Abstract
Phomopsis canker is one of the major devastating stem diseases that occur in tea plants caused by the fungal pathogen Phomopsis theae. Rapid development of this disease leads to a capital loss in the tea industry which demands an ecofriendly disease management strategy to control this aggressive pathogen. A total of 245 isolates were recovered from the tea rhizosphere and screened for in vitro plant growth promoting (PGP) traits and antagonism against P. theae. Among them, twelve isolates exhibited multifarious PGP traits including phytohormones, siderophore, hydrogen cyanide, salicylic acid production, phosphate solubilization, 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity, and antifungal activity. In vitro studies on morphological, biochemical, and phylogenetic analyses classified the selected isolates as Pseudomonas fluorescens (VPF5), Bacillus subtilis (VBS3), Streptomyces griseus (VSG4) and Trichoderma viride (VTV7). Specifically, P. fluorescens VPF5 and B. subtilis VBS3 strains showed the highest level of PGP activities. On the other hand, VBS3 and VTV7 strains showed higher biocontrol efficacy in inhibiting mycelia growth and spore germination of P. theae. A detailed investigation on hydrolytic enzymes produced by antagonistic strains, which degrade the fungus cell wall, revealed that highest amount of chitinase and β-1,3- glucanase in VTV7 and VBS3 strains. Further, the key antifungal secondary metabolites from these biocontrol agents associated with suppression of P. theae were identified using gas chromatography mass spectrometry. The above study clearly recognized the specific traits in the isolated microbes, which make them good candidates as plant growth-promoting rhizobacteria (PGPR) and biocontrol agents to improve plant growth and health. However, greenhouse trials and field application of these beneficial microbes is required to further confirm their efficacy for the management of stem canker in tea cultivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tea is a refreshing and aromatic drink made from the leaves of Camellia sinensis. Next to water, it is the second most consumed beverage in the world. The major tea-growing.
areas are Tamil Nadu, Kerala, and Karnataka in South India (Ponmurugan et al. 2013). Being a perennial cash crop, and are mostly grown as monoculture in a warm and humid climate, it is continuously prone to soil-borne disease (Baby 2002). Among different tea plant diseases, collar (stem) canker caused by the fungus Phomopsis theae Petch belongs to Deuteromycetes is recognized as threatening stem disease in tea growing region around the globe (Ponmurugan and Baby 2007). This sporadic disease spreads mostly in young tea clones and also in mature tea bushes to a small extent (Baby et al. 2001). This devastating disease is a major problem in southern India and is considered to be of great importance. There may be crop loss of about 10–15% due to this disease (Ponmurugan et al. 2006).
Current agricultural practices involve the usage of chemical fungicides including carbendazim to suppress these phytopathogens. However, prolonged application of these chemicals leads to a hazardous effect on surroundings. Also, intense use of these fungicides on crops generates resistant strains; thereby, the efficacy of several important chemical fungicides may be reduced (Yang et al. 2008). Broad host range and increased diversity of phytopathogen infestation are due to easy dispersal with the wind, farm machinery, water, drastic climate changes and large-scale genetically uniform intensive monoculture crops (Pan et al. 2010).
At present, plant disease control using beneficial microbes is considered a better alternative to synthetic fungicides. The wide spectrum of bioactive antifungal secondary metabolites of biocontrol agents is environmentally safe to host plants and thus it increases the organic value of food (Kaur et al. 2016). The antifungal metabolites of biological origin are easily biodegradable, more transient, and confined to the rhizosphere when compared to the agrochemicals (Lugtenberg and Kamilova 2009). Hence, chemical fungicides can be replaced by biocontrol agents and exploited for controlling various fungal phytopathogens (Kulimushi et al. 2017).
The plant rhizosphere is an important resource associated with a wide variety of beneficial microbes that potentially stimulate plant growth and protect the plant’s health from phytopathogens. The rhizosphere is habituated with a diverse group of microorganisms and the bacteria colonizing this region are called rhizobacteria. The rhizobacteria that enhance plant health by stimulating growth and suppressing soil-borne disease are termed as the plant growth promoting rhizobacteria (PGPR). Commonly, a single PGPR holds the property of multiple actions including biocontrol activity against plant disease (Antoun and Kloepper 2001). Similarly, fungi are also associated with beneficial effects on promoting plant growth development and as a biocontrol agent against different kinds of plant pathogens (Harman et al. 2004). Some important direct mechanisms of plant growth-promoting traits exerted by PGPR include phytohormone production, phosphate solubilization, sequestering iron, nitrogen fixation (Glick 1995) while the indirect mechanisms include suppression of harmful plant pathogens and elicit induced systemic resistance (ISR) (Kloepper and Beauchamp 1992; Dobbelaere et al. 2003).
Soil microbes that reside in the rhizosphere can produce a diversified group of antifungal compounds that promote their efficacy as biocontrol agents against various phytopathogens. Microbial metabolites have specific modes of action according to the nature of the antifungal compound produced. A synergistic combination of volatile and nonvolatile antimicrobial compounds secreted by biocontrol agents promoted protection for plants from fungal diseases (Fernando et al. 2005). Earlier, it was reported that Pseudomonas spp., Bacillus spp., Trichoderma spp. and Streptomyces spp. were employed for biological control of plant disease and are well reported to produce secondary metabolites that play a significant role in the control of plant pathogens (Yang et al. 2017; Jayaprakashvel and Mathivanan 2011). A diverse group of antifungal compounds from several beneficial microbes have been reported, but plant protection requires more demand for bioactive metabolites with novel properties.
The major classes of antibiotic compounds include phenazines, phloroglucinols, pyoluteorin, pyrrolnitrin, hydrogen cyanide (a volatile compound), biosurfactants, and cyclic lipopeptides (diffusible compounds), which gained more attention due to their broad-spectrum biocontrol potential against phytopathogens (Haas and Defago 2005; Raaijmakers and Mazzola 2012). The diverse traits of heterogeneous plant growth-promoting microbes include biofertilization, phytostimulation, and biological control that can be exploited to develop formulations to control pathogens, increase yield, and increase food production (Bhattacharyya and Jha 2012). In this work, four strains of different species were isolated from the tea rhizosphere. The main objective of the present study was to explore the antagonistic potential of rhizospheric strains as biocontrol agents to control the growth of the sporadic stem pathogen Phomopsis theae under in vitro conditions. Plant growth-promoting traits of these selected strains were also characterized, which could be eventually used for the growth and development of tea plants. Additionally, the gas chromatography-mass spectrometry (GC-MS) technique was adopted to identify the possible significant volatile organic compounds emitted from biocontrol agents, which are involved in antifungal activity.
Materials and methods
Soil sampling
For isolation of plant growth promoting rhizospheric microbes, the rhizospheric soil samples were taken from the healthy UPASI-9 tea clone of 30 years old (high leaf yielder but sensitive to tea diseases), which was selected for soil sampling and was collected from different estates of Parry Agro Tea Industries, Valparai located at 10° 19’ 36.88” N and 76° 57’ 4.18” E, with elevation about 1,059 m above MSL Tamil Nadu, India and Mattupatty estate, Kanan Devan Hills Plantations Company (P) Limited, Mattupatty, Idukki district, Munnar located at 09º 59.938’ N and 77º 09.902’ E with elevation about 5336 feet above MSL, Kerala, India. The rhizospheric soil samples were taken from a depth of 0–15 cm, kept in plastic bags, and carried to the laboratory.
Isolation of rhizospheric strains from tea soil
Each rhizospheric soil sample, strongly adhering to the roots, was collected and 10 g were poured into a 250 mL conical flask containing 90 mL of sterile distilled water. Serial dilution technique was used for isolation of bacterial and fungal species. The flask was shaken for 24 h on a rotary shaker, and a tenfold serial dilution was prepared from the extract. About 0.1 mL of each dilution (for bacterial isolation (dilution from 10− 6 to 10− 8 and for fungal isolation dilution from 10− 4 to 10− 6) was spread on the plates with respective medium (King’s B media, Tryptic soy agar and starch casein agar, TSM) amended with suitable antibiotics and incubated at 28–30 °C for 1–7 d. Well isolated single colonies were picked up and re-streaked into fresh specific media and incubated similarly. The technique was perpetrated thrice and culture was made to result in pure single colony type.
Phenotypic characterization of rhizospheric strains
The phenotypic features of all the rhizospheric isolates were characterized and compared to phenotypic data of known organisms described in the Bergey’s Manual of systematic Bacteriology (Holt et al. 1994; Vos et al. 2009). The plates were examined after the optimum incubation period. The suspected colonies were identified based on cultural and morphological characteristics. Physiological characteristics of rhizospheric isolates were tested which includes growth at different pH, temperature and salt ranges. In the case of fungal isolates, lacto phenol cotton blue staining was carried out. Two techniques, visual observation on Petri dishes (colony appearance, radial growth rate and sporulation pattern) and micro-morphological studies in slide culture, were adopted for identification of Trichoderma species. For micromorphological studies, a slide culture technique was used. Examination of the shape, size, arrangement and development of conidiophores or phialides provided a tentative identification of Trichoderma spp. (Bissett, 1991a, b; Samuels et al. 2002). Suspected Trichoderma colonies were sub-cultured on new PDA plates to obtain pure cultures for further observation. Above phenotypic tests were performed and all experiments were done following complete randomized design (CRD) with three replications for each isolate and repeated once.
