor EC, Tanner ME (2011) 5’-methylthioadenosine nucleosidase is implicated in playing a key role in a modified futalosine pathway for menaquinone biosynthesis in Campylobacter jejuni. J Biol Chem 286(22):19392–19398
Xu JZ, Yan WL, Zhang WG (2017) Enhancing menaquinone-7 production in recombinant Bacillus amyloliquefaciens by metabolic pathway engineering. RSC Adv 7(45):28527–28534
Yang SM, Cao YX, Sun LM, Li CF, Lin X, Cai ZG, Zhang GY, Song H (2019) Modular pathway engineering of Bacillus subtilis to promote de novo biosynthesis ofmenaquinone-7. ACS Synth Biol 8(1):70–81
Yang Q, Zheng ZM, Zhao GH, Wang L, Wang H, Ding XM, Jiang CX, Li C, Ma GL, Wang P (2022) Engineering microbial consortia of Elizabethkingia meningoseptica and Escherichia coli strains for the biosynthesis of vitamin K2. Microb Cell Fact (2022) 21:37
Yu Y, Aairm R, Liu HR, Lv B, Chang PC, Song H, Wang Y, Li C (2020) Engineering Saccharomyces cerevisiae for high yield production of α-amyrin via synergistic remodeling of α-amyrin synthase and expanding the storage pool. Metab Eng 62:72–83
Yuan P, Cui S, Liu Y, Li J, Lv X, Liu L, Du G (2020) Combinatorial engineering for improved menaquinone-4 biosynthesis in Bacillus subtilis. Enzyme Microb Technol 141:109652
Yuan P, Sun G, Cui S, Wu Y, Lv X, Liu Y, Li J, Du G, Liu L (2021) Engineering a ComA quorum-sensing circuit to dynamically control the production of menaquinone-4 in Bacillus subtilis. Enzyme Microb Tech 147:109782
Zada B, Wang C, Park JB, Jeong SH, Park JE, Singh HB, Kim SW (2018) Metabolic engineering of Escherichia coli for production of mixed isoprenoid alcohols and their derivatives. Biotechnol Biofuels 11:210
Zhi XY, Yao JC, Tang SK, Huang Y, Li HW, Li WJ (2014) The futalosine pathway played an important role in menaquinone biosynthesis during early prokaryote evolution. Genome Biol Evol 6:149–160
Zou D, Maina SW, Zhang F, Yan Z, Xin Z (2020) Mining new plipastatins and increasing the total yield using Crispr/cas9 in genome modified Bacillus Subtilis 1A751. J Agric Food Chem 68(41):11358–11367
Acknowledgements
The study was supported by the National Nature Science Foundation of China (No. 32372295), Outstanding Youth Research Project in Anhui Province Universities (No. 2023AH020013), Anhui university natural science research key project (2023AH050938), and Anhui Provincial Undergraduate Innovation and Entrepreneurship Program (No. 202310363254).
Author information
Authors and Affiliations
Contributions
Yan Liu : conceptualization, review and editing, supervision, investigation, visualization. Jian Wang, Jun-bao Huang: methodology, writing—review and editing. Xiang-fei Li, Yu Chen, Kun Liu, Ming Zhao , Xi-lin Huang, Xu-li Gao, Ya-ni Luo, Wei Tao, Jing Wu: methodology, writing—original draft, review and editing. Zheng-lian Xue: supervision, writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Wang, J., Huang, Jb. et al. Advances in regulating vitamin K2 production through metabolic engineering strategies. World J Microbiol Biotechnol 40, 8 (2024). https://doi.org/10.1007/s11274-023-03828-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03828-5