Isolation of pathogen, culture condition, and pathogenicity test
The fungal pathogen Phomopsis theae was isolated from infected stems of tea plants collected from Valparai, Tamil Nadu, (11.35°N 76.82°E) and Munnar, Kerala (10.106°N 77.124°E) of southern India. The infected plant material was placed in a moist chamber to develop fruiting bodies. Spore masses exuded from pycnidia were transferred to a 2% water agar medium supplemented with streptomycin sulphate (50 mg/l). Actively growing mycelial tips of the fungus were transferred to potato dextrose agar (PDA supplemented with antibiotic chloramphenicol) plates for two weeks at 25ºC and purified. Initially, fungal species were identified based on morphology and microscopy Also, a phytopathogenicity test was conducted using Koch’s postulates (Agrios 1997) with the tea clone, UPASI-3 under nursery conditions.
In vitro screening of rhizospheric strains with plant growth promoting traits and antagonistic activity against Phomopsis theae
Primary screening of rhizospheric strains for plant growth promoting (PGP) traits was performed and determined according to Berg et al (2001). Similarly, screening of antagonistic activity was performed (Petatán-Sagahón et al. 2011). For analysis of PGP and biocontrol traits (Supplementary Mat. S1), an assessment using bonitur scale was done in order to select the most potential rhizospheric strains having PGP as well as biocontrol traits (EI-Sayed et al. 2014).
Molecular identification and phylogenetic analysis of selected antagonistic strains
The identity of the most promising selected plant growth-promoting microbes with antifungal activity (Pseudomonas VP5, Bacillus VBS3, Streptomyces VSG4, and Trichoderma VTV7) was confirmed using 16 S rDNA and ITS region sequence analysis. Briefly, the genomic DNA from the selected strains was isolated using the CTAB method (Sambrook and Russell 2001). The 16 S rDNA gene of bacterial strains was amplified using bacterial universal forward primer 27 F (5′-AGAGTTTGATCCTGGCTCAG-3′) and reverse primer 1492R (5′-GGTTACCTTGTTACGACTT-3′; Maatallah et al. 2002). In the case of fungi, Trichoderma, 18 S rRNA genes were amplified with ITS primers viz., ITS-1 (5-TCCGTAGGTGAACCTGCGG-3), and ITS-4 (5-TCCTCCGCTTATTGATATGC-3) following a standard protocol (White et al. 1990). The DNA isolation, amplification, and 16 S rDNA and ITS gene sequencing were performed with Oscimum lab Biotech, Hyderabad. The sequences were subjected to BLAST analysis and homology was compared with deposited sequences in the GenBank database of NCBI, Maryland, USA. A phylogenetic tree was constructed for amplified sequences (16 S rDNA and ITS) of the selected strains and their closely related sequences using MEGA 7.0 software (Tamura et al. 2011; Martínez-Absalón et al. 2014). The phylogeny was tested using the neighbor-joining method by 1,000 bootstrap replications and condensed with a cut-off value of 70%.
Determination of plant growth promoting traits
Estimation of phytohormone production
The estimation of indole acetic acid (IAA) was quantitatively assessed according to the method of Brick et al. (1991) with slight modifications adapted by Goswami et al. (2013). The culture supernatant was mixed with Salkowski reagent. The absorbance was measured at 530 nm using a spectrophotometer and expressed in µg mL− 1. For estimation of gibberellic acid, the pH of the culture supernatant was adjusted at 2.5 by using 15% HCl. The filtrate was extracted with ethyl acetate (1:3 of filtrate is to solvent ratio) and the extract was used for its gibberellic acid determination. The experiment was conducted in triplicates. In this assay gibberellic acid is converted into gibberellenic acid and is estimated at 254 nm absorbance (Pandya and Desai 2014). For the cytokinin assay, a modified method of Letham (1971) was adopted according to Ereful et al. (2007), in which the culture supernatant was partitioned with ethyl acetate and butanol. The organic portions were reduced to residue using N2 gas. The presence of cytokinin in the extract was evaluated using a radish cotyledon expansion assay and the amount of active compound was quantitatively determined using spectrophotometrically at 269 using a UV-Visible spectrophotometer (Shimadzu UV-1800 Japan).
Qualitative and quantitative estimation of phosphate solubilization
Primary screening of phosphate solubilization of selected strains was assayed qualitatively on Pikovskaya’s agar plates as described by Gaur (1990). Pikovskaya broth was prepared from the cultures and the extracted supernatant was mixed with chloromolybdic acid and chlorostannous acid. The absorbance of blue color was read at 600 nm using a spectrophotometer. A standard curve was prepared from KH2PO4 to detect the amount of phosphorus solubilized in µg mL− 1 (Saxena and Annapurna 2002). Simultaneously, the pH and acidity of the culture medium were measured using a pH meter and titration method. The production of acid and alkaline phosphatase enzymes in the medium was estimated following the procedure of Tabatabai (1994).
Screening for siderophore production
Qualitative analysis of siderophore production was done using universal medium chrome Azul S Agar (CAS) (Schwyn and Neilands 1987) and Quantitative estimation of siderophore production of the selected strains was performed using CAS-shuttle assay (Payne 1994). Broth cultures grown in the iron-free minimal medium were withdrawn and centrifuged at 12,298 x g for 15 min. The culture supernatant was mixed with an equal volume of CAS assay solution and allowed to stand for 20 min. The presence of siderophore was detected with the development of blue color intensity recorded at 630 nm. For the measurements, the minimal medium was used as a blank, and % siderophore units were calculated by the following formula [(Ar–As)/Ar] × 100 = % siderophore units. Where, Ar = absorbance of reference (minimal media + CAS assay solution), As = absorbance of the sample.
Production of hydrogen cyanide, ammonia, and nitrogen fixation
Hydrogen cyanide production was qualitatively determined by the modified method according to Akhtar and Siddiqui (2009). The color change in filter paper from yellow to orange-brown was identified to be the HCN production. Qualitative and quantitative production of ammonia was determined as described by Cappuccino and Sherman (1992). A standard curve was prepared using ammonium sulfate in the range of 0.1-5 µmol mL− 1 to measure the ammonia production at 450 nm. Screening of the nitrogen-fixing property of selected strains was carried out by using semisolid malate medium. For preliminary screening, bromothymol blue (BTB) present in nitrogen-free malate media was used as an indicator and the plates were incubated at 37ºC and 50ºC up to 24 h. The culture broth (bacterial and fungal) 10 µl grown to logarithmic growth phase was dropped to BTB plate and cultured at 28ºC for 48 h. The inactivated bacterial broth was set as control. The experiments were repeated three times. The blue-colored zone formation by the isolates was recognized as nitrogen fixers in the solid culture conditions (Baldani et al. 2014).
Estimation of salicylic acid and assay of ACC deaminase activity
For the estimation of salicylic acid, the cell-free supernatant was acidified and followed as per the method of Visca et al. (1993). The absorbance of the purple iron complex was read at 527 nm using a spectrophotometer and compared with a standard curve of salicylic acid. The assay for ACC deaminase activity was based on the method described previously (Penrose and Glick 2003). The crude bacterial cell-free extract was incubated with ACC, HCl, and 2,4-dinitrophenylhydrazine. The absorbance of the resulting mixture was read at 540 nm. The activity was expressed as units required to produce 1µmole of α-ketobutyrate/min. Protein concentrations were determined following the Bradford method (Bradford 1976).
Determination of antagonistic traits
Dual culture technique
Antagonistic strains were tested for antagonistic activity against tea pathogen by the dual culture technique (Rabindran and Vidyasekaran 1996). Besides, abnormalities in the fungal hyphae were studied using lacto phenol-cotton blue staining and observed under a light microscope.
Post interactional studies of scanning electron microscopy
The mechanism of biocontrol agents against P. theae was studied under a scanning electron microscope. The cultures grown on small pieces of the coverslip (> 1 cm), which were taken out from the interaction zone of dual culture plates was transferred to another separate Petri-dish. Likewise, the coverslip sample was prepared from another plate grown with fungal mycelium alone (control). The sample specimens were vacuum dried for one hour and fixed overnight at 4 °C in 2.5% glutaraldehyde. This was followed by dehydration with ethanol of increasing concentrations (50–100%) (v/v), dried in a desiccator, and coated with gold and palladium in a sputter-coater (QUORUM) for 90 s; then, it was examined with a scanning electron microscope (ZEISS (EVO1) to observe the fungal hyphae abnormalities (Chen et al. 2016).
Assay of hydrolytic enzymes
Primary screening of hydrolytic enzymes was performed by spot inoculation and streaking the antagonistic strains on medium containing colloidal chitin, laminarin, cellulose, skimmed milk, and tributyrin amended agar plates, and incubated at room temperature. The zone of clearance by the corresponding enzyme was recorded after 5 d. Quantitative assays of hydrolytic enzymes: chitinase (Reissig et al. 1955), β-1,3- glucanase (Pan et al. 1991), cellulase (Miller 1959), protease (Tsuchida et al. 1986), lipase (Xu et al. 2002), and total protein content (Bradford 1976) in the crude extracts of the soluble enzymes were determined (Supplementary Mat. S2).
Antifungal activity of solvent extraction of crude metabolites of antagonistic strains against P. theae by agar well diffusion
Antifungal activity of antagonistic strains was determined using the agar well diffusion method with slight modifications (Perez et al. 1990). The microbial crude extract was prepared at a concentration of 1 mg mL− 1 in different solvents viz. chloroform, ethyl acetate, methanol, and water selected based on their polarity. About 100 µl (100 µg) aliquots of different solvent extracts were added using a sterile syringe into the wells and allowed to diffuse at room temperature for 2 h. Standard controls (nystatin 1 mg/disc used as positive control and DMSO as negative control) were maintained and compared with the test samples for calculating the activity index (AI- activity index = IZ of test sample / IZ of standard). The plates were incubated at 28 °C for 48 h. The diameter of the inhibition zone (mm) was measured and calculated. Triplicates were maintained and the average values were recorded. Minimal inhibitory and minimum fungicidal concentration of ethyl acetate extract was determined by the 96 well microtitre plate method (Dellavalle et al. 2011).
Effect of cell-free culture supernatant of antagonistic strains on mycelial biomass of Phomopsis theae
The effect of solvent (water and ethyl acetate 1:1 ratio) extracts of culture filtrate of antagonistic strains on reduction of fungal biomass was carried out. This solvent extract at 25 and 50% concentration was mixed with 100 mL potato dextrose broth medium in a conical flask. The solvent in the flask containing the PDB medium without culture filtrates served as a control. All flasks were inoculated with pathogenic fungi (6 mm disc) and incubated at 28˚C. After 7 d, the fungal mats were harvested to determine the mycelial dry weight. The percentage of mycelial growth inhibition of the pathogenic fungi was determined according to the formula: Reduction (%) = control - treatment / Control × 100 (Li et al. 2015a).
Effect of antagonistic culture filtrates on spore germination of Phomopsis theae
A hanging drop technique was followed to study the spore germination with different antagonist culture filtrates. Bacterial suspensions were prepared and their concentrations were adjusted to 107 CFU/mL by the dilution technique. Likewise, 1 mL of spore suspension of fungal antagonist (104 CFU/mL) was inoculated in the medium from 7-d old culture. The inhibitory effect of culture filtrate of selected strains on spore germination of P. theae was done by taking 100 µl of PDB grown fungal spore suspension (105 spores/mL) mixed with 100 µl of culture filtrate with different concentrations (10, 25, and 50% v/v) and incubated at 28 °C. In the case of control, 100 mL of sterile distilled water was taken instead of culture filtrate. After an incubation period of 8 h, 50 µl of each suspension were placed on a sterile cavity slide. Then, the coverslip was placed over the slide and observed under a microscope (Li et al. 2015a). The germination percentage was calculated from 100 spores randomly selected. Three slides were prepared for each treatment and the means were recorded according to the formula: Percentage of germination = Number of germinated spores x 100/Total number of spores.
Antifungal activity of volatile and non-volatile compounds of antagonistic strains against Phomopsis theae
The effect of volatile compounds from the selected antagonist on the radial growth of P. theae was analyzed according to the method of Dennis and Webster (1971). The two bottom portions of Petri plates containing PDA were inoculated with mycelia of the pathogen and the antagonist respectively, inoculated bottom plates were placed facing each other and sealed with cellophane adhesive tape. The control plate consisted of PDA without an antagonist. The radial growth of the test fungus was noted after 7 d of incubation at 25 ± 1ºC. Similarly, the effects of nonvolatile metabolites produced by the antagonist were determined by the method of Dennis and Webster (1971). The cell-free culture filtrate of four antagonists was inoculated in 100 mL of respective broth in 250 mL conical flasks and incubated at 28 ± 2 °C for 3–7 d. After incubation, the cultures were filtered through a Millipore filter, and culture filtrates (10%) were added to molten PDA medium (40 ◦C). After solidification of the medium in Petri plates, a 3 mm disc of the P. theae was placed centrally and incubated at 25 ± 2 °C. The control plates were maintained in absence of the culture filtrate. The percent of growth inhibition in all the above experiments was calculated by the formula: I = C-T × 100/C; Where I = Percentage of inhibition, C = Growth of mycelium in control T = Growth of mycelium in treatment.
Gas chromatography mass spectrometry analysis of secondary metabolites of selected antagonists for suppression of Phomopsis theae
The extraction of the crude antifungal compound from the antagonistic strains was done following Prapagdee et al. (2008). Crude metabolites of the antagonistic strains were analyzed by GC-MS (GC Ultra DSQ II, Thermo Scientific, Germany) according to the method of Dheepa et al. (2016), for the detection of the volatile metabolites responsible for the suppression of P. theae. The detected compounds were identified and confirmed using the National Institute of Standards and Technology (NIST) library 2006.
Statistical analysis
All the in vitro experiments were repeated thrice under identical conditions. Statistical analyses were performed using SPSS (Version 17) software package. A one-way ANOVA (analysis of variance) was applied to confirm the significance of the data according to Duncan’s multiple range test (DMRT) at 5% (P = 0.05) probability level using SPSS 17 software. The values were displayed as the mean of three replicates ± standard deviation.
Results
Isolation and identification of rhizobial strains with antifungal activity
A total of 245 (73, 57, 60, 55) plant growth promoting rhizospheric isolates were obtained from different regions of tea rhizospheric soil samples. Due to the vastness of data, we have provided only the details of twelve isolates (VP3, VP5, VP7, VB1, VB2, VB3, VS2, VS4, VS6, VT4, VT7 and VT9) with their beneficial traits in the supplementary material. These isolates displayed good capability to produce plant growth promoting traits and showed in vitro antagonism against P. theae in a preliminary screening. Further, based on morphological, physiological, and biochemical properties, they were grouped into Pseudomonas spp., Bacillus spp., Streptomyces spp. and Trichoderma spp (Supplementary Tables 1–3). Among twelve strains, four potential strains (VP5, VB3, VS4 and VT7) with PGP trait and antifungal activity against P. theae were selected for further studies based on the bonitur assessment scale and the ranking score achieved in the primary screening (Supplementary Tables 4–6). However, the selected antifungal strains were subjected to molecular identity where BLAST analysis of 16 S rRNA and ITS gene regions confirmed that the strains VPF5 showed 96–97% homology with P. fluorescense. Likewise, the VBS3 strain showed 94–98% identity with B. subtilis, the other strains VSG4 and VTV7 showed 99% similarity with S. griseus and T. viride. The gene sequence of selected antagonistic strains was submitted to the GenBank database of NCBI, Maryland, USA (accession numbers: MG889456, MG875052, MG875053, and MG875332). The phylogenetic analysis included twenty reference strains of different species belonging to the same genus of nucleotide sequences available in NCBI selected for each biocontrol strain. The sequences of the antagonistic strains were compared with reference strains holding the property of biocontrol activity against plant diseases (Fig. 1).
The phylogenetic tree was constructed for amplified sequences (16 S rDNA and ITS) of the selected strains and their closely related sequences holding the property of PGPR and biocontrol activity using MEGA 7.0 software. The phylogeny was tested using the neighbor-joining method by 1,000 bootstrap replications and condensed with a cut-off value of 70%
In vitro plant growth-promoting trait exhibited by antagonistic strains
Characterization of various plant growth-promoting (PGP) activities exhibited by native antagonistic strains was carried out. There was a significant variation between indole acetic acid and gibberellic acid production by the selected strains (Table 1). The selected strains produced a considerable amount of these phytohormones in the basal medium supplemented with suitable substrates in which the production ranged from 2 to 13 µg/mL of IAA (L-TRP -), 9–21 µg/mL of IAA (L-TRP +), and 17–34 µg/mL of GA3. The maximum production of these two phytohormones was detected after 96 and 72 h of incubation by these selected strains (Table 1). Among the four antagonistic strains, the highest amount of cytokinin (7 µg/mL) was detected in P. fluorescens VPF5 and the lowest in T. viride VTV7 with 3 µg/mL.
In the growth of CAS liquid medium, VBS3 strain produced about 71% of siderophore units when compared to other strains tested (Table 2). Besides, the chemical nature of siderophore types was determined for the antagonistic strains. Our results demonstrated the presence of both hydroxamate and catecholate types of siderophore production in the range of 17–35 and 11–29% respectively (Table 3). Further, the quantification of available phosphorus released by the strains was evaluated using rock phosphate as a source of phosphorus in the basal medium. The VPF5 strain potentially solubilized 352 µg mL− 1 followed by B. Subtilis VBS3 which accounted for 283 µg mL− 1 of soluble phosphate content (Table 2). This indicated that the selected strains could solubilize the insoluble form of phosphorous for efficient uptake by the plant.
Organic acids produced by antagonistic strains were determined by estimating titratable acidity in the basal medium. The strains VPF5 and VBS3 produced greater amounts of organic acids than the other strains (Table 2). Moreover, secretion of organic acids by the strains caused a reduction in pH of the medium with an increase in titrable acidity. Further, these strains were analyzed for the synthesis of acid and alkaline phosphatase under in vitro conditions. Among them, VPF5 (41.9 U mL− 1) and VBS3 (23.6 U mL− 1) strains were the highest producers of acid and alkaline phosphatases, respectively when compared with other strains (Fig. 2).
Acid and alkaline phosphatase activity of four selected antagonistic strains (Pseudomonas fluorescens VPF5, Bacillus subtilis VBS3, Streptomyces griseus VSG4 and Trichoderma viride VTV7). Mean values in each cone followed by different letters are significantly different at P = 0.05 according to DMRT.
The four antagonistic strains showed positive responses to HCN production, where distinctively reddish-orange and brick red colors were observed. All the strains produced ammonia which ranged between 2.4 and 3.4 µmol mL− 1. Among the different strains, B. subtilis VBS3 produced the greatest quantity of salicylic acid (21 µg mL− 1) followed by P. fluorescens VPF5 (17 µg mL− 1). The antagonistic culture filtrate was used for the ACC deaminase enzyme assay and the amount of α-ketobutyrate produced was detected. Among the four strains, VPF5 showed the higher enzyme activity (0.545µM) compared with the other strains used (Table 2).
Antagonistic activity of selected biocontrol agents using the dual culture technique
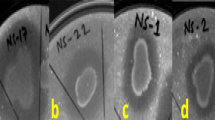
This technique involves simultaneous inoculation of two different cultures on solid growth medium containing nutrients for both, biocontrol agents, and pathogens to test for antagonism. There was a significant variation in mycelial growth inhibition of P. theae exhibited by the four indigenous antagonistic strains (Table 4). Among them, the T. viride VTV7 (78.9% inhibition) showed the greatest antagonism against the pathogen when compared to other strains. This was followed by B. subtilis VBS3 with 76.7% of inhibition. The percentage of inhibition on P. theae ranged from 66.7 to 78.9% in which antagonism potency of the strains was VSG4 < VPF5 < VBS3 < VTV7. The least antagonism was detected in VSG4 and in control plates, where the pathogen reached maximum linear growth of 90 mm in 10 d (Fig. 3).
In vitro antifungal activity of four selected antagonistic strains observed after inoculation of plates at 5th day (left side) and 10th day (right side) in dual-culture assays. (a) P. fluorescens VPF5 (b) B. subtilis VBS3 (c) S. griseus VSG4 (d) T. viride VTV7. Bacterial antagonistic strains inoculated as single-line streaks and fungal antagonistic strain inoculated as mycelium agar plug placed on top of the Petri plate against the phytopathogenic fungi Phomopsis theae. (e) Control plates of P. theae VPT02 as white colonies without antagonistic strains are shown at the extreme bottom
Scanning electron microscopy analysis of post-interaction events of the dual culture
Morphological abnormalities in the hyphae of P. theae obtained from the zone of interaction of selected four antagonistic strains during dual cultures were observed under scanning electron microscopy (SEM) investigation. In the absence of antagonists, hyphae of P. theae were healthy, regular in shape with a smooth outer surface, and showed well-established mycelial growth (Fig. 4a.). In contrast, in the presence of bacterial antagonistic strains, several abnormal morphological features were clearly visible (Fig. 4b-d). The indirect inhibition may be related to a group of certain diffusible antifungal metabolites and antibiotic secretion into the medium. Later, this was followed by shriveling, clump formation and loss of structural integrity (Fig. 4b), perforation and breakage of the hyphal tip (Fig. 4c and d), and lysis. The above process led to the release of cytoplasmic contents. This suggests that the antagonistic mechanism of T. viride VTV7 may be categorized as direct or contact inhibition where the hyphae of two fungi interact at the intersection zone. Subsequently, a dense growth of the hyphae of T. viride VTV7 was extended and more branched, whereas the hyphae of the pathogen was swelled up, enlarged, and vacuolized (Fig. 4f). Consequently, the fungal antagonist coiled around the mycelium and invaded the pathogen by encircling it, and finally degraded it.
Scanning electron micrographs showing morphological abnormalities in the hyphae of P. theae due to the antifungal effect of antagonistic strains isolated from the tea soil. (a) Normal mycelium of P. theae VPT02, (b) Swelling of mycelium (VPF5 vs. VPT02), (c) Shriveling and lysis with release of cytoplasmic contents (VBS3 vs. VPT02), (d) Perforation, vacuolization and bulb formation (VSG4 vs. VPT02), (e) T. viride mycelium with spores (VTV7), (f) Coiling, penetration swelling and collapsing (VTV7 vs. VPT02). Grid observed at 10 kV and bar = 20 μm
Detection and quantification of hydrolytic enzymes
A potential antagonist secretes an array of hydrolytic enzymes to hydrolyze the cell wall components of the pathogen. Preliminary screening of the selected native biocontrol strains revealed the production of chitinase, β-1,3-glucanase, cellulase, protease, and lipase, with a zone of clearance on test plates (Fig. 5). Further, under in vitro conditions, secondary screening of these enzymes was carried out under a liquid medium using specific substrates. The culture filtrates were harvested every day and the enzyme assay, as well as the protein content, were estimated for 10 days. The selected antagonistic strains showed varied levels of enzyme production (Fig. 6). Among the four different strains, the highest amount of chitinase (75.2 U mL− 1) was produced in the T. viride VTV7. The maximum amount of β-1,3- glucanase and protease was recorded in B. subtilis VBS3 with 68.4 and 86.4 U mL− 1 respectively. In general, the amounts of enzymes gradually increased and reached the optimum on the third and fifth day of incubation (both bacteria and fungi), and then gradually decreased.
Hydrolytic enzyme production by antagonistic strains observed with the zone of clearance. Chitinase production – (a) Control and (b) Test sample strain; β-1,3 glucanase production – (c) Control and (d) Test sample strain; Cellulase production – (e) Control and (f) Test sample strain; Protease production – (g) Control and (h) Test sample strains; Lipase production- (i) Control and (j) Test sample strain
Assay of antifungal activity using the agar well diffusion method
Four different solvents were used to extract crude secondary metabolites from selected antagonistic strains to study the in vivo antifungal activity by the agar well diffusion assay. Among them, ethyl acetate and methanol extracts showed the optimum antifungal activity against P. theae. Specifically, the ethyl acetate extract of T. viride VTV7 and B. subtilis VBS3 showed a maximum zone of inhibition (24.7 and 23 mm) and hence, the activity index (0.91 and 0.84) increased accordingly (Table 5). In contrast, there was no significant difference observed in the chloroform extract of the four bioagents. The aqueous extract showed the lowest zone of inhibition (7.3 mm with AI = 0.26) when compared with the rest of the solvent extract. The efficiency of the solvents extracting antifungal metabolites against a pathogen can be sequenced in descending order as follows: ethyl acetate > methanol > chloroform > aqueous. The values of MIC and MFC of crude ethyl acetate extract of selected antagonistic strains are presented in Table 6. All the tested biocontrol agents showed significant antifungal activity against P. theae; among them, VTV7 showed the least MIC and MFC values of 25 and 100 µg mL− 1.
Impact of cell-free culture filtrate of antagonistic strains on the reduction of mycelial biomass
Cell-free bacterial and fungal culture filtrates taken after 3–7 d of growth on a specific medium, were evaluated for a reduction in mycelial biomass of P. theae (Table 7). The ethyl acetate extract showed a potential inhibitory effect on growth suppression of P. theae when compared to the aqueous extract. The suppression in the growth of mycelium by the culture filtrate aqueous extract decreased from 242 to 121 mg/100 mL where the reduction percentage varied from 30.2 to 65.4%. Among the culture filtrates of four different bioagents tested, the ethyl acetate extract (50%) of T. viride VTV7 achieved the highest reduction percentage of 79.4% with decreased biomass of 71 mg of dry weight of P. theae when compared to control. In the case of the bacterial antagonist, B. subtilis VBS3 exhibited a reduction of 75% with decreased biomass of 86 mg of dry weight while P. fluorescens VPF5 exhibited 70.7% and S. griseus VSG4 exhibited 69.5% reduction with decreased biomass of 101 and 86 mg respectively. In contrast, the untreated control was registered with a maximum gain of dry weight of 346 mg/100 mL. In the presence of culture supernatant, there was a significant reduction in the mycelial dry weight of the pathogen. The concentration of toxicity of bioactive metabolites present in the culture extract may be related to the suppression rate of mycelial growth.
In vitro evaluation of antagonistic culture filtrates on inhibition of spore germination of Phomopsis theae
The impact of the culture supernatant of antagonistic strains on spore germination of the pathogen was studied with different concentrations (10, 25, and 50%). At a lower concentration of culture filtrate (10%), the germination was not appreciably affected. In comparison with control, culture filtrate at 25% showed medium inhibition on spore germination. It was established that there was a significant reduction and almost all the spores were found near to lethal at 50% concentration. Among four different antagonistic strains, the culture supernatant from T. viride VTV7 exhibited maximum inhibition (86.7%) of spore germination. Comparatively, the culture supernatant from VBS3 also showed a considerable rate of inhibition (81.3%) on spore germination (Table 8). On the other hand, culture supernatant from VPF5 and VSG4 strains exhibited more than 70% inhibition effect on spore germination. In the above treatments, the morphological changes were associated with loss of pigmentation and shrinkage of spores. The untreated control was registered with the highest rate of 94% spore germination.
3.9 Evaluation of crude volatile and non-volatile metabolites of antagonist strains against the radial growth ofPhomopsis theae.
The effect of crude volatile and non-volatile metabolite activity against pathogen growth is presented in Table 9. It was clearly demonstrated that the radial growth of the pathogen completely covered the control plate due to the absence of antifungal metabolites. In the presence of crude metabolites, while in contact with volatile compounds of antagonistic strains, the radial growth of the pathogen was significantly restricted. The maximum inhibition (72.2%) against radial growth of P. theae was produced by the fungal antagonist VTV7 strain. This was followed by the VPF5 strain with 70.1% of radial growth inhibition. However, the performance of this bacterial strain was on par with the fungal strain. Similarly, in the case of non-volatile inhibition, the VTV7 strain exhibited the highest inhibition (81.1%) against P. theae. Apart from this mycelial inhibition, conidia formation was completely arrested by VTV7 strain. In comparison with the untreated control, the metabolites of antagonistic strains not only arrested the mycelial growth but also reduced mycelial density.
Identification of the main secondary metabolites from the antagonist for suppression of Phomopsis theae
Analysis of crude secondary metabolites of selected antagonist strains was carried out using GC/MS to detect the presence of significant volatile compounds responsible for antifungal activity. A few groups of secondary metabolites detected in the selected biocontrol agents were identified for peak area and relative abundance. These are quite likely, the compounds responsible for the antagonistic activity against P. theae (Fig. 7). The prominent peaks exhibited by P. fluorescens VPF5 include 1,2-benzenedicarboxylic acid, 2,5-piperazinedione, dodecanoic acid, bis(2-methylpropyl) ester and 3-methyl-6-(1-methylethyl), and in case of B. subtilis VBS3, important identified compounds were 1,2-benzenedicarboxylic acid, bis(2-methylpropyl) ester, 2,5-dimethylpyrazine and 2-pentadecanone. The predominant antifungal compounds characterized from S. griseus VSG4 included dibutyl thalate, eicosane, and phenol, 2,5, bis (1,1-dimethyl ethyl). The chief antifungal compounds detected in the fungal antagonist VTV7 included cadinene, β-bisabolene, and α- farnesene. Aldehyde, pyrazines, esters, ketones, alcohols, pyridines, acids and benzene were some of the nature of the volatile organic compounds detected through GC-MS (Fig. 7).
Discussion
The plant rhizosphere is a highly dynamic environment for root interaction that provides niche and support for both beneficial and pathogenic microorganisms due to the availability of rich nutrients and for plant-microbe interactions (Raaijmakers et al. 2009). This interaction is necessary for the plant developmental process as it makes the absorption of nutrients possible, as well as protects the plant from phytopathogens (Lynch and Whipps 1990). The bioactive metabolites of beneficial microbes in the root niche directly influence crop health, yield, and soil fertility (Malik and Sindhu 2011). In this study, the selected four strains isolated from the tea rhizosphere presented several features for plant growth-promoting property associated with biocontrol potential which suggests their multiple actions are beneficial for the tea plant growth and health protection. We characterized the selected strains under various in-vitro microbiological techniques to study their potential for plant growth promotion and antagonistic nature against the stem pathogen P. theae.
The rhizospheric soil of healthy plants is considered an excellent source of plant growth-promoting microbes, which stimulates plant growth through various mechanisms (Yang et al. 2012). It has been reported that plant growth and nutrient uptake were directly influenced by plant growth regulators produced by PGPR (Glick 1995). An important plant-growth-promoting trait of PGPR is associated with phytohormone production including indole-3-acetic acid, cytokinin, and gibberellins (Kapoor et al. 2016). In our study, all four strains could produce these plant growth regulators; specifically, a significant production was observed in the P. fluorescens VPF5 strain. Therefore, phytohormones produced by these strains may be associated with stimulation of root proliferation, aid apical dominance, cell differentiation, stem elongation, axillary bud growth, and flowering in tea plants.
Soil microorganisms can convert insoluble phosphate complexes into a soluble orthophosphate form resulting in improved availability of phosphate for plant uptake and utilization (Rodríguez and Fraga 1999). Screening for phosphate solubilization was carried out for plant growth-promoting strains on Pikovskaya’s agar plates. Clear halo zones were observed around the colonies which indicated that the selected strains were good phosphate solubilizers. Further, quantitative estimation in Pikovskaya broth revealed that a positive relationship exists between the amount of soluble phosphate and titratable acidity and, in contrast, a negative relationship exists between the amount of soluble phosphate and pH. A similar observation was recorded by Braz and Nahas (2012). This study exhibited the ability of selected strains for organic acid production which would facilitate the solubilization of the insoluble phosphates in tea plants.
Siderophore production is one of the biocontrol mechanisms belonging to plant growth-promoting microbes. They inhibit the proliferation of phytopathogens by sequestering Fe3+ in the rhizosphere, making it unavailable to pathogens (Martinez-Viveros et al. 2010). In our findings, all the strains showed the ability for siderophore production under the CAS liquid assay. This important strategy, when adopted by the selected microbes, would inhibit pathogen growth by deprivation of the essential iron in the tea rhizosphere. Indeed, two types of siderophores; namely, hydroxamate and catecholate, were detected in the selected strains. Earlier, Hipol et al. (2019) reported that most soil fungi produced a hydroxamate type of siderophore, while bacteria secrete siderophores with various functional groups. These were consistent with our findings in which a catecholate type of siderophore was only produced by bacteria and absent in fungi.
Another PGP trait exhibited by biocontrol agents is the production of hydrogen cyanide, a gaseous volatile substance that directly blocks electron transport, which inhibits the energy supply to the cells and eventually causes cell death. According to Siddiqui et al. (2006), root-knot disease caused by Meloidogyne javanica was effectively controlled by hydrogen cyanide released by P. fluorescens. Therefore, this metabolite may have a role to suppress stem canker disease in the tea plant. Earlier Jha et al. (2012) discussed the role of beneficial microbes producing ammonia creating an alkaline environment in the rhizosphere that possibly defeats the growth of fungal phytopathogens. Our findings also demonstrated the property of these beneficial microbes in the conversion of complex nitrogenous substances to ammonia. Thus, this study indicates that the release of these microbes in the soil may suppress the growth of tea pathogens by this mechanism as well.
Salicylic acid is a key signaling elicitor compound recognized for the induction of induced systemic resistance (ISR) in the host (Shanmugam and Narayanasamy 2008). In our findings, the selected strains hold the property of salicylic acid production which revealed that it can be involved in endogenous signaling, mediating thus a defense strategy against the stem pathogen in the tea plant. Several rhizospheric bacteria exhibited the presence of 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity, identified as one of the primary mechanisms for regulation of ACC (Glick et al. 2007). The selected PGP strains from this study contained a considerable amount of ACC deaminase activity and hence this process may have a role to modulate ethylene levels in the tea plant under stress conditions.
The dual culture technique was adopted to study the effectiveness of the antagonistic nature of the selected strains against P. theae. The formation of an intersection zone in the dual culture plates indicates the presence of biometabolite diffused in the culture medium to arrest the mycelial growth of the pathogen produced by the different bacterial antagonists. A similar finding was reported by Sawai et al. (2014), where Streptomyces spp. showed high antifungal activity to S. rolfsii and R. solanacearum indicating the secretion of antibiotic substances dissolved in the agar medium of dual culture plates. Among different strains, the greatest radial growth inhibition was shown by the T. viride VTV7 strain. This may be due to the antagonistic ability of the Trichoderma species which controls the pathogenicity through different mechanisms including mycoparasitism, secretion of secondary metabolites and antibiotics. In addition, competition for nutrients and space altogether may contribute to a synergistic action of the biocontrol agent against the stem pathogen. This findings were consistent with the report of Rahman et al. (2009).
For Investigation on the possible mechanism of pathogen control, chronological events associated with the interaction between antagonistic strains and P. theae, and modification of the hyphal cell wall through scanning electron microscopy were studied. According to SEM, several remarkable features of morphological abnormalities were observed (Fig. 4). These morphological alterations of fungal mycelia appear to be due to the combined effect of metabolites and cell wall degrading enzymes. For example, the morphological abnormalities of the mycelia of (A) flavus, M. phaseolina and Pythium arrhenomanes were observed to include deformed and swollen mycelia, when treated with P. fluorescens, (B) cereus, S. aureofaciens and T. viride (Akocak et al. 2015; Shrivastava et al. 2017 and John et al. 2010).
In our study, the production of hydrolytic enzymes by the antagonistic strains possibly.
played a significant role in breaking the cell wall of the P. theae fungi. It has been reported that a significant positive correlation existed between the percentage of growth inhibition of test fungus and the number of hydrolytic enzymes secreted by antagonist T. viride (Parmer et al. 2015). Among them, chitinase and glucanase might be responsible for strong antagonistic activity against the fungal pathogen. This could be one of the possible mechanisms of biocontrol of the pathogen because the cell wall of fungi consists of polysaccharides such as chitin and glucan. Our finding was supported by the report of Kang et al. (2018) in which the architecture of the cell wall of the pathogenic fungus Aspergillus fumigatus when observed under solid-state NMR spectroscopy, revealed that chitin and α-1,3-glucan build a tight cross-link hydrophobic scaffold that contributes to cell wall rigidity and fungal virulence.
The antagonistic potential of the selected strains can be attributed to the nature of the production of extracellular compounds such as cell wall degrading enzymes and antibiotics. This ultimately leads to restriction of the pathogen growth with random breaks in the walls of pathogen hyphae which causes protoplast release and ultimate dissolution and disintegration of mycelial structure as observed under SEM. The above significant contributions determined the efficacy of antagonistic strains that assist the selection of biocontrol agents. This diverse mode of action of an antagonistic strain is essential to achieve optimum stem disease control when exploited under tea plantation soil. Our observations coincided with the earlier report of Köhl et al. (2019).
In the agar well diffusion assay, the antifungal activity exhibited by the selected biocontrol strains was mainly due to the secretion of components into the culture filtrate. In general, these extracellular antifungal compounds are secreted as proteins, enzymes, peptides, or volatile metabolites in nature (Hazarika et al. 2019). Kaur et al. (2016), stated that based on the nature of pathogenic fungi, MIC and MFC values of crude extract of biocontrol agents vary and accordingly suppress the growth of plant pathogenic fungi. In our findings, antagonistic strains showed MIC and MFC values identified in the range of 25–100 µg/mL and 75–200 µg/mL to suppress the fungal growth. Thus, an evaluation of the dosage optimization of culture filtrate is required for greenhouse and field trials in tea plants.
A study on the suppression effect of the culture supernatant of different antagonistic strains on the dry biomass of P. theae was carried out in a liquid medium. The ethyl acetate extract from the VTV7 strain (50%) potentially restrained the mycelia growth at an appreciable level when compared to other treatments and control. Manhas and Kaur (2016) demonstrated that when compared to control, the dry biomass of the fungal pathogen in the test sample was significantly reduced in the presence of culture supernatant of S. hydrogenans. It has been reported that the suppression of mycelial growth and spore germination inhibition may be related to the abundance of antifungal metabolites present in the culture filtrate of the antagonist (Lal et al. 2009). In our study, it has been observed that the suppression of both mycelial growth and conidial germination depends upon the concentration of culture supernatant and nearly complete arrest was noticed at higher concentration. Our above findings were similar to those from Li et al. (2011).
The possible volatile organic compounds secreted by the biocontrol agents were identified using the GC-MS technique. The detected compounds were antifungal secondary metabolites responsible for the antagonistic activity against P. theae. Apart from the compounds identified based on relative abundance, the other antifungal compounds detected in P. fluorescens and B. subtilis included phenol and benzaldehyde (Raza et al. 2016). Similarly, dimethyl disulphide and 1-undecene detected in B. subtilis were classified as sulphur and hydrocarbon-containing compound recognized for antifungal activity which was confirmed previously by Farag et al. (2006). Similarly, the compound 2-pentadecanone produced in B. subtilis holds biocontrol properties against P. theae which was earlier reported by Li et al. (2015b). Certain soil bacteria also produce benzothiazole which has a fungicidal activity that causes lethal to surviving spores in the soil and thus controls the production and occurrence of the disease (Santos et al. 2023; Raza et al. 2016).
The eicosane and dibutyl thalate compounds identified in S. griseus (VSG4) were related to antifungal activity and there was an earlier report to support this finding (Ahsan et al. 2017). The above compounds were considered bioactive substances against P. theae. The plant growth-promoting property of Trichoderma species was assigned to a blend of VOC including 3-methyl butanol and 2-methylbutanol (Di Francesco at al. 2020). It has been also reported that the compounds dl-limonene; cadinene; beta-bisabolene and alpha-farnesene, displayed antifungal effects, and hence, the biocontrol activity of Trichoderma species can be related to them. These VOCs could be an effective synergistic resource for natural fungicides to prevent crops from the attack of fungal disease (Di Francesco at al. 2020). Therefore, the above VOC detected in the identified potential strains may have a role in the suppression of stem canker disease.
Conclusion
The results of the present study are encouraging because the selected four strains isolated from the tea soil exhibited multifarious PGP traits and promising antifungal activity against tea stem pathogen P. theae. In addition, concentrated crude metabolites of these strains inhibited the growth of phytopathogenic fungi in vitro, highlighting their potential to be used as biocontrol agents. A combination of antifungal volatile metabolites secreted by these biocontrol agents could mediate a synergistic protection for tea plants from fungal diseases. Several beneficial microbes have been reported to produce a diverse group of antifungal compounds; however, more demand exists for bioactive metabolites with novel properties for different varieties of pathogens to achieve plant protection. The above findings indicate that the selected four antagonistic strains and their culture metabolites can be developed as biofertilizers and bio fungicides for soil application as well as a wound healing to control stem canker disease in tea plants. This study could pave the way for an integrated management of the tea plant disease with a combination of these antifungal compounds. However, nursery and field trial studies have to be carried out to confirm the real efficacy of plant growth-promoting potential and biocontrol activities of these promising strains when exploited in tea plantations.
Data Availability
The data analysed during this study are included in this article /Supplementary material, further the datasets generated during the current study are available from the corresponding author on reasonable request.
References
Agrios GN (1997) Plant Pathology, 4th edn. Academic Press, San Diego
Ahsan T, Chen J, Zhao X, Irfan M, Wu Y (2017) Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Expr 7(1):54. https://doi.org/10.1186/s13568-017-0351-z
Akhtar MS, Siddiqui ZA (2009) Use of plant growth-promoting rhizobacteria for the biocontrol of root-rot disease complex of chickpea. Aust Plant Pathol 38:44–50
Akocak PB, Churey JJ, Worobo RW (2015) Antagonistic effect of chitinolytic Pseudomonas and bacillus on growth of fungal hyphae and spores of aflatoxigenic aspergillus flavus. Food Biosci 10:48–58. https://doi.org/10.1016/j.fbio.2015.01.005
Antoun H, Kloepper JW (2001) Plant growth promoting rhizobacteria. In: Brenner S, Miller JH (eds) Encyclopedia of Genetics. Academic, New York, pp 1477–1480
Baby UI (2002) An overview of blister blight disease of tea and its control. J Plant Crops 30:1–12
Baby UI, Ponmurugan P, Premkumar R, Radhakrishnan B, Udhayabhanu KG, Cox S (2001) Incidence of Phomopsis canker in south indian tea plantations. Planter’s Chron 97:303–307
Baldani JI, Reis VM, Videira SS, Boddey LH, Baldani VLD (2014) The art of isolating nitrogen-fixing bacteria from nonleguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil 384:413–431. https://doi.org/10.1007/s11104-014-2186-6
Berg G, Fritze A, Roskot N, Smalla K (2001) Evaluation of potential biocontrol rhizobacteria from different host plants of Verticillium dahliae Kleb. J Appl Microbiol 91(6):963–971. https://doi.org/10.1046/j.1365-2672.2001.01462.x
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28(4):1327–1350. https://doi.org/10.1007/s11274-011-0979-9
Bissett J (1991a) A revision of the genus Trichoderma. II. Infrageneric classification. Can J Bot 69:2357–2372
Bissett J (1991b) A revision of the genus Trichoderma. III. Section pachybasium. Can J Bot 69:2373–2417
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Braz RR, Nahas E (2012) Synergistic action of both Aspergillus niger and Burkholderia cepacea in co-culture increases phosphate solubilization in growth medium. FEMS Microbiol Lett 332:84–90. https://doi.org/10.1111/j.1574-6968.2012.02580.x
Brick JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57(2):535–538. https://doi.org/10.1128/aem.57.2.535-538
Cappuccino JC, Sherman N (1992) Biochemical activities of microorganisms In: Microbiology: A Laboratory Manual 3rd edn. Benjamin-Cummings Publ., Co, Redwood City, California, USA, 1992, pp. 125–179
Chen X, Zhang Y, Fu X, Li Y, Wang Q (2016) Isolation and characterization of Bacillus amyloliquefaciens PG12 for the biological control of apple ring rot. Postharvest Biol and Technol 115:113–121. https://doi.org/10.1016/j.postharvbio.2015.12.021
Dellavalle PD, Cabrera A, Alem D, Larrañaga P, Ferreira F, Rizza MD (2011) Antifungal activity of medicinal plant extracts against phytopathogenicfungus Alternaria spp. Chil J Agric Res 71(2):231–239. https://doi.org/10.4067/S0718-58392011000200008
Dennis C, Webster J (1971) Antagonistic Properties of Species-Groups of Trichoderma: II. Production of volatile antibiotics. Trans Br Mycol Soc 57(1):41–48 IN4. https://doi.org/10.1016/S0007-1536(71)80078-5
Dheepa R, Vinodkumar S, Renukadevi P, Nakkeeran S (2016) Phenotypic and molecular characterization of chrysanthemum white rust pathogen Puccinia horiana (Henn) and the effect of liquid based formulation of Bacillus spp. for the management of Chrysanthemum white rust under protected cultivation. Biol Control 103:172–186. https://doi.org/10.1016/j.biocontrol.2016.09.006
Di Francesco A, Zajc J, Gunde-Cimerman N et al (2020) Bioactivity of volatile organic compounds by Aureobasidium species against gray mold of tomato and table grape. World J Microbiol Biotechnol 36:171. https://doi.org/10.1007/s11274-020-02947-7
Dobbelaere S, Vanderleyden J, Okon Y (2003) Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit Rev Plant Sci 22:107–149. https://doi.org/10.1080/713610853
El-Sayed WS, Akhkha A, El-Naggar MY, Elbadry M (2014) In vitro antagonistic activity, plant growth promoting traits and phylogenetic affiliation of rhizobacteria associated with wild plants grown in arid soil. Front Microbiol 5:651. https://doi.org/10.3389/fmicb.2014.00651
Ereful NC, Paterno ES, Merca FE (2007) Assessment of cytokinin production in some plant growth-promoting bacteria. Asian Int J Life Sci 16(2):137–152
Farag MA, Ryu CM, Sumner LW, Paré PW (2006) GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 67(20):2262–2268. https://doi.org/10.1016/j.phytochem.2006.07.021
Gaur AC (1990) Physiological functions of phosphate solubilizing microorganisms. In: Gaur AC (ed) Phosphate Solubilizing Micro-organisms as Biofertilizers. Omega Scientific Publishers, New Delhi, pp 16–72
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41(2):109–117. https://doi.org/10.1139/m95-015
Glick BR, Todorovic B, Czarny J, Cheng Z, Duan J, McConkey B (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26:227–242. https://doi.org/10.1080/07352680701572966
Goswami D, Vaghela H, Parmar S, Dhandhukia P, Thakker JN (2013) Plant growth promoting potentials of Pseudomonas spp. strain OG isolated from marine water. J Plant Interact 8:281–290. https://doi.org/10.1080/17429145.2013.768360
Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319. https://doi.org/10.1038/nrmicro1129
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species-opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56. https://doi.org/10.1038/nrmicro797
Hazarika DJ, Goswami G, Gautom T et al (2019) Lipopeptide mediated biocontrol activity of endophytic Bacillus subtilis against fungal phytopathogens. BMC Microbiol 19:71. https://doi.org/10.1186/s12866-019-1440-8
Hipol RM, Baldelomar JA, Bolinget KC, Solis AFF (2019) The soil fungi producing siderophores of Mt. Yangbew, Tawang, La Trinidad, Benguet. Stud Fungi 4(1):1–13. https://doi.org/10.5943/sif/4/1/1
Holt JG, Krieg NR, Sneath PHA, Stanley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology, 9th edn. Williams & Wilkins Co., Baltimore, Maryland USA
Jayaprakashvel M, Mathivanan N (2011) Management of plant diseases by microbial metabolites. In: Harikesh BS, Chetan K, Reddy MS, Estibaliz S, Carlos GE (eds) Bacteria in Agrobiology: Plant Nutrient Management. Sringer-Verlag, Berlin, pp 237–265
Jha CK, Patel B, Sarf M (2012) Stimulation of the growth of Jatropha curcas by the plant growth bacterium Enterobacter cancerogenus MSA2. World J Microbiol Biotechnol 28(3):891–899. https://doi.org/10.1007/s11274-011-0886-0
John RP, Tyagi RD, Prévost D, Brar SK, Pouleur S, Surampalli RY (2010) Mycoparasitic Trichoderma viride as a biocontrol agent against Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes and as a growth promoter of soybean. Crop Prot 29:1452–1459. https://doi.org/10.1016/j.cropro.2010.08.004
Kang X, Kirui A, Muszyński A, Widanage MCD, Chen A, Azadi P, Wang P, Mentink-Vigier F, Wang T (2018) Molecular architecture of fungal cell walls revealed by solid-state NMR. Nat Commun 9(1):2747. https://doi.org/10.1038/s41467-018-05199-0
Kapoor R, Soni R, Kaur M (2016) Gibberellins production by fluorescent Pseudomonas isolated from Rhizospheric soil of Malus and Pyrus. Int J of Agric Env and Biotechnol 9(2):193–199. https://doi.org/10.5958/2230-732X.2016.00026.7
Kaur T, Kaur A, Sharma V, Manhas RK (2016) Purification and characterization of a new antifungal compound 10-(2,2-dimethyl-cyclohexyl)-6,9-dihydroxy-4,9-dimethyl-dec-2-enoic acid Methyl Ester from Streptomyces hydrogenans strain DH16. Front Microbiol 7:1004. https://doi.org/10.3389/fmicb.2016.01004
Kloepper JW, Beauchamp CJ (1992) A review of issues related to measuring colonization of plant roots by bacteria. Can J Microbiol 38(12):1219–1232. https://doi.org/10.1139/m92-202
Köhl J, Kolnaar R, Ravensberg WJ (2019) Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance Beyond Efficacy. Front Plant Sci 10:845. https://doi.org/10.3389/fpls.2019.00845
Kulimushi PZ, Arias AA, Franzil L, Steels S, Ongena M (2017) Stimulation of fengycin-type antifungal lipopeptides in Bacillus amyloliquefaciens in the presence of the maize fungal pathogen Rhizomucor variabilis. Front Microbiol 8:850. https://doi.org/10.3389/fmicb.2017.00850
Lal RJ, Sinha OK, Bhatnagar S et al (2009) Biological control of sugarcane smut (Sporisorium scitamineum) through botanicals and Trichoderma viride. Sugar Tech 11:381–386. https://doi.org/10.1007/s12355-009-0065-x
Letham DS (1971) Regulators of cell division in plant tissues XII. A cytokinin Bioassay using excised Radish Cotyledons. Physiol Plant 25:391–396. https://doi.org/10.1111/j.1399-3054.1971.tb01462.x
Li Q, Jiang Y, Ning P, Zheng L, Huang J, Li G et al (2011) Suppression of Magnaporthe oryzae by culture filtrates of Streptomyces globisporus JK-1. Biol Control 58:139–148. https://doi.org/10.1016/j.biocontrol.2011.04.013
Li Z, Guo B, Wan K, Cong M, Huang H, Ge Y (2015a) Effects of bacteria-free filtrate from Bacillus megaterium strain L2 on the mycelium growth and spore germination of Alternaria alternata. Biotechnol Equip 29(6):1062–1068. https://doi.org/10.1080/13102818.2015.1068135
Li XY, Mao ZC, Wu YX, Ho HH, He YQ (2015b) Comprehensive volatile organic compounds profiling of Bacillus species with biocontrol properties by head space solid phase microextraction with gas chromatography-mass spectrometry. Biocontrol Sci Technol 25:132–143. https://doi.org/10.1080/09583157.2014.960809
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Ann Rev Microbiol 63:541–556. https://doi.org/10.1146/annurev.micro.62.081307.162918
Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant Soil 129:1–10. https://doi.org/10.1007/BF00011685
Maatallah J, Berraho EB, Munoz S, Sanjuan J, Lluch C (2002) Phenotypic and molecular characterization of chickpea rhizobia isolated from different areas of Morocco. J Appl Microbiol 93(4):531–540. https://doi.org/10.1046/j.1365-2672.2002.01718.x
Malik DK, Sindhu SS (2011) Production of indole acetic acid by Pseudomonas sp.: effect of coinoculation with Mesorhizobium sp. Cicer on nodulation and plant growth of chickpea (Cicer arietinum). Physiol Mol Biol Plants 17(1):25–32. https://doi.org/10.1007/s12298-010-0041-7
Manhas RK, Kaur T (2016) Biocontrol potential of Streptomyces hydrogenans strain DH16 toward Alternaria brassicicola to control damping off and black leaf spot of Raphanus sativus. Front Plant Sci 7:1869. https://doi.org/10.3389/fpls.2016.01869
Martínez-Absalón S, Rojas-Solís D, Hernández-León R et al (2014) Potential use and mode of action of the new strain Bacillus thuringiensis UM96 for the biological control of the grey mould phytopathogen Botrytis cinerea. Biocontrol Sci Technol 24:1349–1362. https://doi.org/10.1080/09583157.2014.940846
Martinez-Viveros O, Jorquera MA, Crowley DE, Gajardo G, Mora ML (2010) Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant Nutr 10(3):293–319. https://doi.org/10.4067/S0718-95162010000100006
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Pan SQ, Ye XS, Kuc J (1991) A technique for detection of chitinase, β-1,3- glucanase, and protein patterns after a single separation using polyacrylamide gel electrophoresis or isoelectrofocusing. Phytopathol 81:970–974
Pan Z, Li X, Yang XB, Andrade D, Xue L, McKinney N (2010) Prediction of plant diseases through modelling and monitoring airborne pathogen dispersal. In: Hemming D (ed) CAB reviews: perspectives in Agriculture, Veterinary Science. Nutrition and Natural Resources CABI, UK, pp 191–202
Pandya ND, Desai PV (2014) Screening and characterization of GA3 producing Pseudomonas monteilii and its impact on plant growth promotion. Int J Curr Microbiol App Sci 3(5):110–115
Parmer HJ, Bodar NP, Lakhani HN, Patel SV, Umrania VV, Hassan MM (2015) Production of lytic enzymes by Trichoderma strains during in vitro antagonism with Sclerotium rolfsii the causal agent of stem rot of groundnut. Afr J Microbiol Res 9(6):365–372. https://doi.org/10.5897/AJMR2014.7330
Payne SM (1994) Detection, isolation and characterization of siderophores. Method Enzymol 235:329–344. https://doi.org/10.1016/0076-6879(94)35151-1
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118(1):10–15. https://doi.org/10.1034/j.1399-3054.2003.00086.x
Perez C, Pauli M, Bazerque P (1990) An antibiotic assay by agar-well diffusion method. Acta Biologiae et Medecine Experimentalis 15:113–115
Petatán-Sagahón I, Anducho-Reyes MA, Silva-Rojas HV et al (2011) Isolation of bacteria with antifungal activity against the phytopathogenic fungi Stenocarpella maydis and stenocarpella macrospora. Int J Mol Sci 12(9):5522–5537. https://doi.org/10.3390/ijms12095522
Ponmurugan P, Baby UI (2007) Evaluation of fungicides and biocontrol agents against Phomopsis canker of tea under field conditions. Aus Plant Pathol. https://doi.org/10.1071/AP06084
Ponmurugan P, Baby UI, Gopi C (2006) Efficacy of certain fungicides against Phomopsis theae under in vitro conditions. Afr J Biotechnol 5(5):434–436. https://doi.org/10.5897/AJB05.411
Ponmurugan P, Gnanamangai BM, Baby UI (2013) Incidence, prevalence and clonal susceptibility of Phomopsis canker disease in southern indian tea plantations. Indian Phytopathol 66(1):46–52. https://epubs.icar.org.in/index.php/IPPJ/article/view/28109
Prapagdee B, Kuekulvong C, Mongkolsuk S (2008) Antifungal potential of Extracellular Metabolites produced by Streptomyces hygroscopicus against Phytopathogenic Fungi. Int J Biol Sci 4:330–337
Raaijmakers JM, Mazzola M (2012) Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Ann Rev Phytopathol 50:403–424. https://doi.org/10.1146/annurev-phyto-081211-172908
Raaijmakers JM, Paulitz TC, Steinberg C et al (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361. https://doi.org/10.1007/s11104-008-9568-6
Rabindran R, Vidyasekaran P (1996) Development of a formulation of P. fluorescens PfALR2 for management of rice sheath blight. Crop Protect 15(8):715–721. https://doi.org/10.1016/S0261-2194(96)00045-2
Rahman MA, Begum MF, Alam MF (2009) Screening of Trichoderma isolates as a Biological Control Agent against Ceratocystis paradoxa Causing Pineapple. Disease of Sugarcane Mycobiol 37(4):277–285. https://doi.org/10.4489/MYCO.2009.37.4.277
Raza W, Ling N, Yang L et al (2016) Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Sci Rep 6:24856. https://doi.org/10.1038/srep24856
Reissig JL, Strominger JL, Leloir LF (1955) A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem 217(2):959–966
Rodríguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17(4–5):319–339. https://doi.org/10.1016/s0734-9750(99)00014-2
Sambrook J, Russell DW (2001) Molecular cloning: a Laboratory Manual. Cold Spring Harbor Laboratory Press, New York
Samuels GJ, Dodd SL, Gams W, Castlebury LA, Petrini O (2002) Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 94(1):146–170. https://doi.org/10.2307/3761854
Santos JEd, de Brito MV, Pimenta ATÁ et al (2023) Antagonism of volatile organic compounds of the Bacillus sp. against Fusarium kalimantanense. World J Microbiol Biotechnol 39: 60 (2023). https://doi.org/10.1007/s11274-022-03509-9
Saxena AK, Lata Annapurna K (2002) Training manual on Plant Growth promoting Rhizobacteria. Division of Microbiology, Indian Agricultural Research Institute, New Delhi, India, p 38
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Shanmugam P, Narayanasamy M (2008) Optimization and production of salicylic acid by rhizobacterial strain Bacillus licheniformis MML2501. Internet J Microbiol 6:1–8. https://print.ispub.com/api/0/ispub-article/11025
Shrivastava P, Kumar R, Yandigeri MS (2017) In vitro biocontrol activity of halotolerant Streptomyces aureofaciens K20: a potent antagonist against Macrophomina phaseolina (Tassi) Goid. Saudi J Biol Sci 24(1):192–199. https://doi.org/10.1016/j.sjbs.2015.12.004
Siddiqui IA, Shaukat SS, Sheikh IH et al (2006) Role of cyanide production by Pseudomonas fluorescens CHA0 in the suppression of root-knot nematode Meloidogyne javanica in tomato. World J Microbiol Biotechnol 22:641–650. https://doi.org/10.1007/s11274-005-9084-2
Statements & Declarations
Tabatabai MA (1994) Soil enzymes. In: Weaver RW, Angle JS, BottomLey PS (eds) Methods of soil analysis. Microbiological and biochemical properties. Soil Science Society of America, Madison, WI, pp 775–833
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739. https://doi.org/10.1093/molbev/msr121
Tsuchida O, Yamagata Y, Ishizuka T et al (1986) An alkaline proteinase of an alkalophilic Bacillus sp. Curr Microbiol 14:7–12. https://doi.org/10.1007/BF01568094
Visca P, Ciervo A, Sanfilippo V, Orsi N (1993) Iron-regulated salicylate synthesis by Pseudomonas spp. J Gen Microbiol 139(9):1995–2001. https://doi.org/10.1099/00221287-139-9-1995
Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA et al (2009) Bergey’s Manual of systematic bacteriology. NewYork, Springer
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Xu Y, Wang D, Mu XQ, Zhao GA, Zhang KC (2002) Biosynthesis of ethyl esters of short-chain fatty acids using whole-cell lipase from Rhizopus chinensis CCTCC M201021 in non-aqueous phase. J Mol Cat B: Enzymat 18:29–37
Yang L, Xie J, Jiang D, Fu Y, Li G, Lin F (2008) Antifungal substances produced by Penicillium oxalicum strain PY-1 potential antibiotics against plant pathogenic fungi. World J Microbiol Biotechnol 24:909–915. https://doi.org/10.1007/s11274-007-9626-x
Yang W, Xu Q, Liu HX et al (2012) Evaluation of biological control agents against Ralstonia wilt on ginger. Biol Control 62(3):144–151. https://doi.org/10.1016/j.biocontrol.2012.05.001
Yang JH, Zhang WW, Zhuang YQ, Xiao T (2017) Biocontrol activities of bacteria from cowdung against the rice sheath blight pathogen. J Plant Dis Prot 124:131–141. https://doi.org/10.1007/s41348-017-0080-1
Acknowledgements
The authors are thankful to the Department of Science and Technology, Ministry of Science and Technology, New Delhi, India for financial support to carry out this study. The authors are also thankful to the management of the Vellore Institute of Technology University, Vellore for providing the necessary facilities to carry out this work.
Funding
This work was supported by the Department of Science and Technology (DST), [grant number: DST/DISHA/SoRF-PM/049/2013(G)], Ministry of Science and Technology, New Delhi, India. Author MK has received the research support from DST.
Author information
Authors and Affiliations
Contributions
PP and AKAM conceived, planned, and designed the experiment, involved in data analysis and interpretation. MK did sample collection, performed lab experiments, assembled and interpreted data analysis and drafted manuscript. MGB was involved in data evaluation of statistical analysis and discussed the manuscript write-up. AKAM supervised the study, mobilized the consumable for the study, edited, commented and critically revised the final manuscript. PP co-supervised the study, provided suggestions during manuscript preparation. MK wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kolandasamy, M., Mandal, A.K.A., Balasubramanian, M.G. et al. Multifaceted plant growth-promoting traits of indigenous rhizospheric microbes against Phomopsis theae, a causal agent of stem canker in tea plants. World J Microbiol Biotechnol 39, 237 (2023). https://doi.org/10.1007/s11274-023-03688-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03688-z